Abstract
Asplenic individuals are compromised not only in their ability to destroy infectious agents, but are at increased risk of death from autoimmune disease, certain tumors, and ischemic heart disease. Enhanced mortality is attributed to lack of phagocytes sequestered in spleen that efficiently engulf and destroy appropriate targets, though related cells are found elsewhere. To determine whether a unique population regulates RBC-pathogen clearance and filtration of altered self, we reviewed the anatomic literature and analyzed in situ by immunohistochemistry and immunofluorescence the expression patterns of a little-characterized cell that dominates the splenic red pulp of man and closely related primates-the venous sinus lining or littoral cell (LC). High expression of the formin FHOD1 outlines the LC population. Though LCs are endothelial-like in distribution they express several macrophage directed proteins, the RBC antigen DARC and T-cell co-receptor CD8α/α yet they lack lineage-associated markers CD34 and CD45. Strikingly, SIRPα (CD172a) expression in human spleen concentrates on LCs, consistent with recent demonstration of a key role in RBC turnover and elimination versus release of infected or altered self. Our results indicate human LCs (SIRPα+, FHOD1+, CD8α/α+, CD34−, CD45−) comprise a highly plastic barrier cell population that emerged late in primate evolution coordinate with CD8 expression. Unique to Hominidae, LCs may be the ultimate determinant of which cells re-circulate after passage through human spleen.
Keywords: Spleen, littoral cell, angioma, RBC, FHOD1, DARC, CD8α/α, SIRPα, primate
Introduction
Individuals who are asplenic or functionally asplenic (hyposplenic) are unable to eliminate many bacterial and parasite pathogens [1]. They also display an increased risk of autoimmune disease [1], some cancers [2] and ischemic cardiac disease[2, 3]. Perhaps this is not surprising as the spleen is the largest secondary immune system organ in mammals. However, although the overall organization of splenic white pulp is similar to that of lymph nodes, the spleen is not connected to the lymphatic system [4]. Rather, soluble and particulate antigens, pathogens, RBCs, altered, apoptotic, necrotic and tumor cells are all delivered to and leave the spleen via the circulation. In contrast to white pulp, the red pulp of mammalian spleen serves as a filter that mediates the ultimate retention and destruction of senescent and modified cells [5, 6].
Opsonization with antibody and/or complement enhances clearance of foreign antigens, cell debris and altered self by phagocytes [1, 7]. In man, inflammatory particles that are opsonized and immobilized by complement fragments are regularly transported through the circulation tethered by complement receptor type 1 (CR1, CD35) on the RBC membrane for efficient phagocytosis at distant locations [4, 8, 9]. This process, a sophisticated mechanism known as immune adherence clearance (IAC) evolved with mammals to remove inflammation-inducing particles from blood [10]. However, utilization of RBCs for IAC is a recent development in the evolution of the immune system restricted to humans and closely related primates, as non-primates including rodents primarily rely on platelet-based transport mechanisms [11, 12]. Much evidence indicates liver and spleen are where CD35-bound immune complexes (IC) are delivered [9], though precisely how ICs are removed at each location is not well understood.
RBCs themselves (residual IC loaded, infected and aging) are sequestered, variably internalized, and removed as they course through the red pulp of spleen [13]. Precisely where and how RBCs as well as other altered cell types delivered by the circulation are destroyed or filtered is unknown. The stroma of human red pulp is comprised of the cords of Billroth and the sinusoids [14]. In contrast to man, the mouse has an asinusal spleen and thus it is architecturally and functionally distinct [4, 15–21]. Human red pulp has a high content of diverse phagocytes, however to return to the venous circulation most splenic constituents must pass between or through the sinus lining cell or littoral cell (LC). Although the morphologic appearance of the LC suggests it may be a key mediator of particle clearance and cellular filtration [13], direct demonstration of function is lacking. In fact, little is known about these cells.
Littoral, literally means shoreline or tidal, which accurately describes the polarized cells that line the venous sinusoids of human spleen with cytoplasmic fronds protruding into the sinus. LCs comprise ~30% of the red pulp and thus are a major constituent of human spleen [22]. Morphologically LCs are elongate and contain prominent cytoplasmic filaments (stress fibers) [23]. Their abundant cytoplasm is filled with many pinocytic vesicles, lysosomes, and dense deposits that surround the nucleus. Phagocytosed RBCs, leukocytes, hemosiderin, and other debris can be visualized within these cells [24–26] – even more so in the setting of certain diseases [27, 28]. RBCs can also be seen coursing between adjacent LCs [10, 13].
Based on their perisinusal distribution, it was long assumed LCs derive from endothelium. However, in 1983–1985, in situ staining showed [22, 29] that LCs also express several antigens found on histiocytes as well as CD8, which at that time was believed to be T-cell restricted. Though CD8 expression was verified [14], the close proximity of LCs to other red pulp components including diverse phagocytes, endothelial cells and lymphocytes often resulted in discrepant characterization. Since then work has been limited. However, the LC angioma (LCA), an unusual splenic tumor believed to derive from LCs, has been more often described despite its rarity [24]. LCAs are low-grade tumors that contain few atypical mitoses. Though related by morphology, angioma cells appear enlarged, disorganized and interestingly they lack CD8 [24, 30], a consistent marker of normal LCs. A striking clinical observation is frequent association of LCAs with malignancy (often lymphoma or solid tumors) at distant sites, mandating investigation for occult malignancy in any patient diagnosed with LCA [31]. LCAs have also been associated with several forms of autoimmune disease (lupus, inflammatory bowel disease), with certain hemoglobinopathies (sickle cell disease, hemoglobin Punjab) and a storage diorders (Gaucher’s). This diversity suggests recognition, contact, internalization and/or filtration of abnormal cell types or cell products (possibly entosis) [28, 31, 32] drives aberrant LC activation and development of the LCA.
We report that FHOD1, a member of the formin family of diaphanous-related formins (DRFs), is highly expressed on human LCs distinguishing them from other splenocytes [33]. Using high FHOD1 and CD8 expression together with perisinusal localization as an initial guide we examined and/or re-examined when discrepant, previously reported patterns of antigen expression on LCs from normal human spleen. We contrasted this with the LCA, other red pulp inhabitants, rodent and a spectrum of primate splenocytes. Endothelial cells that line the venous sinuses of human liver although similar in distribution are distinct from LCs which express a unique spectrum of antigens including FHOD1, SIRPα/CD172a, CD8 α/α, as well as DARC/CD234 [34], CD206 [35], stabilin-1[36], and other distinctive proteins-despite lacking common lineage associated markers CD34 (endothelial) and CD45 (hematopoietic). Perhaps most revealing, the unexpectedly high polarized expression of SIRPα on LCs when compared with surrounding red pulp phagocytes hints at mechanism. SIRPα, a transmembrane protein primarily localized to myeloid cells [37], has been increasingly implicated as a major regulator of RBC turnover and innate self-recognition [38, 39]. This is achieved through interaction with CD47 a ubiquitous ligand expressed on neighboring cells that upon engaging SIRPα transduces a negative intracellular signal that blocks phagocytosis. The final decision to destroy or not to release pathogen-bearing and senescent RBCs, circulating tumor and/or other altered cells marked by reduced or absent CD47 into the venous circulation may ultimately be determined by the LC - a hypothesis examined herein.
Materials and Methods
Tissue
Normal discarded and unidentified human spleen consented for research was obtained from the Pathology Departments of Beth Israel Deaconess Medical Center (BIDMC). Brigham and Women’s Hospital (BWH) and from the New England Organ Bank (NEOB) The tissues were obtained in accordance with the policies of the Institutional Review Board at each of the respective sites. Splenic tissues were processed immediately to optimize conservation of cell morphology and composition. LCA slides were provided by the BWH Pathology Department (to SR, generously provided by Dr. Christopher D. M. Fletcher). Archived formalin-fixed paraffin embedded non-human primate spleens were obtained from a repository at the New England and Southwest Primate Research Centers. Archived formalin-fixed paraffin embedded normal mouse (BALB/c) and rat (Sprague Dawley) spleen was obtained from the Dana-Farber Cancer Institute (DFCI).
Immunohistochemistry (IHC) of mammalian spleens
IHC staining and analysis were performed per routine protocol on human, non-human primate, and rodent splenic tissues as previously described [40]. Antibody concentrations and reaction conditions were varied as described in Table 1. All sections were 5-um-thick formalin-fixed, paraffin embedded splenic tissue sections on individual slides. Two independent pathologists assessed reactivity for enumerated antigens. Positive staining of lymphocytes, endothelial cells and distributed neutrophils and macrophages served as positive internal controls. Positive staining for LCs was defined as evidence of peroxidase reaction within the linear and circular LC network either as continuous cytoplasmic or polarized linear membrane staining.
Table 1.
Antibodies used for IHC with method information.
| Antibody | Clone | Species* | Source | Pre-Treatment ** | Detection System *** | Dil. (1:X) |
|---|---|---|---|---|---|---|
| CD3 | SP7 | RM | Thermo Scientific | TUF (30′) | RPV | 300 |
| CD4 | 4B12 | MM | Vector lab | EDTA (50′) | MPV | 200 |
| CD8 alpha (human) | C8/144B | MM | Dako | EDTA (30′) | MEV | 100 |
| CD8 (monkey) | VP-C325 Clone 1A5 | MM | Vector lab | Trilogy Solution | MEV | 50 |
| CD8 beta (human) | F-5 | MM | Santa Cruz (sc-25277) | EDTA (30′) | MEV | 50 |
| CD14 | 7 | MM | Abcam (ab49755) | EDTA | MEV | 100 |
| CD19 | LE-CD19 | MM | Serotec (MCA24254T) | Citrate | MEV | 50 |
| CD20 | L26 | MM | Dako (N1502) | Dako RTU | MEV | RTUb |
| CD21 | IF8 | MM | Dako (M0784) | DAKO PTS (PC 3′) | MPV | 40 |
| CD31 | JC/70A | MM | Dako | EDTA (30′) | MPV | 100 |
| CD34 | QBEnd-10 | MM | Beckman-Coulter | Citrate | MPV | 70 |
| CD35 | Ber-MARC-DRC | MM; RMa | Dako | DAKO PTS (PC 3′) | RPV | 30 |
| CD45 | 2B11+PD7/26 | MM | Dako | Citrate | MPV | 500 |
| CD56 | 123C3.D5 | MM | Cell Marque | EDTA (30′) | MPV | 50 |
| CD68 | PGM1 | MM | Dako (M0876) | Dako RTU | MEV | 200 |
| CD163 | 10D6 | MM | NovoCastra | DAKO PTS (PC 3′) | MPV | 400 |
| CD172a | Human (syn. peptide) | RP | Abcam (ab53721) | Citrate | REV | 2000 |
| CD234 (Darc) | 358307 | MM | R&D Systems (MAB4139) | Citrate | MEV | 150 |
| D2-40 | D2-40 | MM | Covance | Citrate (PC) | MPV | 100 |
| FHOD-1 | Human protein (full-length, aa1-1165) Human (N-term aa 1-965) |
MP RP |
Abcam (ab73443) Fingeroth lab |
Citrate | MEV | 200 |
| TSP1 | A6.1 | MM | Abcam ab1823 | Citrate (PC) | MEV | 25 |
RM = Rabbit Monoclonal; RP = Rabbit Polyclonal; MM = Mouse Monoclonal; MP = Mouse Polyclonal
TUF = Tissue Unmasking Fluid; PTS = Pre-treatment solution (proprietary); PC = Pressure Cooker
RPV = Rabbit Powervision; MPV = Mouse Powervision; REV = Rabbit Envision; MEV = Mouse Envision
A mouse-anti-human is used as primary followed by a rabbit-anti-mouse and then Rabbit Powervision
Ready to Use
A rabbit antisera to the N terminal portion of FHOD-1 was also used in some assays.
Detection of CD8 expression on primate spleen by IHC
An IHC protocol for detection of CD8 was performed on primate spleen as previously described [41]. In brief, formalin fixed paraffin embedded tissues were sectioned at 5 μm, deparaffinized in xylene, rehydrated in sequential ethanol/water washes and incubated with 3% hydrogen peroxide in phosphate buffered saline (PBS) for 5 minutes followed by antigen retrieval consisting of microwave pre-treatment in Trilogy solution (Cell Marque, Rocklin, CA). Dual endogenous enzyme block (5 minutes) and protein block (10 minutes) were used to eliminate nonspecific staining (DakoCytomation, Carpinteria, CA). Sections were then incubated with primary antibody (anti-CD8 clone 1A5 (Vector)) at 1:50 for 60 minutes at room temperature followed by incubation with Envision Labeled Polymer (DakoCytomation) for 30 minutes. Antigen-antibody complex formation was detected by use of 3,3′ diaminobenzidine (DAB) chromogen (DakoCytomation). An isotype matched (IgG1) control antibody (Mouse IgG1 negative control, catalog number MCA928, Serotec) was used as irrelevant control antibody along with standard positive control rhesus tissue.
Co-localization of CD8 and CD163 by Immunofluorescence Microscopy
Sections of formalin fixed paraffin embedded tissue (6 uM) were prepared and then deparaffinized in xylene twice for 10 minutes each, followed with two steps each in 100% ethanol, 95% and 75% ethanol, three steps in dH2O, all for 2 minutes each. The sections were placed in 1× target retrieval solution (DakoCytomation) and boiled in a Pascal pressure chamber (DakoCytomation) at 125°C for 30 seconds, 90°C for 10 seconds, and then allowed to cool down to room temperature. To evaluate co-expression of CD8 and CD163, primary antibodies (Mouse anti-human CD163 monoclonal antibody, clone 10D6 (Leica, Buffalo Grove, IL) and Rabbit anti-human CD8 monoclonal antibody, clone SP-16 (Abcam, Cambridge, MA)) were incubated on the tissue sections overnight at 4°C. After washing out unbound primary antibody with Tris buffered saline-0.05% Tween 20 (TBST), the sections were incubated with the appropriate secondary antibodies (Alexa Fluor 594 donkey anti-mouse IgG (Invitrogen, Carlsbad, CA) or Alexa Fluor 488 donkey anti-rabbit IgG (Invitrogen)) at room temperature for 1 hour. After washing out unbound secondary antibodies, slides were then placed under coverslips with ProLong Gold Anti-Fade containing DAPI (Invitrogen). Sections were analyzed with a BX51/BX52 microscope (Olympus America Inc, Melville, NY, USA). Images were captured using the CytoVision 3.6 software (Applied Imaging, San Jose, CA, USA).
RT-PCR
RT-PCR to detect FHOD1 message in murine spleen compared with human spleen was performed as described [34] using specific (forward MG182, reverse MG196) and control (GAPDH - forward MG121, reverse MG122) primers as detailed.
Microscopy
Visualization of all stained tissues was performed on an Olympus BX41 microscope. Splenic tissue was photographed on an Olympus BX41 microscope using OlympusQColor3 and analyzed with acquisition software QCapture v2.60 (QImaging) and Adobe Photoshop 6.0 (Adobe).
Results
Human LCs express a unique spectrum of hybrid antigens
In recent years discovery of many protein markers has greatly refined understanding of the heterogeneity of cells resident in human splenic white pulp, however little is known about red pulp populations. To begin to uncover the role(s) of the human splenic LC with the goal of determining its relevance to different disease states (e.g. malaria infection), and to optimize purification for mechanistic investigations we determined the spectrum of antigens expressed (or absent) on human LCs based on study of twenty normal spleens. This was combined with a literature review and a more limited comparison with the LCA. These results are summarized in Table 2. New observations relevant to the unique phenotype and evolution of the human splenic LC, its relationship to the LCA and the implications of these findings for the regulation of splenic filtration were then further analyzed and are selectively described.
TABLE 2.
Multi-lineage Antigen Expression Pattern of Splenic LCs and the LCA
| Cell type | Molecule/Marker | Functions | LC | LCA | Reference** |
|---|---|---|---|---|---|
| Endothelial | vWF | Factor VIII-related antigen, clotting | + | + | Pusztaszeri et al. 2006 Bi et al. 2007 |
| CD31* | PECAM, adhesion, signalling | + | + | Ruck et al. 1994 Arber et al. 1997 Pusztaszeri et al. 2006 |
|
| CD34* | Endothelial lineage marker (also on heme stem cells) | − | − | Pusztaszeri et al. 2006 Bi et al. 2007 |
|
| CD62P | P-selectin, adhesion molecule | +/− | − | Korkusuz et al. 2002 | |
| CD105 | Endoglin, adhesion and signaling | + | ND | Korkusuz et al. 2002 | |
| CD106 | VCAM1, adhesion | + | ND | Korkusuz et al. 2002 | |
| CD141 | Thrombomodulin, thrombin binding protein | + | ND |
Korkusuz et al. 2002 Steininger et al. 2007 |
|
| CD146 | MCAM, adhesion and cohesion | + | ND | Korkusuz et al. 2002 | |
| CD144 | VE-cadherin adhesion | + | ND | Pusztaszeri et al. 2006 | |
| VEGFR3 | FLT4, growth factor receptor | ND | − | Yamate et al. 2009 | |
| D2-40* | Lymphatic endothelium marker | − | − |
Korkusuz et al. 2002 Steininger et al. 2004 |
|
| BMA-120 | Unknown glycoprotein | + | − | Falk et al 1991 | |
| UEA-1 | Ulex europeus Lectin I, binds fucose | − | + | Falk et al 1991 | |
| Macrophage/Monocyte | LYVE-1 | Lymphatic endothelium hyaluron receptor | + | ND | Martinez-Pomares et al 2005 |
| CD172A | SIRPα, inhibitory/migratory receptor | + | + | Ogembo et al | |
| HLA II | Histocompatibility antigen | + | + | Buckley et al. 1985 | |
| Stabilin-1 | Scavenger receptor recognizing phosphatidylserine | + | ND |
Goerdt et al 1991 Toomarian et al 2011 |
|
| CD36 | Scavenger receptor, phagocytosis | + | ND | Korkusuz et al. 2002 | |
| CD54 | ICAM1, adhesion molecule | + | − | Korkusuz et al. 2002 | |
| CD68* | Macrophage marker | − | + | Falk et al 1991 Steininger et al. 2004 Martinez-Pomares et al 2005 |
|
| CD163* | Macrophage receptor for bacteria | − | + | Lau et al. 2004 | |
| CD169 | Sialoadhesin, adhesion | + | ND | Marmey et al. 2006 | |
| CD14 | Recognition of LPS, peptidoglycan | − | ND | Ogembo et al | |
| CD26 | Peptidase | + | ND | Korkusuz et al. 2002 | |
| CD35 | Complement receptor I | − | ND | Ogembo et al | |
| CD11b/CD18 | Complement receptor III | − | ND | Buckley et al. 1985 | |
| CD71 | Transferrin receptor | − | + |
Buckley et al. 1985 Korkusuz et al. 2002 |
|
| Ferroportin | Iron transporter | − | ND | Ogembo et al | |
| CD45 | LCA, heme lineage marker | − | − | Ogembo et al | |
| CD205 | DEC, Antigen presentation | − | ND | Pack et al. 2008 | |
| CD206 | Macrophage mannose receptor (endocytosis and phagocytosis) | + | ND | Pusztaszeri et al. 2006 | |
| CD209 | DC-Sign, adhesion receptor | − | ND | Bi et al. 2007 | |
| Receptor PTPγ | Phosphatase, suppression inflammation | + | ND | Lissandrini et al. 2006 | |
| NK cell | CD16 | FcγRIIIa and FcγRIIIb | − | − | Buckley et al. 1991 |
| CD56 | NCAM1, adhesion | − | ND | Ogembo et al. | |
| Dendritic cell | Ki-M9 | PEST domain protein on FDCs, bone marrow sinus lining cells | − | ND | Wacker et al. 1997 |
| Red Blood cell | CD234* | DARC, pan chemokine receptor | + | + | Buckley et al. 1987 |
| T-cell | CD3* | Pan T-cell marker | − | − | Buckley et al. 1984 |
| CD8α | HLAI receptor, T- and NK cells, macrophages, dendritic cells | + | − | Ogembo et al. | |
| CD8β | T-cell co-receptor, HLAI receptor | − | ND | Ogembo et al. | |
| CD4* | T-cell co-receptor, helper cell | − | − | Stuart et al. 1983 | |
| CD231 | TALLA1, tetraspanin 7 | + | ND | Korkusuz et al. 2002 | |
| PD-1 | Member extended CD28/CTLA-4 family of T-cell regulators | − | ND | Ogembo et al | |
| CD102 | ICAM2, adhesion | + | ND | Korkusuz et al. 2002 | |
| B-cell | CD21* | Complement receptor II | − | + | Arber et al. 1997 |
| CD19 | B-cell activation antigen | − | ND | Ogembo et al. | |
| Bcl-2 | Apoptosis regulatory protein | − | ND | Jiang et al. 2005 | |
| CD20* | B-cell restricted surface marker | − | − | Buckley et al. 1985 | |
| Mesenchymal/Smooth Muscle cell | FHOD1 | Formin, cytoskeletal organization | + | − | Ogembo et al |
| SM myosin/myosin | Excitation-contraction coupling | + | ND | Pinkus et al 1986 Drenckhahn et al 1986 |
|
| Vimentin | Intermediate filament | + | + | Giorno et al 1985 Falk et al. 1991 Arber et al 1997 |
|
| Alpha-actinin | Stress fiber | + | ND | Drenckhahn et al 1986 | |
| Epithelial cell | Cytokeratins, AE1-AE3* | Epithelial cytoskeleton | − | − | Michal et al. 1993 |
| Epithelial membrane antigen* | Pan epithelial marker | − | − | Michal et al. 1993 | |
| Enzymes | Lysozyme | Muraminidase | + | + | Buckley et al. 1985 |
| Alpha1-anti-chymotrypsin | Chymotrypsin antagonist | + | + | Falk et al. 1991 | |
| Cathepsin D | Aspartic proteinase | + | + | Reid et al. 1986 | |
| Nonspecific esterase | α-Naphthyl acetate and butyrate | + | + | Heusermann et al. 1975 Ruck et al. 1994 |
|
| Alkaline phosphatase | Phosphatase | − | + | Heusermann et al. 1975 | |
| Megakaryocyte | Thrombospondin 1 | Cell adhesion, proliferation, motility, survival. | − | ND | Ogembo et al. |
Several molecules/markers are multi-lineage-Table is based on majority literature search.
Represent antigen staining re-performed in this current manuscript to clear the discrepancy in the literature.
For conservation of space, typically only the first literature reference to staining is provided although several antibody studies were performed and demonstrated in multiple works.
LCs express the CD8αα isoform of CD8
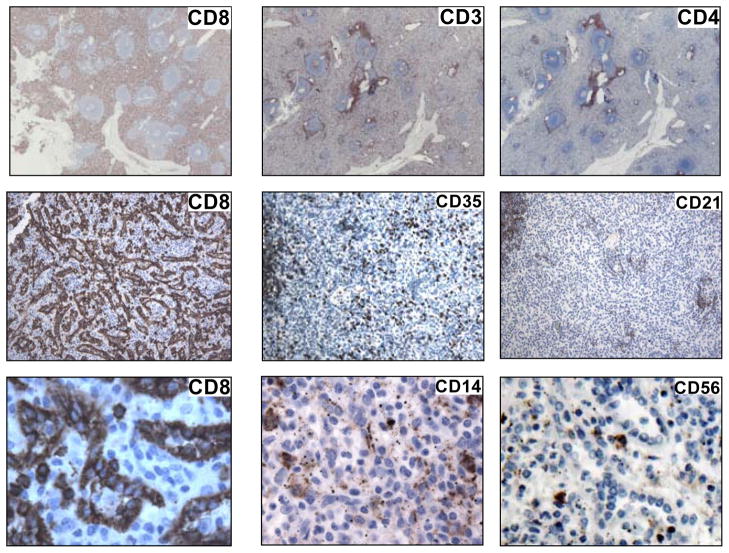
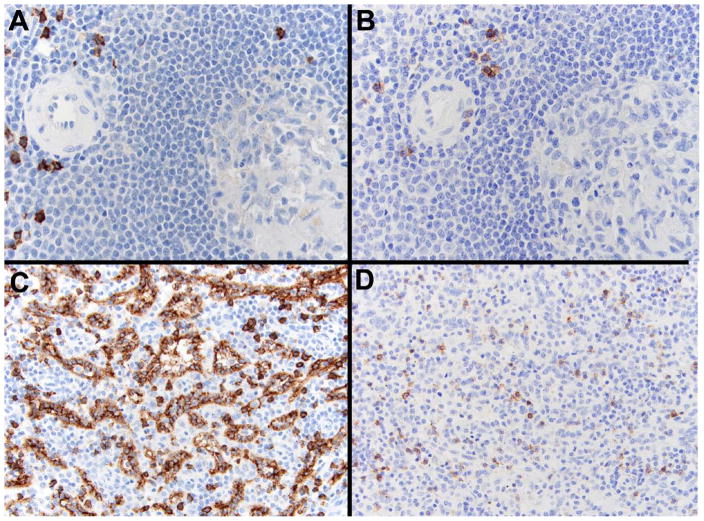
CD8, a co-receptor of the T-cell receptor that binds HLA Class I, was originally believed to be a unique marker of cytotoxic T-cells. Subsequent investigations revealed certain NK as well as monocyte/macrophage and dendritic cells could sometimes express CD8 [42]. However, the pattern of peptide dimerization (α/β versus α/α chain) and post-translational modifications often differed. The distribution and composition of CD8 on LCs remained unknown. Upon staining of human splenocytes (Fig. 1) prominent CD8 expression on T-cells in white pulp as well as on the sinus lining cells within red pulp was confirmed. There was no significant staining of red pulp LCs with antibody to CD4 (alternate T /macrophage subset), to CD3 (pan T cell) or to CD21 (B cell and FDC) despite clear staining of B (CD21) and T-cell (CD3, CD4) zones within nearby germinal centers (Fig. 1). CD14 positive monocytes as well as free CD14 appeared randomly distributed in red pulp and within some sinusoids, but did not uniformly associate with LCs; likewise, CD56, a protein primarily expressed by NK cells was not identified on the LC population (Fig. 1). Within red pulp, cells lining the cords of Billroth and cells within the sinusoids were rarely CD8 positive. In contrast to LCs these scattered cells showed prominent CD8 membrane expression, whereas LCs appeared to accumulate CD8 protein both on the cell membrane and diffusely within the cytoplasm (Fig. 1, bottom). Using a recently available monoclonal antibody, F-5 (Santa Cruz), that specifically detects human CD8β upon IHC analysis together with a human CD8α specific monoclonal antibody (Table 1), it was apparent that although CD8α is highly expressed on LCs (Fig. 2, C), CD8β (Fig. 2, D) is not. Control staining of T-cells that surround a small arteriole is displayed in panel A (CD8α) and panel B (CD8β).
Figure 1. CD8+ LCs form a major component of human splenic red pulp.
(1) Left panels top to bottom (at increasing magnification of 20×, 200×, 400×). CD8 in human splenic red pulp is highly expressed by the LC population and distributes both at the cell surface and throughout the cytoplasm (bottom). Middle and right panels. No other T-cell specific (CD3, CD4), B cell specific (CD35, CD21), monocyte/macrophage associated (CD14, also CD35) nor predominant NK cell (CD56) receptors are uniformly expressed by LCs.
Figure 2.
LCs express the CD8α but not the CD8β chain of CD8. Immunohistochemical analysis. T-cells (control) express both CD8α (A) and CD8β (B) as shown by staining of sequential sections of a periarteriolar sheath from normal spleen (300×). In contrast, the LC network in red pulp stains for CD8α (C), but no CD8β staining (D) is visualized, although background T-cells display dual staining. Also note the presence of individual CD8α positive cells between LCs that likely represent CD8aa macrophages (200×).
High expression of formin homology domain protein 1 (FHOD1) distinguishes LCs from the LCA
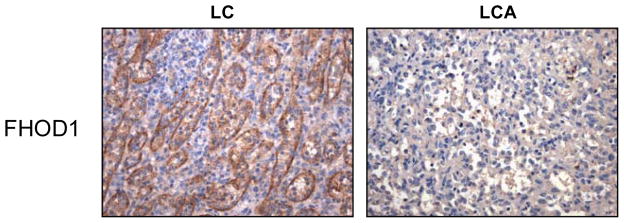
In the course of investigation of proteins that interact with the cytoplasmic domain of CD21 we identified FHOD1 [33], a member of the drosophila-related formin family of actin regulatory proteins. Similar to other family members FHOD1 functions in cytoskeletal regulation through actin cable formation [43]. FHOD1 has further been implicated in dynamic remodeling of the cytoskeleton, establishment of cell polarity, cell elongation, vesicle trafficking, plasma membrane blebbing, and downstream signaling to the nucleus [33, 44–46]. Most recently a role in the differentiation of smooth muscle cells was uncovered [47, 48]. Reports that FHOD1 could interact with cell surface and transport proteins [33, 49], suggested it could also function in relation to antigen/pathogen or cell invasion/internalization or in the prevention thereof. As CD21 was expressed on the LCA [30], when FHOD1 was prominently detected on splenic LCs by in situ hybridization [33], we speculated this might relate in part to CD21 interaction. However, direct analysis revealed CD21, a known activation antigen was absent on the normal LC equivalent (Fig. 1). Using an anti-FHOD1 antiserum produced by our laboratory (Table 1) as well as a mouse polyclonal antibody to human FHOD1 (Abcam), we did confirm that FHOD1 protein is highly expressed by normal LCs, but is not expressed by the LCA (Fig. 3).
Figure 3. FHOD1 protein is highly expressed on normal LCs but not on the LCA.
Immunohistochemical analysis. Expression of FHOD1 on normal LCs (left) compared with an adjacent section of spleen containing a LCA (right, 200×).
High expression of Duffy Antigen Receptor for Chemokines (DARC) distinguishes human LCs from adjacent splenocytes, as well as from venous sinus lining cells of other organs
DARC (CD234, gp-Fy) was identified in 1950 as an antigen expressed on the surface of human RBCs [50]. It was subsequently shown to be the receptor for Plasmodium vivax and Plasmodium knowlesi, as well as a promiscuous receptor for C-C and C-X-C chemokines. DARC is absent on erythrocytes of most West Africans and their descendents due to a RBC-specific promoter polymorphism that confers protection from malaria. However, all persons regardless of whether their RBCS are DARC positive or negative, express DARC on immediate post-capillary endothelial cells and certain epithelial cells of kidney, lung, colon and brain Purkinje cells [50]. In spleen, DARC was atypically noted to be highly expressed on the sinus lining cell [34], an observation confirmed herein (Fig. 4), however, no DARC expression could be detected on cells that lined the venous sinusoids of liver and bone marrow [34]. Similarly, neither CD8 nor FHOD1 was detected on/in the sinus lining cells of human liver and bone marrow (not shown), emphasizing that a distinct pattern of protein expression characterizes the human LC.
Figure 4.
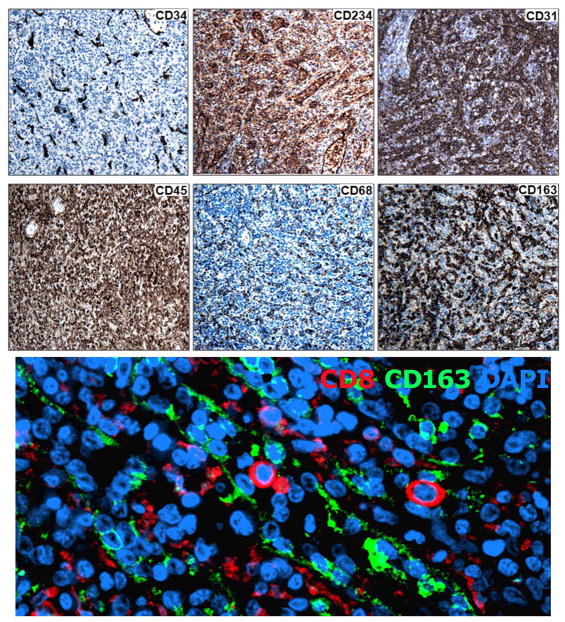
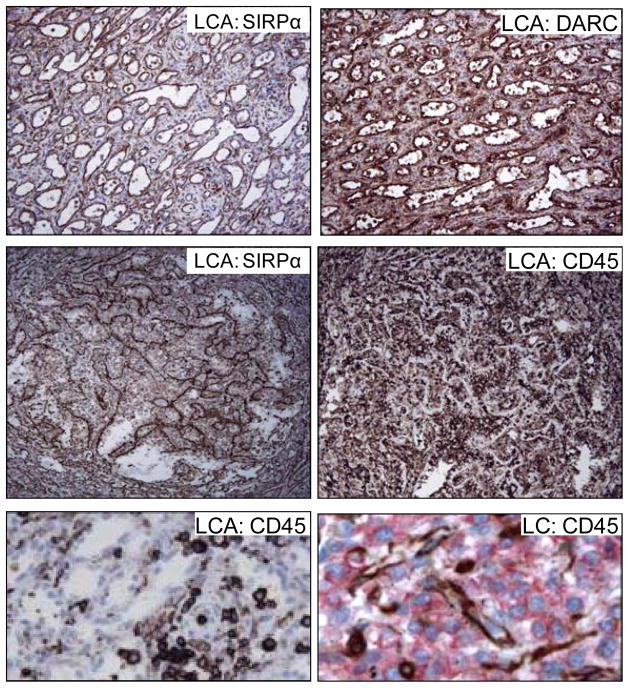
Figure 4A. LCs do not express either CD34 or CD45, though cellular proteins characteristic of both endothelial and hematopoietic (RBC, macrophage) lineages are variably expressed (CD234, CD31, CD68, CD163). Immunohistochemical analysis. Left, top and bottom, lineage markers CD34 and CD45 respectively. Middle top, RBC marker CD234. Right top, endothelial marker CD31. Bottom, middle and right, macrophage markers CD68 and CD163, respectively. All images are magnified 200×.
Figure 4B. LCs do not express CD163. Dual immunofluorescence analysis with anti-CD8 (green) and anti-CD163 (red) reveals that CD163 does not co-localize with the CD8 positive LC though it is present on nearby cells that line the cords of Billroth (400X).
LCs lack CD34, CD45, CD68 and CD163 but express CD31
CD34 is a marker of most mature cells committed to the endothelial lineage from which normal LCs are reported to derive. The LCA is a low-grade malignancy believed to originate from endothelium, despite expression of several macrophage-associated antigens. The absence of CD34 on LCAs was attributed to changes associated with transformation, however, somewhat unexpectedly CD34 was also absent on normal LCs (Fig. 4A, top, left). CD45, in contrast, delineates cells that originate from hematopoietic stem cells and is expressed on most mature hematopoietic lineage cells with the exception of RBCs. The macrophage-like properties of LCs and expression of CD8 suggested LCs and perhaps the related angioma might alternately derive from a hematopoietic precursor. However, although the majority of cells in human spleen bound a pan anti-CD45 antibody, the splenic LC population (Fig. 4A, bottom, left) along with some small calibre blood vessels and red blood cells excluded stain. In fact, the pattern of DARC and CD45 expression was nearly reciprocal (Fig. 4A, also see Fig. 6). Thus, the lineage derivation of human splenic LCs (and the LCA) remains indeterminate. LCs did strongly express CD31 (Fig. 4A top right) a marker primarily associated with endothelial cells. CD68 (Fig. 4A bottom, middle) did not appear to be expressed on LCs though it was present on nearby cells (Table 2). CD163, a common macrophage marker was detected on cells lining the cords of Billroth (Fig. 4A bottom, right), but not on LCs based on evaluation by dual immunofluorescence analysis with antibodies to both CD163 and CD8 (Fig. 4B).
Figure 6.
LCAs retain expression of SIRPα and DARC and also lack CD45.
Top. Staining of sequential section from a LCA demonstrates that the disorganized sinus lining cells express both SIRPα (left) and DARC (right) (200×). Middle. A reciprocal expression pattern SIRPα+ (left) and CD45− (right) is evident following staining of sequential sections from a LCA (200×). Bottom, left. An enlarged image demonstrates CD45+ cells between the CD45− sinus lining cells of a LCA (1000×). Bottom, right. Dual immunofluorescent analysis of a segment of normal spleen confirms (as shown in Fig. 3) that CD45+ cells (red) and LCs (brown) form mutually exclusive populations, a pattern retained in the LCA (1000×).
LCs express high amounts of SIRPα (SHPS-1, BIT, p84, CD172a)
SIRPα, a type 1 transmembrane glycoprotein, is primarily expressed on the surface of myeloid lineage (macrophages, dendritic cells, neutrophils) cells [50]. It is closely related by structure to the major antigen recognition proteins [50, 51], and like these receptors, SIRP genes first appear coordinate with development of an adaptive immune system [52, 53]. SIRPα binds its cognate receptor, CD47 (integrin-associated protein, IAP), a ubiquitous surface membrane protein and marker of “self”, as well as certain other ligands [37, 54]. Interaction with CD47 results in transmission of a negative intracellular signal that restricts the ability of CD172A+ macrophages to phagocytose and destroy adjacent cells. LPS exposure reduces SIRPα expression, releasing macrophages from quiescence [48] and thereby enhancing their ability to phagocytose other nearby immune cells to resolve an inflammatory response. Senescent RBCs downregulate CD47 resulting in phagocytosis by environmental macrophages. Recently several human tumor types were found to upregulate CD47, providing a relevant mechanism for escape from macrophage immune surveillance [55–57].
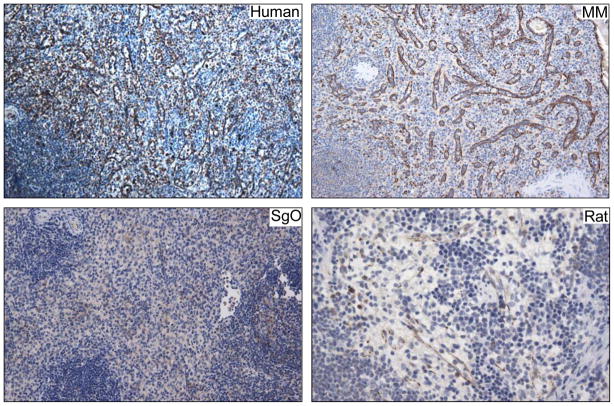
Though it has long been recognized that mammalian spleen is the site where most RBC elimination occurs and furthermore that turnover is mediated by splenic macrophages, the precise subpopulation(s) responsible has remained largely unknown. Recent work in mice identified a major requirement for SIRPα and red pulp macrophages for efficient RBC phagocytosis [58] and regulation of iron homeostasis [59]. As man and mouse are separated by 50 million years of evolution many differences in splenic structure and immune functions exist; nevertheless, emerging evidence suggests major SIRPα functions are conserved [60]. To identify SIRPα expressing cells in normal human spleen, IHC analyses were performed which revealed that not only were SIRPα expressing cells primarily located within the red pulp, but expression was highly localized to the LC population (Fig. 5, top left). Interestingly, SIRPα expression was also strongly detected on sinus lining cells in the spleens of old world primates, new world primates, and even rat spleen contained a number of slit like sinusoids that were lined by cells expressing SIRPα (Fig. 5).
Figure 5.
SIRPα is highly expressed on human splenic LCs and is variably detected on primate and rodent sinus lining cells.
Top, left Human LCs as well as the sinus lining cells of MM (old world monkey) (right) express high amounts of SIRPα, whereas expression among SGO (new world monkey) (bottom, left) is more subtle. Most sinus lining cells in rat spleen do not express SIRPα, however, a subpopulation that does express SIRPα can be detected (bottom, right). All images are 200X original magnification
Is the LCA a bona fide derivative of LCs?
The LCA is a tumor-like proliferation that occurs only in spleen. Frank atypia and metastasis are extremely rare [24, 30, 61]. The LCA is believed to derive from normal LCs based on morphologic appearance including disorganized sinuses, debris-filled sinus lining cells as well as admixture with a population of taller cells between and within the sinuses. The appearance of two potentially distinct cell types and an immunophenotype that only partially overlaps (atypically CD8−, FHOD1−, CD21+, CD68hi) its putative normal counterpart raised questions about whether the LC was the authentic cell of origin [62]. An IHC analysis displayed in Fig. 6 top showed both SIRPα and DARC were strongly expressed on cells lining the disorganized sinuses of the LCA and similar to normal LCs, CD45 was absent. These results provide clear evidence of the relatedness of the LCA to its normal cell counterpart and establish a firm basis for distinguishing the LCA from other splenic vascular tumors.
Littoral cell evolution: rodents, non-human primates, man
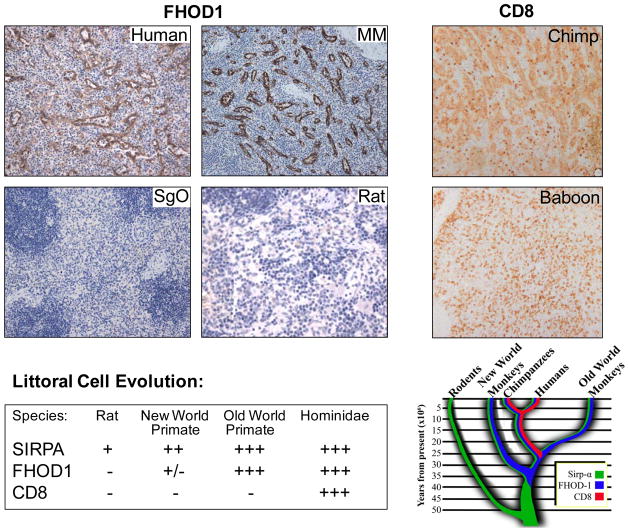
Much current understanding of splenic function derives from murine studies. However, mice (Mus domesticus) have asinusal spleens in which the red pulp vasculature consists of small non-anastomosing primitive veins. Rats (Ratus norvegicus) do have sinusal spleens, as red pulp is filled by large irregular venous sinuses, however bona fide endothelial cells with typical lateral extensions line most of the rat splenic sinuses [15]. CD8 expression in rodent spleen has been well documented; specific antibody binds T, as well as NK, monocyte and macrophage populations in both rodent’s white and red pulp, but not the venous sinus-lining cell (rat). Although the respective genes for murine and rat FHOD1 are predicted to be 85% identical to human FHOD1, we did not detect FHOD1 in mouse or rat spleen by RT PCR (personal observation). Furthermore, no FHOD1 protein was detected in cells forming the sinsuoids of rat spleen by IHC (Fig. 7). Interestingly rare elongated cells that line a subset of rat splenic sinusoids did express SIRPα (Fig. 5). These curious results suggested that during mammalian evolution alteration in the composition and function of splenic red pulp, specifically changes involving the sinus-lining cell occurred.
Figure 7.
The CD8+ splenic LC is an evolutionary newcomer.
Top, left panel. FHOD1 is expressed in sinus lining cells of humans and MM (old world monkey) and man, but cannot be detected in SGO (new world monkey) or in rats. Top, right. CD8 is highly expressed on the sinus lining cells of a Chimpanzee (Hominidae), whereas no CD8 protein is detected on the sinus lining cells of a Baboon (old world monkey). Bottom, summary and diagram of expression patterns that describe LC evolution. All images are magnified 200×.
In light of the observation that a critical switch from primary utilization of platelets to utilization of RBC for systemic transport of IC was documented coincident with evolution of Hominidae [9–11], we queried whether an unknown, though temporally related set of events might have driven LC evolution as a cache and/or sieve for the now IC bearing RBCs. Whereas SIRPα expression was readily detected on almost all primate sinus-lining cells with the exception of Sanguinus oedipus (cotton top tamarin) (Fig. 5), FHOD1 was absent on the New World Primates Sanguinus oedipus and Saimiri sciureus (squirrel monkey), though moderately expressed on Callithrix jacchus (common marmoset). All Old World Primates studied including Macaca mulatta (rhesus), Macaca fascicularis (cynomologus), and Chlorocebus sebaeus (African green monkey) expressed FHOD1. A monoclonal antibody, IA5 that detects CD8 on all primates, stained scattered cells in the red pulp but did not outline the sinus lining cell population of any New or Old World primates with the exception of Chimpanzees (Pan troglodytes), the non-human primate closest to man (Fig. 7, top right).
Discussion
A primordial spleen-like tissue first appeared as a condensation of the lymphomyeloid complex in the intestine of primitive vertebrates (cyclostomes) [63–65] together with the earliest form of RBC - and ever since their development has remained tightly linked. Although splenic evolution proceeded along a highly variable course relative to location, shape and function(s), the vascular organization of red pulp [66] remained the major determinant of structure and function. Typical venous sinsuoids, first arose in mammals [67, 68], but they were only variously adapted by different species, thus splenic circulation either persisted as typical vascular channels or re-organized as venous lakes [66]. In the latter case, cells percolate through the red pulp stroma before re-entering the circulation on traversing the sinus-lining cell barrier. Both vessel wall and sinus-lining cells were long assumed to be of endothelial origin. However, with the advent of advanced light microscopy Weidenreich (1901) and Mollier (1911) noted that human and certain simian sinus lining cells were distinct in shape, were surrounded by annular rings rather than reticulin fiber sheaths and they lacked the abundant side processes typical of mammalian endothelial junctions (reviewed in [23, 26, 66, 69–71]. Application of electron microscopy and development of antigen-specific antibodies for phenotypic analysis confirmed that these lining cells were different from those of most other mammals, as well as from the vascular lining cells (endothelium) of other human organs [29, 72, 73].
In 1985 Buckley et al [22] discovered that the human splenic lining cell or LC atypically expressed CD8, then believed to be entirely T-cell specific. They confirmed that LCs were CD6 (T cell, scavenger receptor-B) and CD20 (B cell) negative, but noted expression of several intracellular enzymes suggesting a possible macrophage lineage. These results were extended in a subsequent study [74] comparing different macrophage and dendritic subpopulations in human spleen; and though the phagocytic potential of LCs (nonspecific esterase +, lysozyme+) and other monocyte/macrophage-like characteristics (HLA-DR+, CD36+) were described (see Table 2), LCs remained classified as endothelial cells [23, 45].
Despite development of many new reagents for delineation of cellular phenotype few studies in the intervening decades focused on the human LC. This was notwithstanding a dominant presence in red pulp and greater than one hundred years of accumulated microscopy suggesting LCs formed a uniquely selective barrier that filtered RBCs (senescent, pathogen infected), other leukocytes and altered self. Consequently, mechanistic information is lacking and in vitro culture systems do not exist. The LCA, a rare proliferative lesion found only in human spleen is characterized by disorganized sinus-lining cells, few atypical mitoses and is curiously associated with unrelated tumors at distant sites. Though the LCA was believed to originate from LCs, many of the applied cell markers were discordant posing diagnostic difficulty when compared with other splenic vascular tumors (manuscript in preparation). Therefore, we reviewed the published literature and analyzed as well as re-analyzed, the expression pattern of the normal human LC and the LCA (Table 2) providing salient new information for distinguishing and isolating LCs from other splenic populations. Our findings verify the relatedness of the LCA and LC, as SIRPα and DARC are highly expressed on both normal and angiomatous sinus lining cells, whereas the markers CD8 (negative), FHOD1 (negative) and CD21 (positive) discriminate neoplastic from normal cells.
The high expression and the unique distribution of CD8α/α, FHOD1 and SIRPα, together with DARC and specific subsets of adhesion and phagocytic/endocytic receptors (e.g. stabilin-1 recently implicated in phosphatidylserine-dependent phagocytosis of RBCs and cell corpses in mice [36, 75, 76] provide much in the way of new insights into the likely mechanisms underlying the function(s) of the human LC, a cell that appears in current form (CD8α/α+) only upon evolution of Hominidae (humans, chimps). Because splenic and RBC evolution are historically tightly linked [63, 66], it is particularly noteworthy that coincident with the rapid evolution of the LC, systemic transport of ICs switches from platelets to RBCs accompanied by acquisition of RBC CR1/CD35 [11]. The LC appears well equipped to process membrane altered RBCs or RBCs that bear residual ICs (particularly if ICs were not eliminated during earlier stages of hepatic or splenic transport), as its most important role may be sorting of the cleared RBC. Many of the described LC surface receptors (Table 2) have known adhesive and/or phagocytic functions; however, it is notable that the subset of receptors currently identified on LCs predominantly predicts anti-inflammatory rather than pro-inflammatory response patterns (e.g. DARC, CD206, CD163, CD31, enzymes, chemokines) [37, 77, 78], reviewed in [79].
The strategic location of the LC compartment and electron microscopic evidence that RBCs and other cells (lymphocytes, other mononuclear cells, possibly tumor cells) pass between (paramigration) as well as through (transmigration) LCs or are destroyed within the LC is consistent with a major role in regulating the cellular composition of the systemic circulation. We speculate that similar to a customs checkpoint, the panel of antigens expressed on LCs initially determine which cells (documents) are appropriate for passage, then re-check their content (baggage) and finally engulf (reject) or contract (open the gate) permitting entry into the circulation (country). Multiple cell-cell interactions are surely involved that we have just begun to identify. The significance of CD8 expression is uncertain although it is notable that RBCs do not express and many tumors downmodulate HLA I, suggesting that the absence of a “self”-interaction signals a requirement for additional review. Evidence already exists that similar to mice, the SIRPα-CD47 axis in man is of major import in regulating the turnover of RBCs, as well as survival of stem cells and tumor cells [55, 80, 81]. Upon arrival at the LC periphery failure to engage SIRPα on the basis of reduced CD47 (e.g. typical of senescent RBCs) would stimulate an “eat me” signal leading to cell destruction, whereas the absence of such a signal could facilitate passage to the sinus. In recent work, the actin nucleator FHOD1 was discovered to be activated downstream of RhoA-ROCK (in addition to Rac), thereby promoting development of a smooth muscle cell phenotype with contractile function [23, 47]. Thus upon delivery of an integrated checkpoint signal from ligation of multi-receptor surface complexes, contraction of LCs might promote rapid local release of cells stored in spleen–such as RBCs, tumor cells, or perhaps global release of monocytes for cardiac repair as recently described in murine spleen [82]. The multi-lineage characteristics or plasticity of LCs as demonstrated by expression of a spectrum of antigens associated with endothelial, mono/macrophage/dendritic as well as smooth muscle cells reveals a newly arrived and highly specialized barrier cell whose role as a major component of human spleen awaits precise definition.
Acknowledgments
This work was supported by National Institutes of Health Grants R01AI063571 (JDF) and K23AI072033 (DAM), by an American Heart Association Grant-in-Aid (JDF) and by a fellowship from the Cancer Research Institute (JGO).
We sincerely thank the donor families and the staff of the New England Organ Bank, Scott Johnson, Seth Karp, Amy Evenson and Douglas Hanto of the Department of Surgery (BIDMC) for their support. We thank Christine Unitt, Corey Marvin, Tyler Caron, Mei Zheng, Jason Hornick, Qian Zhan, Alyson Smeedy Campbell, Sara Akhavandord, and John Iafrate for advice and/or technical assistance. We thank Dr. Michael Gimbrone (HMS), Professor Dyann Wirth (Harvard School of Public Health), and Professor Terrie Taylor (Michigan State University) for their support and mentorship of Dr. Milner for this work. We thank Dr. Anne Nicholson-Weller for her valuable comments and Dr. Johanna Daily for her continued collaboration.
Footnotes
Authorship Contribution: JDF, JGO and DAM designed and analyzed all experiments, reviewed the literature, and wrote the manuscript. JGO and DAM performed most experiments. KGM, GSP, GFM and JLK provided expertise and additional samples for analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References
- 1.Patel S, Kramer N, Rosenstein ED. Evolving connective tissue disease influenced by splenectomy: beneath the sword of Dameshek. J Clin Rheumatol. 2010 Sep;16(6):280–3. doi: 10.1097/RHU.0b013e3181eeb761. [DOI] [PubMed] [Google Scholar]
- 2.Linet MS, Nyren O, Gridley G, Adami HO, Buckland JD, McLaughlin JK, et al. Causes of death among patients surviving at least one year following splenectomy. American journal of surgery. 1996 Oct;172(4):320–3. doi: 10.1016/S0002-9610(96)00196-1. [DOI] [PubMed] [Google Scholar]
- 3.Robinette CD, Fraumeni JF., Jr Splenectomy and subsequent mortality in veterans of the 1939–45 war. Lancet. 1977 Jul 16;2(8029):127–9. doi: 10.1016/s0140-6736(77)90132-5. [DOI] [PubMed] [Google Scholar]
- 4.Cesta MF. Normal structure, function, and histology of the spleen. Toxicologic pathology. 2006;34(5):455–65. doi: 10.1080/01926230600867743. [DOI] [PubMed] [Google Scholar]
- 5.Rosse WF. The spleen as a filter. The New England journal of medicine. 1987 Sep 10;317(11):704–6. doi: 10.1056/NEJM198709103171110. [DOI] [PubMed] [Google Scholar]
- 6.Brozman M, Jakubovsky J. The red pulp of the human spleen. Structural basis of blood filtration. Zeitschrift fur mikroskopisch-anatomische. Forschung. 1989;103(2):316–28. [PubMed] [Google Scholar]
- 7.Hart SP, Smith JR, Dransfield I. Phagocytosis of opsonized apoptotic cells: roles for ‘old-fashioned’ receptors for antibody and complement. Clinical and experimental immunology. 2004 Feb;135(2):181–5. doi: 10.1111/j.1365-2249.2003.02330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fearon DT. Identification of the membrane glycoprotein that is the C3b receptor of the human erythrocyte, polymorphonuclear leukocyte, B lymphocyte, and monocyte. The Journal of experimental medicine. 1980 Jul 1;152(1):20–30. doi: 10.1084/jem.152.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hebert LA. The clearance of immune complexes from the circulation of man and other primates. Am J Kidney Dis. 1991 Mar;17(3):352–61. doi: 10.1016/s0272-6386(12)80488-4. [DOI] [PubMed] [Google Scholar]
- 10.Nelson RA., Jr The immune-adherence phenomenon; an immunologically specific reaction between microorganisms and erythrocytes leading to enhanced phagocytosis. Science (New York, NY. 1953 Dec 18;118(3077):733–7. doi: 10.1126/science.118.3077.733. [DOI] [PubMed] [Google Scholar]
- 11.Birmingham DJ, Hebert LA. CR1 and CR1-like: the primate immune adherence receptors. Immunological reviews. 2001 Apr;180:100–11. doi: 10.1034/j.1600-065x.2001.1800109.x. [DOI] [PubMed] [Google Scholar]
- 12.Jacobson AC, Weis JH. Comparative functional evolution of human and mouse CR1 and CR2. J Immunol. 2008 Sep 1;181(5):2953–9. doi: 10.4049/jimmunol.181.5.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirasawa Y, Tokuhiro H. Electron microscopic studies on the normal human spleen: especially on the red pulp and the reticulo-endothelial cells. Blood. 1970 Feb;35(2):201–12. [PubMed] [Google Scholar]
- 14.Korkusuz P, Dagdeviren A, Asan E. Immunophenotypic analysis of human spleen compartments. Ann Anat. 2002 Sep;184(5):431–41. doi: 10.1016/S0940-9602(02)80075-9. [DOI] [PubMed] [Google Scholar]
- 15.Udroiu I. Evolution of sinusal and non-sinusal spleens of mammals. Hystrix. 2006;17(2):99–116. [Google Scholar]
- 16.Hataba Y, Kirino Y, Suzuki T. Scanning electron microscopic study of the red pulp of mouse spleen. Journal of electron microscopy. 1981;30(1):46–56. [PubMed] [Google Scholar]
- 17.Snook T. A comparative study of the vascular arrangements in mammalian spleens. The American journal of anatomy. 1950 Jul;87(1):31–77. doi: 10.1002/aja.1000870103. [DOI] [PubMed] [Google Scholar]
- 18.Weiss L. The red pulp of the spleen: structural basis of blood flow. Clinics in haematology. 1983 Jun;12(2):375–93. [PubMed] [Google Scholar]
- 19.Schmidt EE, MacDonald IC, Groom AC. Microcirculation in rat (sinusal) spleen studied by means of corrosion casts, with particular reference to intermediate pathways. Journal of morphology. 1985;186:1–16. doi: 10.1002/jmor.1051860102. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt EE, MacDonald IC, Groom AC. Microcirculation in mouse (nonsinusal) spleen studied by means of corrosion casts. Journal of morphology. 1985;186:17–29. doi: 10.1002/jmor.1051860103. [DOI] [PubMed] [Google Scholar]
- 21.McCuskey RS, McCuskey PA. In vivo and electron microscopic studies of the splenic microvasculature in mice. Experientia. 1985 Feb 15;41(2):179–87. doi: 10.1007/BF02002611. [DOI] [PubMed] [Google Scholar]
- 22.Buckley PJ, Dickson SA, Walker WS. Human splenic sinusoidal lining cells express antigens associated with monocytes, macrophages, endothelial cells, and T lymphocytes. J Immunol. 1985 Apr;134(4):2310–5. [PubMed] [Google Scholar]
- 23.Drenckhahn D, Wagner J. Stress fibers in the splenic sinus endothelium in situ: molecular structure, relationship to the extracellular matrix, and contractility. The Journal of cell biology. 1986 May;102(5):1738–47. doi: 10.1083/jcb.102.5.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kutok JL, Fletcher CD. Splenic vascular tumors. Seminars in diagnostic pathology. 2003 May;20(2):128–39. doi: 10.1016/s0740-2570(03)00011-x. [DOI] [PubMed] [Google Scholar]
- 25.Barnhart MI, Lusher JM. The human spleen as revealed by scanning electron microscopy. American journal of hematology. 1976;1(2):243–64. doi: 10.1002/ajh.2830010209. [DOI] [PubMed] [Google Scholar]
- 26.Fujita T, Kashimura M, Adachi K. Scanning electron microscopy (SEM) studies of the spleen--normal and pathological. Scanning electron microscopy. 1982;(Pt 1):435–44. [PubMed] [Google Scholar]
- 27.Pongponratn E, Riganti M, Bunnag D, Harinasuta T. Spleen in falciparum malaria: ultrastructural study. The Southeast Asian journal of tropical medicine and public health. 1987 Dec;18(4):491–501. [PubMed] [Google Scholar]
- 28.Jiskoot PM, Halsey C, Rivers R, Bain BJ, Wilkins BS. Unusual splenic sinusoidal iron overload in sickle cell/haemoglobin D-Punjab disease. Journal of clinical pathology. 2004 May;57(5):539–40. doi: 10.1136/jcp.2002.004481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stuart AE, Warford A. Staining of human splenic sinusoids and demonstration of unusual banded structures by monoclonal antisera. Journal of clinical pathology. 1983 Oct;36(10):1176–80. doi: 10.1136/jcp.36.10.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arber DA, Strickler JG, Chen YY, Weiss LM. Splenic vascular tumors: a histologic, immunophenotypic, and virologic study. The American journal of surgical pathology. 1997 Jul;21(7):827–35. doi: 10.1097/00000478-199707000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Bisceglia M, Sickel JZ, Giangaspero F, Gomes V, Amini M, Michal M. Littoral cell angioma of the spleen: an additional report of four cases with emphasis on the association with visceral organ cancers. Tumori. 1998 Sep-Oct;84(5):595–9. doi: 10.1177/030089169808400516. [DOI] [PubMed] [Google Scholar]
- 32.Gupta MK, Levin M, Aguilera NS, Pastores GM. Littoral cell angioma of the spleen in a patient with Gaucher disease. American journal of hematology. 2001 Sep;68(1):61–2. doi: 10.1002/ajh.1151. [DOI] [PubMed] [Google Scholar]
- 33.Gill MB, Roecklein-Canfield J, Sage DR, Zambela-Soediono M, Longtine N, Uknis M, et al. EBV attachment stimulates FHOS/FHOD1 redistribution and co-aggregation with CD21: formin interactions with the cytoplasmic domain of human CD21. Journal of cell science. 2004 Jun 1;117(Pt 13):2709–20. doi: 10.1242/jcs.01113. [DOI] [PubMed] [Google Scholar]
- 34.Peiper SC, Wang ZX, Neote K, Martin AW, Showell HJ, Conklyn MJ, et al. The Duffy antigen/receptor for chemokines (DARC) is expressed in endothelial cells of Duffy negative individuals who lack the erythrocyte receptor. The Journal of experimental medicine. 1995 Apr 1;181(4):1311–7. doi: 10.1084/jem.181.4.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pack M, Trumpfheller C, Thomas D, Park CG, Granelli-Piperno A, Munz C, et al. DEC-205/CD205+ dendritic cells are abundant in the white pulp of the human spleen, including the border region between the red and white pulp. Immunology. 2008 Mar;123(3):438–46. doi: 10.1111/j.1365-2567.2007.02710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goerdt S, Walsh LJ, Murphy GF, Pober JS. Identification of a novel high molecular weight protein preferentially expressed by sinusoidal endothelial cells in normal human tissues. The Journal of cell biology. 1991 Jun;113(6):1425–37. doi: 10.1083/jcb.113.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annual review of immunology. 2005;23:901–44. doi: 10.1146/annurev.immunol.23.021704.115816. [DOI] [PubMed] [Google Scholar]
- 38.van den Berg TK, van der Schoot CE. Innate immune ‘self’ recognition: a role for CD47-SIRPalpha interactions in hematopoietic stem cell transplantation. Trends in immunology. 2008 May;29(5):203–6. doi: 10.1016/j.it.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Kinchen JM, Ravichandran KS. Phagocytic signaling: you can touch, but you can’t eat. Curr Biol. 2008 Jun 24;18(12):R521–4. doi: 10.1016/j.cub.2008.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodig SJ, Ouyang J, Juszczynski P, Currie T, Law K, Neuberg DS, et al. AP1-dependent galectin-1 expression delineates classical hodgkin and anaplastic large cell lymphomas from other lymphoid malignancies with shared molecular features. Clin Cancer Res. 2008 Jun 1;14(11):3338–44. doi: 10.1158/1078-0432.CCR-07-4709. [DOI] [PubMed] [Google Scholar]
- 41.Yearley JH, Pearson C, Shannon RP, Mansfield KG. Phenotypic variation in myocardial macrophage populations suggests a role for macrophage activation in SIV-associated cardiac disease. AIDS research and human retroviruses. 2007 Apr;23(4):515–24. doi: 10.1089/aid.2006.0211. [DOI] [PubMed] [Google Scholar]
- 42.Gibbings DJ, Marcet-Palacios M, Sekar Y, Ng MC, Befus AD. CD8 alpha is expressed by human monocytes and enhances Fc gamma R-dependent responses. BMC immunology. 2007;8:12. doi: 10.1186/1471-2172-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Faix J, Grosse R. Staying in shape with formins. Developmental cell. 2006 Jun;10(6):693–706. doi: 10.1016/j.devcel.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Gasteier JE, Schroeder S, Muranyi W, Madrid R, Benichou S, Fackler OT. FHOD1 coordinates actin filament and microtubule alignment to mediate cell elongation. Experimental cell research. 2005 May 15;306(1):192–202. doi: 10.1016/j.yexcr.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Hannemann S, Madrid R, Stastna J, Kitzing T, Gasteier J, Schonichen A, et al. The Diaphanous-related Formin FHOD1 associates with ROCK1 and promotes Src-dependent plasma membrane blebbing. The Journal of biological chemistry. 2008 Oct 10;283(41):27891–903. doi: 10.1074/jbc.M801800200. [DOI] [PubMed] [Google Scholar]
- 46.Young KG, Copeland JW. Formins in cell signaling. Biochimica et biophysica acta. 2010 Feb;1803(2):183–90. doi: 10.1016/j.bbamcr.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 47.Staus DP, Blaker AL, Medlin MD, Taylor JM, Mack CP. Formin homology domain-containing protein 1 regulates smooth muscle cell phenotype. Arteriosclerosis, thrombosis, and vascular biology. 2010 Feb;31(2):360–7. doi: 10.1161/ATVBAHA.110.212993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kong XN, Yan HX, Chen L, Dong LW, Yang W, Liu Q, et al. LPS-induced down-regulation of signal regulatory protein {alpha} contributes to innate immune activation in macrophages. The Journal of experimental medicine. 2007 Oct 29;204(11):2719–31. doi: 10.1084/jem.20062611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tojo H, Kaieda I, Hattori H, Katayama N, Yoshimura K, Kakimoto S, et al. The Formin family protein, formin homolog overexpressed in spleen, interacts with the insulin-responsive aminopeptidase and profilin IIa. Molecular endocrinology (Baltimore, Md. 2003 Jul;17(7):1216–29. doi: 10.1210/me.2003-0056. [DOI] [PubMed] [Google Scholar]
- 50.Barclay AN. Signal regulatory protein alpha (SIRPalpha)/CD47 interaction and function. Current opinion in immunology. 2009 Feb;21(1):47–52. doi: 10.1016/j.coi.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hatherley D, Graham SC, Harlos K, Stuart DI, Barclay AN. Structure of signal-regulatory protein alpha: a link to antigen receptor evolution. The Journal of biological chemistry. 2009 Sep 25;284(39):26613–9. doi: 10.1074/jbc.M109.017566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van den Berg TK, Yoder JA, Litman GW. On the origins of adaptive immunity: innate immune receptors join the tale. Trends in immunology. 2004 Jan;25(1):11–6. doi: 10.1016/j.it.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 53.van Beek EM, Cochrane F, Barclay AN, van den Berg TK. Signal regulatory proteins in the immune system. J Immunol. 2005 Dec 15;175(12):7781–7. doi: 10.4049/jimmunol.175.12.7781. [DOI] [PubMed] [Google Scholar]
- 54.Sarfati M, Fortin G, Raymond M, Susin S. CD47 in the immune response: role of thrombospondin and SIRP-alpha reverse signaling. Current drug targets. 2008 Oct;9(10):842–50. doi: 10.2174/138945008785909310. [DOI] [PubMed] [Google Scholar]
- 55.Jaiswal S, Chao MP, Majeti R, Weissman IL. Macrophages as mediators of tumor immunosurveillance. Trends in immunology. 2010 Jun;31(6):212–9. doi: 10.1016/j.it.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD, Jr, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009 Jul 23;138(2):286–99. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chan KS, Espinosa I, Chao M, Wong D, Ailles L, Diehn M, et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proceedings of the National Academy of Sciences of the United States of America. 2009 Aug 18;106(33):14016–21. doi: 10.1073/pnas.0906549106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science (New York, NY. 2000 Jun 16;288(5473):2051–4. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 59.Kohyama M, Ise W, Edelson BT, Wilker PR, Hildner K, Mejia C, et al. Role for Spi-C in the development of red pulp macrophages and splenic iron homeostasis. Nature. 2009 Jan 15;457(7227):318–21. doi: 10.1038/nature07472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsai RK, Rodriguez PL, Discher DE. Self inhibition of phagocytosis: the affinity of ‘marker of self’ CD47 for SIRPalpha dictates potency of inhibition but only at low expression levels. Blood cells, molecules & diseases. 2010 Jun 15;45(1):67–74. doi: 10.1016/j.bcmd.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fernandez S, Cook GW, Arber DA. Metastasizing splenic littoral cell hemangioendothelioma. The American journal of surgical pathology. 2006 Aug;30(8):1036–40. doi: 10.1097/00000478-200608000-00016. [DOI] [PubMed] [Google Scholar]
- 62.Bi CF, Jiang LL, Li Z, Liu WP. Littoral cell angioma of spleen: a clinicopathologic study of 17 cases. Zhonghua bing li xue za zhi Chinese journal of pathology. 2007 Apr;36(4):239–43. [PubMed] [Google Scholar]
- 63.Glomski CA, Tamburlin J, Chainani M. The phylogenetic odyssey of the erythrocyte. III. Fish, the lower vertebrate experience. Histology and histopathology. 1992 Jul;7(3):501–28. [PubMed] [Google Scholar]
- 64.Tanaka Y, Saito Y, Gotoh H. Vascular architecture and intestinal hematopoietic nests of two cyclostomes, Eptatretus burgeri and ammoncoetes of Entosphenus reissneri: a comparative morphological study. Journal of morphology. 1981 Oct;170(1):71–93. doi: 10.1002/jmor.1051700106. [DOI] [PubMed] [Google Scholar]
- 65.Jonsson V. Comparison and definition of spleen and lymph node: a phylogenetic analysis. Journal of theoretical biology. 1985 Dec 21;117(4):691–9. doi: 10.1016/s0022-5193(85)80247-2. [DOI] [PubMed] [Google Scholar]
- 66.Tischendorf F. On the evolution of the spleen. Experientia. 1985 Feb 15;41(2):145–52. doi: 10.1007/BF02002606. [DOI] [PubMed] [Google Scholar]
- 67.Hartman A. Handbuch der mikroskopischen Anatomie des Menschen. 1930;6(pt. 1):397–563. [Google Scholar]
- 68.HM Comparative studies of the spleen in submammalian vertebrates. Part II. Minute structure of the spleen, with special reference to the periarterial lymphoid sheath. Bull Yamaguchi Med Sch. 1959;6:83–105. [Google Scholar]
- 69.Weidenreich E. Arch f mikr Anat u Entwick. 1901;58:247–376. [Google Scholar]
- 70.Mollier S. Arch f mikr Anat. 1911;76:608–657. [Google Scholar]
- 71.Fujita T, Kashimura M, Adachi K. Scanning electron microscopy and terminal circulation. Experientia. 1985 Feb 15;41(2):167–79. doi: 10.1007/BF02002610. [DOI] [PubMed] [Google Scholar]
- 72.Steininger H, Pfofe D, Marquardt L, Sauer H, Markwat R. Isolated diffuse hemangiomatosis of the spleen: case report and review of literature. Pathology, research and practice. 2004;200(6):479–85. doi: 10.1016/j.prp.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 73.Steininger B, Stachniss V, Schwarzbach H, Barth PJ. Phenotypic differences between red pulp capillary and sinusoidal endothelia help localizing the open splenic circulation in humans. Histochemistry and cell biology. 2007 Nov;128(5):391–8. doi: 10.1007/s00418-007-0320-8. [DOI] [PubMed] [Google Scholar]
- 74.Buckley PJ, Smith MR, Braverman MF, Dickson SA. Human spleen contains phenotypic subsets of macrophages and dendritic cells that occupy discrete microanatomic locations. Am J Pathol. 1987 Sep;128(3):505–20. [PMC free article] [PubMed] [Google Scholar]
- 75.Lee SJ, Park SY, Jung MY, Bae SM, Kim IS. Mechanism for phosphatidylserine-dependent erythrophagocytosis in mouse liver. Blood. May 12;117(19):5215–23. doi: 10.1182/blood-2010-10-313239. [DOI] [PubMed] [Google Scholar]
- 76.Park SY, Jung MY, Lee SJ, Kang KB, Gratchev A, Riabov V, et al. Stabilin-1 mediates phosphatidylserine-dependent clearance of cell corpses in alternatively activated macrophages. Journal of cell science. 2009 Sep 15;122(Pt 18):3365–73. doi: 10.1242/jcs.049569. [DOI] [PubMed] [Google Scholar]
- 77.Afenyi-Annan A, Kail M, Combs MR, Orringer EP, Ashley-Koch A, Telen MJ. Lack of Duffy antigen expression is associated with organ damage in patients with sickle cell disease. Transfusion. 2008 May;48(5):917–24. doi: 10.1111/j.1537-2995.2007.01622.x. [DOI] [PubMed] [Google Scholar]
- 78.Privratsky JR, Newman DK, Newman PJ. PECAM-1: conflicts of interest in inflammation. Life sciences. 2010 Jul 17;87(3–4):69–82. doi: 10.1016/j.lfs.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chakera A, Seeber RM, John AE, Eidne KA, Greaves DR. The duffy antigen/receptor for chemokines exists in an oligomeric form in living cells and functionally antagonizes CCR5 signaling through hetero-oligomerization. Molecular pharmacology. 2008 May;73(5):1362–70. doi: 10.1124/mol.107.040915. [DOI] [PubMed] [Google Scholar]
- 80.Garratty G. The James Blundell Award Lecture 2007: do we really understand immune red cell destruction? Transfusion medicine (Oxford, England) 2008 Dec;18(6):321–34. doi: 10.1111/j.1365-3148.2008.00891.x. [DOI] [PubMed] [Google Scholar]
- 81.Takenaka K, Prasolava TK, Wang JC, Mortin-Toth SM, Khalouei S, Gan OI, et al. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nature immunology. 2007 Dec;8(12):1313–23. doi: 10.1038/ni1527. [DOI] [PubMed] [Google Scholar]
- 82.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science (New York, NY. 2009 Jul 31;325(5940):612–6. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]