Summary
In monoderm (single membrane) Gram-positive bacteria, the majority of secreted proteins are first translocated across the cytoplasmic membrane into the inner wall zone. For a subset of these proteins, final destination is within the cell envelope either as membrane-anchored or cell wall-anchored proteins, whereas another subset of proteins is destined to be transported across the cell wall into the extracellular milieu. Although the cell wall is a porous structure, there is evidence that, for some proteins, transport is a regulated process. This review aims at describing what is known about the mechanisms that regulate the transport of proteins across the cell wall of monoderm Gram-positive bacteria.
Keywords: monoderm Gram-positive bacteria, protein transport, protein secretion, cell wall, metalloenzymes, proproteins, toxins
This MicroReview relates to the transport of proteins across the cell wall of monoderm (single membrane) Gram-positive bacteria. Therefore, it does not include Mycobacteria, which are considered diderm (two membranes) because of the mycolate outer layer, or any other diderm bacteria classified as Gram-positive (Sutcliffe, 2010).
The cell envelope of monoderm Gram-positive bacteria
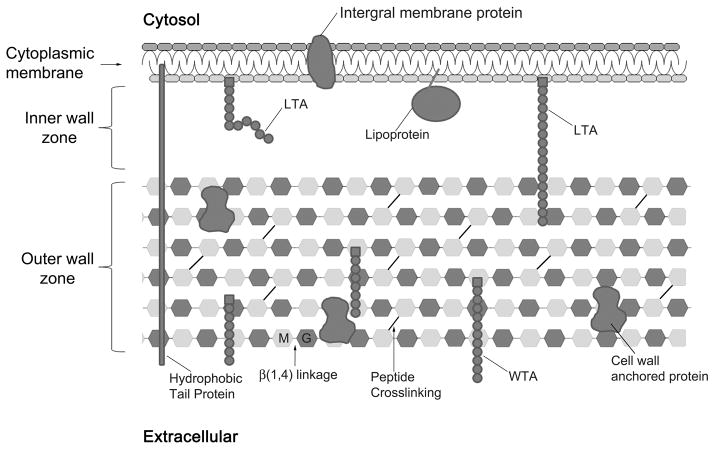
The cell envelope of monoderm Gram-positive bacteria is comprised of a cytoplasmic membrane (CM) and a cell wall (CW), which can be divided into the inner wall zone (IWZ) and outer wall zone (OWZ) (Matias & Beveridge, 2005, Zuber et al., 2006) (Fig. 1). Anchored to the CM are proteins and zwitterionic polymers called lipoteichoic acid (LTA) (Navarre & Schneewind, 1999). The IWZ is about 30 nm thick and is presumably filled with proteins and chains of LTA (Matias & Beveridge, 2006, Reichmann & Grundling, 2011). The IWZ has been occasionally described as a periplasmic space, although this term is generally restricted to a space delimited by two selective permeable barriers. The OWZ, 25–50 nm thick, is comprised of peptidoglycan, wall teichoic acid polymers (WTA), and a variety of proteins (Navarre & Schneewind, 1999, Vollmer et al., 2008). The peptidoglycan is made of disaccharide glycan chains of various lengths that are cross-linked by short peptides. In Bacillus subtilis, it is estimated that the glycan chain length is 1,300 disaccharides in average, and that approximately 20% of the peptide chains are cross-linked (Atrih et al., 1998, Hayhurst et al., 2008, Ward, 1973). These glycan chains form helices of ≈ 50 nm widths, and it was proposed that these cable-like structures coil around the narrow axis of the bacterium and are cross-linked by peptides (Hayhurst et al., 2008). The glycan chains of ovococcal bacteria, such as Streptococcus sp., Enterococcus sp., and Lactococcus sp., are formed of more than 100 disaccharide units in average, whereas the glycan chains of cocci like Staphyloccus sp. are relatively short with 5 to 10 disaccharide units in average (Wheeler et al., 2011). A variety of proteins and WTA polymers are anchored to the peptidoglycan (Kawai et al., 2011, Mazmanian et al., 1999, Navarre & Schneewind, 1999, Weidenmaier & Peschel, 2008). The zwitterionic WTA polymers potentially contribute to the sequestration of divalent cations within the OWZ, including Ca2+, Mg2+, and Fe2+ (Beveridge & Murray, 1980). Overall, the CW of monoderm Gram-positive bacteria is a complex structure that protects them from mechanical and osmotic lysis, and serves as a scaffold for anchoring proteins, glycopolymers, and cations that perform various functions (Navarre & Schneewind, 1999, Vollmer et al., 2008).
Fig. 1.
Schematic of the monoderm Gram-positive cell envelope. LTA: lipoteichoic acid polymers; WTA: wall teichoic acid polymers; M: N-acetyl-muramic acid; G: N-acetyl glucosamine. Refer to text for further details.
The OWZ of monoderm Gram-positive bacteria is a very dynamic structure, as bacterial growth requires constant remodeling of the peptidoglycan, which has a turnover rate of 50% per generation (Koch & Doyle, 1985). Remodeling is mediated by cell-wall anchored autolysins that are active on the outermost layer of the peptidoglycan (Jolliffe et al., 1981). Inhibition of autolysin activity on the newly synthesized peptidoglycan is dependent on the acidic pH that is maintained on the trans side of the CM by the proton motive force (Calamita et al., 2001, Kemper et al., 1993, Krulwich et al., 2011). These observations imply that a pH gradient exists across the CW, with the innermost layer being in an acidic environment and the outermost layer being in equilibrium with the pH of the extracellular milieu. This pH gradient is likely to influence the structure and function of proteins that reside within the CW and of those that are transported through the CW.
Translocation of proteins across the cytoplasmic membrane
Although protein translocation across the CM is not the focus of this MicroReview, a brief overview of the translocation systems that exist in monoderm Gram-positive bacteria is instrumental in gaining a global view of protein secretion. For a detailed description of these translocation systems, the reader is directed toward some recent reviews on that subject (Chen & Dubnau, 2004, Desvaux & Hebraud, 2006, du Plessis et al., 2011, Pohl & Harwood, 2010, Robinson et al., 2011, Sutcliffe, 2011, Wang et al., 2000).
Six protein translocation systems have been described for monoderm Gram-positive bacteria. (i) The Sec (Secretion) translocation system is likely the most important one (du Plessis et al., 2011). Proteins destined for export through the Sec translocon generally contain a N-terminal signal sequence, which is cleaved by signal peptidases upon translocation. During translation, the signal sequence of a newly synthesized protein binds to signal recognition proteins, which target the mRNA-ribosome complex to the Sec translocon where protein synthesis continues with the nascent chain passing through the translocon. Proteins secreted through the Sec translocon fold on the trans side of the CM. In some bacterial species, such as Streptococcus pyogenes, the Sec translocon forms a microdomain called the ExPortal (Rosch & Caparon, 2004). Interestingly, the ExPortal localizes exclusively at the division septum, which could be an area of greater CW porosity due to the intense remodeling activity at that site. (ii) The twin arginine transporter (TAT) allows for the translocation of folded proteins (Robinson et al., 2011). Proteins destined for export through the TAT contain a characteristic twin arginine motif within the N-terminal signal sequence, which is cleaved by signal peptidases upon translocation. This system is well known for the transport of redox proteins that require co-factors for folding, such as cytochrome C. (iii) The fimbrillin-protein exporter (FPE) serves to translocate proteins that form pilin-like structures (Chen & Dubnau, 2004). Pilin-like proteins are synthesized with a N-terminal signal sequence that is cleaved by a Type 4 prepilin peptidase. The FPE is a component of the Com pathway that enables bacteria to uptake DNA. (iv) The flagella export apparatus (FEA) serves to secrete proteins that will form the flagella hook, filament, and cap (Erhardt et al., 2010). There is no evidence that the flagella system of monoderm Gram-positive bacteria has evolved into a type III secretion system as seen in Gram-negative bacteria. (v) Holins are phage-encoded pore forming proteins that insert into the bacterial CM and serve to translocate phage-encoded CW hydrolases during the phage lytic cycle (Wang et al., 2000). (vi) Lastly, the ESAT-6/WXG100 secretion system (WSS) translocates proteins that contain a WXG motif in the middle of their polypeptides (Sutcliffe, 2011). This system contributes to the translocation of virulence factors in some pathogenic bacteria (Burts et al., 2005, Abdallah et al., 2007).
Amongst the protein secretion pathways described above, the FEA and possibly the FPE generate structures that expand across the entire bacterial cell envelope (Chen & Dubnau, 2004, Desvaux et al., 2009, Erhardt et al., 2010). During synthesis, the subunits forming the filament are transported within the emerging shaft and added to the distal end of the structure. To the contrary, SEC, TAT, holins, and WSS deliver proteins to the IWZ and there is no evidence of channels facilitating the transport of proteins across the CW.
Transport of proteins across the bacterial cell wall
Since the CW is a porous structure, secreted proteins may diffuse across it. Using purified peptidoglycan, it was estimated that the permeability of the B. subtilis peptidoglycan is limited to globular proteins with a mass of approximately 25 kDa (Demchick & Koch, 1996). Considering that many secreted proteins are larger than 25 kDa and not all proteins are globular, it would appear that the transport of proteins across the CW is not necessarily an unregulated passive event. However, many factors might influence CW permeability: the average length of glycan chains, the level of crosslinking between glycan chains, the presence or absence of bridges between the crosslinking peptides, electrostatic interactions, and the mechanical tension imposed by cell turgor pressure (Ou & Marquis, 1970, Vollmer et al., 2008). Considering that the CW is constantly being remodeled, particularly during exponential bacterial growth, these factors might fluctuate. There is also evidence that mechanisms that are not related to CW permeability may regulate protein transport across the CW of Gram-positive bacteria. Discussed below are the few examples of secreted proteins whose transport across the CW is regulated.
Alpha-amylase and levansucrase
The first reported observation of regulated protein transport across the CW involved the transport of α-amylase, an enzyme that hydrolyzes starch to generate glucose, and an undefined protease by Bacillus amyloliquefaciens (Gould et al., 1975). It was observed that transport of these two enzymes continues for about 15 minutes after blocking de novo protein synthesis with chloramphenicol. The uncoupler, 2,4-Dinitrophenol, and the ATPase inhibitor sodium azide had no influence on this phenomenon, indicating that it is independent of translocation through the Sec translocon. Transport was influenced by temperature and did not occur at 0°C. Lastly, this pool of enzymes was absent in protoplasts. Conclusions from this study were that bacteria accumulate a pool of proteins on the trans side of the CM and that transport of these proteins across the CW is restricted. The effect of temperature might suggest that transport of these two enzymes is influenced either by enzymatic reactions, rate of protein folding, or the dynamics of complex formation/dissolution within the cell envelope.
Further studies on the mechanism that regulates the transport of α-amylase and levansucrase across the CW of Bacillus spp. revealed that the rate of transport is positively influenced by the rate of protein folding (Stephenson et al., 1998). Levansucrase is an enzyme that catalyzes the transfer of a fructose moiety from sucrose to polyfructose, releasing a molecule of glucose. Interestingly, α-amylase and levansucrase are metalloenzymes whose folding is cation-dependent (Chambert et al., 1990, Haddaoui et al., 1997, Leloup et al., 1997, Petit-Glatron et al., 1993, Stephenson et al., 1998). The in vitro rate of folding of α-amylase and levansucrase are the same in presence of calcium, although anionic polymers can substitute for calcium in modulating the folding of levansucrase, but not of α-amylase (Chambert & Petit-Glatron, 1999). Perhaps, this would explain why the transport of levansucrase is twice as fast as the transport of α-amylase (Leloup et al., 1997). Alternatively, the difference in size might influence the rate of protein transport, as α-amylase is larger (69 kDa) than levansucrase (50 kDa).
Another factor that influences the rate of transport of α-amylase across the CW is the post-translocation chaperone and peptidyl-prolyl cis/trans isomerase, PrsA (Vitikainen et al., 2004, Vitikainen et al., 2001). PrsA is a lipoprotein that is anchored on the trans side of the cytoplasmic membrane by an acyl chain. A linear correlation was observed between the bacterial concentration of PrsA and the amount of α-amylase being secreted by B. subtilis. Interestingly, interruption of the gene coding for a D-alanine transferase (dlt) rescues the α-amylase transport defect associated with a defective PrsA protein (Hyyrylainen et al., 2000). D-alanine transferase mediates the alanylation of WTA and LTA, thereby modulating charges of these polymers (Perego et al., 1995). It was suggested that the net negative charge of the CW influences protein folding, as modification of WTA and LTA by D-alanylation modulates the rate of protein transport across the CW (Hyyrylainen et al., 2000). It would be reasonable to speculate that a more negatively charged CW is likely to bind more cations, facilitating folding of α-amylase in the absence of PrsA.
In summary, the rate of transport of α-amylase and levansucrase is influenced by the rate of protein folding, which is influenced by cations, the net negative charge of the CW, and the peptidyl-prolyl cis/trans isomerase and post-translocation chaperone, PrsA. The role of PrsA on the rate of transport of α-amylase and levansucrase could be direct or indirect. Indirectly, PrsA is likely to influence the makeup of the CW by modulating the folding of many proteins, which might influence the transport of α-amylase and levansucrase. For example, PrsA mediates the folding of penicillin-binding proteins (PBPs), which are a set of enzymes that catalyze transpeptidase and transglycosidase reactions during peptidoglycan biosynthesis (Hyyrylainen et al., 2010).
Subtilisin
Subtilisin is a serine protease secreted by Bacillus spp. It is synthesized as a proenzyme (36 kDa), whose maturation occurs by autocatalysis (Power et al., 1986). The propeptide of subtilisin serves as a folding chaperone and can act in trans (Zhu et al., 1989). Interestingly, the propeptide remains bound to the catalytic domain following autocatalysis, and release of the mature enzyme (28 kDa) requires degradation of the propeptide by the catalytic domain (Yabuta et al., 2001). PrsA is required for protein stability, presumably because it contributes to folding of subtilisin (Jacobs et al., 1993). The mature enzyme contains two Ca2+sites that also contribute to protein stability (Bode et al., 1987, Pantoliano et al., 1988).
It was observed that the proform of subtilisin remains associated with bacteria, whereas the mature form is released in the extracellular milieu (Power et al., 1986). A catalytic mutant of subtilisin that cannot undergo maturation also remains associated with bacteria. However, this mutant can be rescued in trans if a wild-type copy of the gene coding for subtilisin is provided, resulting in transport of a mature but inactive mutant enzyme across the CW. Therefore, transport of subtilisin is dependent on protein folding, stability, and enzymatic activity, which are influenced by its propeptide, PrsA, and Ca2+. It is also possible that the propeptide contributes to complex formation with elements of the cell envelope, precluding transport of the zymogen across the CW.
Enterotoxin B
Staphylococcus aureus secretes several toxins including enterotoxin B, also known as SEB. SEB has no enzymatic activity, but acts as a superantigen by crosslinking major histocompatibility complex II on antigen presenting cells to the T cell receptor on CD4 T lymphocytes, resulting in a massive release of pro-inflammatory cytokines (Muller-Alouf et al., 2001). It was observed that following cleavage of the signal peptide, mature SEB (mSEB, 28 kDa) remains transiently associated with bacteria (Tweten & Iandolo, 1983). Digestion of the CW with lysostaphin released all cell-associated mSEB, but 1M NaCl had no effect. Cell-associated mSEB was resistant to proteolysis by proteinase K and could not be detected with antibodies. Together, these observations suggested that mSEB is temporarily sequestered in the IWZ prior to transport across the OWZ. Perhaps mSEB has a relatively slow rate of folding, slowing down its rate of transport across the CW. Interestingly, the toxin has a basic isoelectric point, a factor that would promote its interaction with anionic CW polymers.
Broad-range phospholipase C and metalloprotease of Listeria monocytogenes
The broad-range phospholipase C, also known as PC-PLC, and the metalloprotease (Mpl) of L. monocytogenes are two zinc-dependent enzymes that contribute to the virulence of this foodborne bacterial pathogen (Portnoy et al., 1992). During infection, L. monocytogenes resides inside host cells (Mackaness, 1962, Tilney & Portnoy, 1989). Following entry into a cell, membrane-bound bacteria rapidly escape vacuoles to access the host cytosol where they multiply. Cell-to-cell spread is mediated by an actin-based mechanism of motility, resulting in entrapment of bacteria in double membrane vacuoles from which they must escape to perpetuate the intracellular growth cycle. Escape from vacuoles is facilitated by PC-PLC (Marquis et al., 1995, Smith et al., 1995, Vazquez-Boland et al., 1992). PC-PLC (30 kDa) is synthesized with a 24-residue N-terminal propeptide that is cleaved by Mpl, to generate the mature enzyme (28 kDa) (Forster et al., 2011a, Marquis et al., 1997, Poyart et al., 1993). Similarly, Mpl is synthesized as a zymogen (55 kDa) with a 200-residue N-terminal propeptide (20 kDa) (Bitar et al., 2008, Mengaud et al., 1991). Both PC-PLC and Mpl are zinc metalloenzymes (Coffey et al., 2000, Zuckert et al., 1998). In addition, Mpl binds several molecules of calcium that increase its stability. Regulation of PC-PLC and Mpl maturation and transport across the CW during intracellular growth is imperative to the virulence of L. monocytogenes (Yeung et al., 2007).
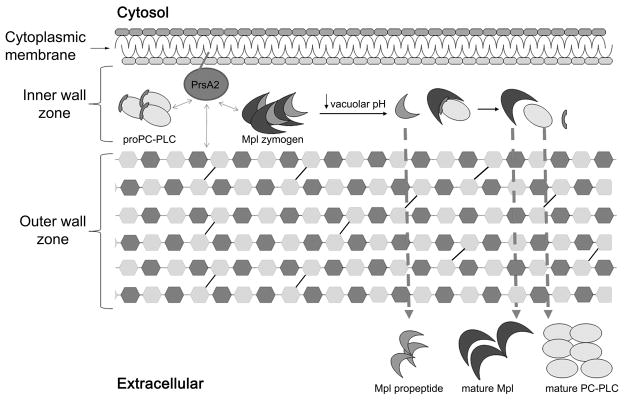
The Mpl zymogen temporarily resides in the IWZ of L. monocytogenes (Forster et al., 2011a, O’Neil et al., 2009). The propeptide of Mpl, which can only act in cis, contributes to Mpl stability, native folding, and localization (Bitar et al., 2008, O’Neil et al., 2009). During infection, Mpl undergoes autocatalysis upon a decrease in vacuolar pH, leading to transport of an intact propeptide (20 kDa) and catalytic domain (35 kDa) across the CW (Forster et al., 2011a). This pH effect may be indirect as the proton motive force maintains the outermost layer of the CW in a highly protonated state (Calamita et al., 2001, Krulwich et al., 2011). PrsA2, an ortholog of the Bacillus chaperone PrsA, also contributes to the localization of Mpl (Forster et al., 2011b). In the absence of PrsA2, the Mpl zymogen is rapidly secreted across the bacterial CW, and the secreted zymogen does not undergo autocatalysis in response to acidic pH.
Similarly to Mpl, the proform of PC-PLC temporarily resides in the bacterial IWZ and this localization is dependent on the presence of its propeptide and PrsA2 (Forster et al., 2011b, Marquis & Hager, 2000, Snyder & Marquis, 2003). Upon a decrease in pH, mature Mpl enables the transport of the pro and mature forms of PC-PLC, indicating that protein size is not the limiting factor in PC-PLC transport (Slepkov et al., 2010, Yeung et al., 2005). The N-terminus of the PC-PLC propeptide is required for maintaining the protein in the IWZ and for Mpl-mediated processing of the propeptide (Slepkov et al., 2010). However, the propeptide of PC-PLC cannot act in trans and cannot retain a Bacillus ortholog of PC-PLC in the IWZ, indicating that the PC-PLC propeptide does not have an intrinsic ability to prevent protein transport (Yeung et al., 2005). A propeptide-less mutant of PC-PLC is rapidly secreted as an active enzyme across the CW, independent of a decrease in pH and of Mpl, suggesting that the propeptide is essential to prevent transport of PC-PLC at physiological pH, and that the catalytic domain folds to its native form independent of the propeptide (Slepkov et al., 2010, Yeung et al., 2005).
In summary, transport of PC-PLC and Mpl is controlled by a combination of factors that include their respective propeptides, PrsA2, and pH (Fig. 2) (Forster et al., 2011a, Forster et al., 2011b, Marquis & Hager, 2000, O’Neil et al., 2009, Slepkov et al., 2010, Snyder & Marquis, 2003, Yeung et al., 2005). During intracellular growth, bacteria maintain a pool of the proform of PC-PLC and the Mpl zymogen in the IWZ. Efficient transport of both enzymes requires a decrease in pH that leads to Mpl autocatalysis, PC-PLC maturation, and transport of both enzymes across the CW. This phenomenon is completely independent of de novo protein synthesis (Marquis & Hager, 2000). In the absence of PrsA2, the proform of either enzyme is secreted independent of pH, affecting their ability to undergo maturation (Alonzo et al., 2009, Forster et al., 2011b, Zemansky et al., 2009). Although there was no evidence of increase in CW permeability in the absence of PrsA2, the mutant was more sensitive to penicillin and to lyzozyme indicating that CW properties were modified (Alonzo III et al., 2011, Forster et al., 2011b). It is possible that the proform of PC-PLC and Mpl are retained in the IWZ by interacting with PrsA2. Alternatively, PrsA2 may be required to stabilize cell envelope proteins that interact with PC-PLC and Mpl, or to maintain the biochemical and biophysical properties of the CW.
Fig. 2.
Model of the mechanism regulating PC-PLC and Mpl maturation and transport across the CW during infection. Refer to text for further details.
Common themes
Monoderm Gram-positive bacteria have the means to regulate the transport of secreted proteins across the CW, although currently we have a limited understanding of these processes. In this review, we discussed six proteins whose transport across the CW is regulated. There are three features that are common to five out of the six proteins: enzymatic activity, the requirement for divalent cations, and a post-translocation chaperone with peptidyl-prolyl cis/trans isomerase activity known as PrsA or PrsA2 (Table 1). In addition, three of these enzymes are made as proproteins whose maturation requires proteolytic cleavage of the propeptide.
Table 1.
Summary of protein characteristicsa
| Protein | Bacterial species | Size (kDa) | Metallo-enzymes | Transport influenced by PrsA/PrsA2 | Other influencing factors |
|---|---|---|---|---|---|
| α - Amylase | Bacillus sp. | 69 | Yes | Yes | D-alanylation of CW polymers |
| Levansucrase | Bacillus sp. | 50 | Yes | Yes | |
| Subtilisin | B. subtilis and other Bacillus sp. | 36/28b | Yes | Yes | |
| SEB | S. aureus | 28 | No | NDc | |
| Mpl | L. monocytogenes | 55/35 | Yes | Yes | pH |
| PC-PLC | L. monocytogenes | 30/28 | Yes | Yes | pH |
References are listed in the text
For proproteins, the size of the pro and mature enzymes are listed
Not determined
Alpha-amylase, levansucrase, subtilisin, Mpl, and PC-PLC are all metalloenzymes that bind divalent cations (Bode et al., 1987, Chambert et al., 1990, Coffey et al., 2000, Haddaoui et al., 1997, Leloup et al., 1997, Pantoliano et al., 1988, Petit-Glatron et al., 1993, Stephenson et al., 1998, Zuckert et al., 1998). Divalent cations are important for protein folding, which is a prerequisite for transport across the CW and for protein stability. These cations are also often required for enzyme activity, leading to autocatalysis, as in the case of subtilisin and Mpl. Taken together, these observations suggest that divalent cations may regulate the transport of metalloenzymes across the CW by supporting protein folding and autocatalysis.
The post-translocation chaperone and peptidyl-prolyl cis/trans isomerase PrsA increases the stability and the rate of transport of α-amylase, levansucrase, and subtilisin possibly by mediating folding of these proteins in the IWZ (Hyyrylainen et al., 2010, Jacobs et al., 1993, Vitikainen et al., 2004, Vitikainen et al., 2001). Mutants that make less PrsA also secrete less α-amylase, levansucrase, and subtilisin. Interestingly, a decrease in D-alanylation of CW polymers reverses the defect associated with the PrsA mutant (Hyyrylainen et al., 2000). This effect may be due to an increase in the CW net negative charge and consequently to an increase in the concentration of divalent cations, which are known to contribute to protein folding, stability, and enzyme activity. To the contrary, in L. monocytogenes, PrsA2 is required to maintain the proforms of Mpl and PC-PLC in the IWZ (Forster et al., 2011b). In the absence of PrsA2, the pro-enzymes are rapidly transported into the extracellular milieu, hindering the ability of Mpl to undergo autocatalysis and to mediate PC-PLC maturation. Perhaps PrsA2 interacts with Mpl and PC-PLC, or promotes interaction with other CW components to transiently retain the proteins within the CW. Alternatively, PrsA2 might modulate the porosity of the OWZ by contributing to the folding of PBPs (Alonzo III et al., 2011, Hyyrylainen et al., 2010). A lower level of peptidoglycan crosslinking is likely to increase the porosity of the CW. Therefore, the post-translocation chaperone and peptidyl-prolyl cis/trans isomerases PrsA and PrsA2 regulate by direct and indirect means the rate of transport of metalloenzymes across the CW.
Three of the proteins discussed in this review are made as proenzymes: subtilisin, Mpl, and PC-PLC. All three are rapidly transported across the CW following propeptide cleavage (Forster et al., 2011a, Marquis & Hager, 2000, Power et al., 1986, Snyder & Marquis, 2003). Arguably, protein size might be an issue, as the mature enzymes are smaller. Although we know that size does not matter for PC-PLC (Slepkov et al., 2010, Yeung et al., 2005), it might play a role for subtilisin and Mpl, which are much bigger enzymes. An alternative hypothesis to protein size would be that the propeptides interact with CW components, retaining the proteins within the CW. PrsA/PrsA2 might indirectly regulate these protein/protein interactions, by contributing to the folding of many proteins post-translocation.
SEB does not have enzymatic activity and it does not bind divalent cations (Papageorgiou et al., 1998). However, it is not known if folding of SEB is influenced by a PrsA-like chaperone. Perhaps the transport of SEB across the CW is regulated by completely different mechanisms than metalloenzymes. Recently, it was reported that anthrax toxin is associated with membrane-derived vesicles, which could be another mean of leaving the IWZ (Rivera et al., 2010).
There are certainly additional factors that modulate the rate of protein transport across the bacterial CW. One might speculate that the tightly regulated CW hydrolases and transglycosylases directly or indirectly control the transport of proteins by modulating the length of the glycan chains as well as the level of peptide cross-linking between them (Hyyrylainen et al., 2010, Jolliffe et al., 1981, Scheurwater et al., 2008). In addition, as suggested by the location of the Sec translocon in S. pyogenes, protein transport across the CW might be more efficient at sites of bacterial division (Rosch & Caparon, 2004).
Conclusion
What would monoderm Gram-positive bacteria gain in regulating the transport of proteins across the CW? Perhaps, we can take examples from the sophisticated transport systems harbored by Gram-negative bacteria, which have refined spatial and temporal means of regulating protein transport for basic biological functions and for virulence (Hayes et al., 2010). Gram-positive bacteria might also benefit in storing virulence factors that can be delivered within seconds of sensing a cue that the perfect target or compartment has been reached. It is up to us to discover their ingenuity.
Acknowledgments
The work related to L. monocytogenes PC-PLC and Mpl was supported by the National Institutes of Health grants AI42800 and AI52154 (H.M.).
References
- Abdallah AM, Gey van Pittius NC, Champion PA, Cox J, Luirink J, Vandenbroucke-Grauls CM, Appelmelk BJ, Bitter W. Type VII secretion--mycobacteria show the way. Nature reviews Microbiology. 2007;5:883–891. doi: 10.1038/nrmicro1773. [DOI] [PubMed] [Google Scholar]
- Alonzo F, 3rd, Port GC, Cao M, Freitag NE. The posttranslocation chaperone PrsA2 contributes to multiple facets of Listeria monocytogenes pathogenesis. Infect Immun. 2009;77:2612–2623. doi: 10.1128/IAI.00280-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonzo F, III, Xayarath B, Whisstock JC, Freitag NE. Functional analysis of the Listeria monocytogenes secretion chaperone PrsA2 and its multiple contributions to bacterial virulence. Mol Microbiol. 2011;80:1530–1548. doi: 10.1111/j.1365-2958.2011.07665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atrih A, Zollner P, Allmaier G, Williamson MP, Foster SJ. Peptidoglycan structural dynamics during germination of Bacillus subtilis 168 endospores. J Bacteriol. 1998;180:4603–4612. doi: 10.1128/jb.180.17.4603-4612.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge TJ, Murray RG. Sites of metal deposition in the cell wall of Bacillus subtilis. J Bacteriol. 1980;141:876–887. doi: 10.1128/jb.141.2.876-887.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitar AP, Cao M, Marquis H. The metalloprotease of Listeria monocytogenes is activated by intramolecular autocatalysis. J Bacteriol. 2008;190:107–111. doi: 10.1128/JB.00852-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode W, Papamokos E, Musil D. The high-resolution X-ray crystal structure of the complex formed between subtilisin Carlsberg and eglin c, an elastase inhibitor from the leech Hirudo medicinalis. Structural analysis, subtilisin structure and interface geometry. Eur J Biochem. 1987;166:673–692. doi: 10.1111/j.1432-1033.1987.tb13566.x. [DOI] [PubMed] [Google Scholar]
- Burts ML, Williams WA, Debord K, Missiakas DM. EsxA and EsxB are secreted by an ESAT-6-like system that is required for the pathogenesis of Staphylococcus aureus infections. Proc Natl Acad Sci U S A. 2005;102:1169–1174. doi: 10.1073/pnas.0405620102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calamita HG, Ehringer WD, Koch AL, Doyle RJ. Evidence that the cell wall of Bacillus subtilis is protonated during respiration. Proc Natl Acad Sci USA. 2001;98:15260–15263. doi: 10.1073/pnas.261483798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambert R, Benyahia F, Petit-Glatron MF. Secretion of Bacillus subtilis levansucrase. Fe(III) could act as a cofactor in an efficient coupling of the folding and translocation processes. Biochem J. 1990;265:375–382. doi: 10.1042/bj2650375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambert R, Petit-Glatron MF. Anionic polymers of Bacillus subtilis cell wall modulate the folding rate of secreted proteins. FEMS Microbiol Lett. 1999;179:43–47. doi: 10.1111/j.1574-6968.1999.tb08705.x. [DOI] [PubMed] [Google Scholar]
- Chen I, Dubnau D. DNA uptake during bacterial transformation. Nature reviews Microbiology. 2004;2:241–249. doi: 10.1038/nrmicro844. [DOI] [PubMed] [Google Scholar]
- Coffey A, van den Burg B, Veltman R, Abee T. Characteristics of the biologically active 35-kDa metalloprotease virulence factor from Listeria monocytogenes. J Appl Microbiol. 2000;88:132–141. doi: 10.1046/j.1365-2672.2000.00941.x. [DOI] [PubMed] [Google Scholar]
- Demchick P, Koch AL. The permeability of the wall fabric of Escherichia coli and Bacillus subtilis. J Bacteriol. 1996;178:768–773. doi: 10.1128/jb.178.3.768-773.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvaux M, Hebraud M. The protein secretion systems in Listeria: inside out bacterial virulence. FEMS Microbiol Rev. 2006;30:774–805. doi: 10.1111/j.1574-6976.2006.00035.x. [DOI] [PubMed] [Google Scholar]
- Desvaux M, Hebraud M, Talon R, Henderson IR. Secretion and subcellular localizations of bacterial proteins: a semantic awareness issue. Trends Microbiol. 2009;17:139–145. doi: 10.1016/j.tim.2009.01.004. [DOI] [PubMed] [Google Scholar]
- du Plessis DJ, Nouwen N, Driessen AJ. The Sec translocase. Biochim Biophys Acta. 2011;1808:851–865. doi: 10.1016/j.bbamem.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Erhardt M, Namba K, Hughes KT. Bacterial nanomachines: the flagellum and type III injectisome. Cold Spring Harb Perspect Biol. 2010;2:a000299. doi: 10.1101/cshperspect.a000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster BM, Bitar AP, Slepkov ER, Kota KJ, Sondermann H, Marquis H. The metalloprotease of Listeria monocytogenes is regulated by pH. J Bacteriol. 2011a;193:5090–5097. doi: 10.1128/JB.05134-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster BM, Zemansky J, Portnoy DA, Marquis H. Posttranslocation chaperone PrsA2 regulates the maturation and secretion of Listeria monocytogenes proprotein virulence factors. J Bacteriol. 2011b;193:5961–5970. doi: 10.1128/JB.05307-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould AR, May BK, Elliott WH. Release of extracellular enzymes from Bacillus amyloliquefaciens. J Bacteriol. 1975;122:34–40. doi: 10.1128/jb.122.1.34-40.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddaoui EA, Leloup L, Petit-Glatron MF, Chambert R. Characterization of a stable intermediate trapped during reversible refolding of Bacillus subtilis alpha-amylase. Eur J Biochem. 1997;249:505–509. doi: 10.1111/j.1432-1033.1997.00505.x. [DOI] [PubMed] [Google Scholar]
- Hayes CS, Aoki SK, Low DA. Bacterial contact-dependent delivery systems. Annu Rev Genet. 2010;44:71–90. doi: 10.1146/annurev.genet.42.110807.091449. [DOI] [PubMed] [Google Scholar]
- Hayhurst EJ, Kailas L, Hobbs JK, Foster SJ. Cell wall peptidoglycan architecture in Bacillus subtilis. Proc Natl Acad Sci USA. 2008;105:14603–14608. doi: 10.1073/pnas.0804138105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyyrylainen HL, Marciniak BC, Dahncke K, Pietiainen M, Courtin P, Vitikainen M, Seppala R, Otto A, Becher D, Chapot-Chartier MP, Kuipers OP, Kontinen VP. Penicillin-binding protein folding is dependent on the PrsA peptidyl-prolyl cis-trans isomerase in Bacillus subtilis. Mol Microbiol. 2010;77:108–127. doi: 10.1111/j.1365-2958.2010.07188.x. [DOI] [PubMed] [Google Scholar]
- Hyyrylainen HL, Vitikainen M, Thwaite J, Wu H, Sarvas M, Harwood CR, Kontinen VP, Stephenson K. D-Alanine substitution of teichoic acids as a modulator of protein folding and stability at the cytoplasmic membrane/cell wall interface of Bacillus subtilis. J Biol Chem. 2000;275:26696–26703. doi: 10.1074/jbc.M003804200. [DOI] [PubMed] [Google Scholar]
- Jacobs M, Andersen JB, Kontinen V, Sarvas M. Bacillus subtilis PrsA is required in vivo as an extracytoplasmic chaperone for secretion of active enzymes synthesized either with or without pro-sequences. Mol Microbiol. 1993;8:957–966. doi: 10.1111/j.1365-2958.1993.tb01640.x. [DOI] [PubMed] [Google Scholar]
- Jolliffe LK, Doyle RJ, Streips UN. The energized membrane and cellular autolysis in Bacillus subtilis. Cell. 1981;25:753–763. doi: 10.1016/0092-8674(81)90183-5. [DOI] [PubMed] [Google Scholar]
- Kawai Y, Marles-Wright J, Cleverley RM, Emmins R, Ishikawa S, Kuwano M, Heinz N, Bui NK, Hoyland CN, Ogasawara N, Lewis RJ, Vollmer W, Daniel RA, Errington J. A widespread family of bacterial cell wall assembly proteins. Embo J. 2011;30:4931–4941. doi: 10.1038/emboj.2011.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper MA, Urrutia MM, Beveridge TJ, Koch AL, Doyle RJ. Proton motive force may regulate cell wall-associated enzymes of Bacillus subtilis. J Bacteriol. 1993;175:5690–5696. doi: 10.1128/jb.175.17.5690-5696.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch AL, Doyle RJ. Inside-to-outside growth and turnover of the wall of gram-positive rods. J Theor Biol. 1985;117:137–157. doi: 10.1016/s0022-5193(85)80169-7. [DOI] [PubMed] [Google Scholar]
- Krulwich TA, Sachs G, Padan E. Molecular aspects of bacterial pH sensing and homeostasis. Nat Rev Microbiol. 2011;9:330–343. doi: 10.1038/nrmicro2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leloup L, Haddaoui el A, Chambert R, Petit-Glatron MF. Characterization of the rate-limiting step of the secretion of Bacillus subtilis alpha-amylase overproduced during the exponential phase of growth. Microbiol. 1997;143(Pt 10):3295–3303. doi: 10.1099/00221287-143-10-3295. [DOI] [PubMed] [Google Scholar]
- Mackaness GB. Cellular resistance to infection. J Exp Med. 1962;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis H, Doshi V, Portnoy DA. The broad-range phospholipase C and a metalloprotease mediate listeriolysin O-independent escape of Listeria monocytogenes from a primary vacuole in human epithelial cells. Infect Immun. 1995;63:4531–4534. doi: 10.1128/iai.63.11.4531-4534.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis H, Goldfine H, Portnoy DA. Proteolytic pathways of activation and degradation of a bacterial phospholipase C during intracellular infection by Listeria monocytogenes. J Cell Biol. 1997;137:1381–1392. doi: 10.1083/jcb.137.6.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis H, Hager EJ. pH-regulated activation and release of a bacteria-associated phospholipase C during intracellular infection by Listeria monocytogenes. Mol Microbiol. 2000;35:289–298. doi: 10.1046/j.1365-2958.2000.01708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matias VR, Beveridge TJ. Cryo-electron microscopy reveals native polymeric cell wall structure in Bacillus subtilis 168 and the existence of a periplasmic space. Mol Microbiol. 2005;56:240–251. doi: 10.1111/j.1365-2958.2005.04535.x. [DOI] [PubMed] [Google Scholar]
- Matias VR, Beveridge TJ. Native cell wall organization shown by cryo-electron microscopy confirms the existence of a periplasmic space in Staphylococcus aureus. J Bacteriol. 2006;188:1011–1021. doi: 10.1128/JB.188.3.1011-1021.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazmanian SK, Liu G, Ton-That H, Schneewind O. Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall. Science. 1999;285:760–763. doi: 10.1126/science.285.5428.760. [DOI] [PubMed] [Google Scholar]
- Mengaud J, Geoffroy C, Cossart P. Identification of a new operon involved in Listeria monocytogenes virulence: its first gene encodes a protein homologous to bacterial metalloproteases. Infect Immun. 1991;59:1043–1049. doi: 10.1128/iai.59.3.1043-1049.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Alouf H, Carnoy C, Simonet M, Alouf JE. Superantigen bacterial toxins: state of the art. Toxicon. 2001;39:1691–1701. doi: 10.1016/s0041-0101(01)00156-8. [DOI] [PubMed] [Google Scholar]
- Navarre WW, Schneewind O. Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol Mol Biol Rev. 1999;63:174–229. doi: 10.1128/mmbr.63.1.174-229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil HS, Forster BM, Roberts KL, Chambers AJ, Bitar AP, Marquis H. The propeptide of the metalloprotease of Listeria monocytogenes controls compartmentalization of the zymogen during intracellular infection. J Bacteriol. 2009;191:3594–3603. doi: 10.1128/JB.01168-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou LT, Marquis RE. Electromechanical interactions in cell walls of gram-positive cocci. J Bacteriol. 1970;101:92–101. doi: 10.1128/jb.101.1.92-101.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoliano MW, Whitlow M, Wood JF, Rollence ML, Finzel BC, Gilliland GL, Poulos TL, Bryan PN. The engineering of binding affinity at metal ion binding sites for the stabilization of proteins: subtilisin as a test case. Biochem. 1988;27:8311–8317. doi: 10.1021/bi00422a004. [DOI] [PubMed] [Google Scholar]
- Papageorgiou AC, Tranter HS, Acharya KR. Crystal structure of microbial superantigen staphylococcal enterotoxin B at 1.5 A resolution: implications for superantigen recognition by MHC class II molecules and T-cell receptors. J Mol Biol. 1998;277:61–79. doi: 10.1006/jmbi.1997.1577. [DOI] [PubMed] [Google Scholar]
- Perego M, Glaser P, Minutello A, Strauch MA, Leopold K, Fischer W. Incorporation of D-alanine into lipoteichoic acid and wall teichoic acid in Bacillus subtilis. Identification of genes and regulation. J Biol Chem. 1995;270:15598–15606. doi: 10.1074/jbc.270.26.15598. [DOI] [PubMed] [Google Scholar]
- Petit-Glatron MF, Grajcar L, Munz A, Chambert R. The contribution of the cell wall to a transmembrane calcium gradient could play a key role in Bacillus subtilis protein secretion. Mol Microbiol. 1993;9:1097–1106. doi: 10.1111/j.1365-2958.1993.tb01239.x. [DOI] [PubMed] [Google Scholar]
- Pohl S, Harwood CR. Heterologous protein secretion by Bacillus species from the cradle to the grave. Adv Appl Microbiol. 2010;73:1–25. doi: 10.1016/S0065-2164(10)73001-X. [DOI] [PubMed] [Google Scholar]
- Portnoy DA, Chakraborty T, Goebel W, Cossart P. Molecular determinants of Listeria monocytogenes pathogenesis. Infect Immun. 1992;60:1263–1267. doi: 10.1128/iai.60.4.1263-1267.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power SD, Adams RM, Wells JA. Secretion and autoproteolytic maturation of subtilisin. Proc Natl Acad Sci USA. 1986;83:3096–3100. doi: 10.1073/pnas.83.10.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyart C, Abachin E, Razafimanantsoa I, Berche P. The zinc metalloprotease of Listeria monocytogenes is required for maturation of phosphatidylcholine phospholipase C: direct evidence obtained by gene complementation. Infect Immun. 1993;61:1576–1580. doi: 10.1128/iai.61.4.1576-1580.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichmann NT, Grundling A. Location, synthesis and function of glycolipids and polyglycerolphosphate lipoteichoic acid in Gram-positive bacteria of the phylum Firmicutes. FEMS Microbiol Lett. 2011;319:97–105. doi: 10.1111/j.1574-6968.2011.02260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera J, Cordero RJ, Nakouzi AS, Frases S, Nicola A, Casadevall A. Bacillus anthracis produces membrane-derived vesicles containing biologically active toxins. Proc Natl Acad Sci U S A. 2010;107:19002–19007. doi: 10.1073/pnas.1008843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson C, Matos CF, Beck D, Ren C, Lawrence J, Vasisht N, Mendel S. Transport and proofreading of proteins by the twin-arginine translocation (Tat) system in bacteria. Biochim Biophys Acta. 2011;1808:876–884. doi: 10.1016/j.bbamem.2010.11.023. [DOI] [PubMed] [Google Scholar]
- Rosch J, Caparon M. A microdomain for protein secretion in gram-positive bacteria. Science. 2004;304:1513–1515. doi: 10.1126/science.1097404. [DOI] [PubMed] [Google Scholar]
- Scheurwater E, Reid CW, Clarke AJ. Lytic transglycosylases: bacterial space-making autolysins. Int J Biochem Cell Biol. 2008;40:586–591. doi: 10.1016/j.biocel.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Slepkov ER, Pavinski Bitar A, Marquis H. Differentiation of propeptide residues regulating the compartmentalization, maturation and activity of the broad-range phospholipase C of Listeria monocytogenes. Biochem J. 2010;432:557–563. doi: 10.1042/BJ20100557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GA, Marquis H, Jones S, Johnston NC, Portnoy DA, Goldfine H. The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect Immun. 1995;63:4231–4237. doi: 10.1128/iai.63.11.4231-4237.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder A, Marquis H. Restricted translocation across the cell wall regulates secretion of the broad-range phospholipase C of Listeria monocytogenes. J Bacteriol. 2003;185:5953–5958. doi: 10.1128/JB.185.20.5953-5958.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson K, Carter NM, Harwood CR, Petit-Glatron MF, Chambert R. The influence of protein folding on late stages of the secretion of alpha-amylases from Bacillus subtilis. FEBS Lett. 1998;430:385–389. doi: 10.1016/s0014-5793(98)00698-x. [DOI] [PubMed] [Google Scholar]
- Sutcliffe IC. A phylum level perspective on bacterial cell envelope architecture. Trends Microbiol. 2010;18:464–470. doi: 10.1016/j.tim.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Sutcliffe IC. New insights into the distribution of WXG100 protein secretion systems. Antonie van Leeuwenhoek. 2011;99:127–131. doi: 10.1007/s10482-010-9507-4. [DOI] [PubMed] [Google Scholar]
- Tilney LG, Portnoy DA. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989;109:1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweten RK, Iandolo JJ. Transport and processing of staphylococcal enterotoxin B. J Bacteriol. 1983;153:297–303. doi: 10.1128/jb.153.1.297-303.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Boland JA, Kocks C, Dramsi S, Ohayon H, Geoffroy C, Mengaud J, Cossart P. Nucleotide sequence of the lecithinase operon of Listeria monocytogenes and possible role of lecithinase in cell-to-cell spread. Infect Immun. 1992;60:219–230. doi: 10.1128/iai.60.1.219-230.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitikainen M, Lappalainen I, Seppala R, Antelmann H, Boer H, Taira S, Savilahti H, Hecker M, Vihinen M, Sarvas M, Kontinen VP. Structure-function analysis of PrsA reveals roles for the parvulin-like and flanking N- and C-terminal domains in protein folding and secretion in Bacillus subtilis. J Biol Chem. 2004;279:19302–19314. doi: 10.1074/jbc.M400861200. [DOI] [PubMed] [Google Scholar]
- Vitikainen M, Pummi T, Airaksinen U, Wahlstrom E, Wu H, Sarvas M, Kontinen VP. Quantitation of the capacity of the secretion apparatus and requirement for PrsA in growth and secretion of alpha-amylase in Bacillus subtilis. J Bacteriol. 2001;183:1881–1890. doi: 10.1128/JB.183.6.1881-1890.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer W, Blanot D, de Pedro MA. Peptidoglycan structure and architecture. FEMS Microbiol Rev. 2008;32:149–167. doi: 10.1111/j.1574-6976.2007.00094.x. [DOI] [PubMed] [Google Scholar]
- Wang IN, Smith DL, Young R. Holins: the protein clocks of bacteriophage infections. Annu Rev Microbiol. 2000;54:799–825. doi: 10.1146/annurev.micro.54.1.799. [DOI] [PubMed] [Google Scholar]
- Ward JB. The chain length of the glycans in bacterial cell walls. Biochem J. 1973;133:395–398. doi: 10.1042/bj1330395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidenmaier C, Peschel A. Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions. Nat Rev Microbiol. 2008;6:276–287. doi: 10.1038/nrmicro1861. [DOI] [PubMed] [Google Scholar]
- Wheeler R, Mesnage S, Boneca IG, Hobbs JK, Foster SJ. Super-resolution microscopy reveals cell wall dynamics and peptidoglycan architecture in ovococcal bacteria. Mol Microbiol. 2011;82:1096–1109. doi: 10.1111/j.1365-2958.2011.07871.x. [DOI] [PubMed] [Google Scholar]
- Yabuta Y, Takagi H, Inouye M, Shinde U. Folding pathway mediated by an intramolecular chaperone: propeptide release modulates activation precision of pro-subtilisin. J Biol Chem. 2001;276:44427–44434. doi: 10.1074/jbc.M107573200. [DOI] [PubMed] [Google Scholar]
- Yeung PS, Na Y, Kreuder AJ, Marquis H. Compartmentalization of the broad-range phospholipase C activity to the spreading vacuole is critical for Listeria monocytogenes virulence. Infect Immun. 2007;75:44–51. doi: 10.1128/IAI.01001-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung PS, Zagorski N, Marquis H. The metalloprotease of Listeria monocytogenes controls cell wall translocation of the broad-range phospholipase C. J Bacteriol. 2005;187:2601–2608. doi: 10.1128/JB.187.8.2601-2608.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemansky J, Kline BC, Woodward JJ, Leber JH, Marquis H, Portnoy DA. Development of a mariner-based transposon and identification of Listeria monocytogenes determinants, including the peptidyl-prolyl isomerase PrsA2, that contribute to Its hemolytic phenotype. J Bacteriol. 2009;191:3950–3964. doi: 10.1128/JB.00016-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XL, Ohta Y, Jordan F, Inouye M. Pro-sequence of subtilisin can guide the refolding of denatured subtilisin in an intermolecular process. Nature. 1989;339:483–484. doi: 10.1038/339483a0. [DOI] [PubMed] [Google Scholar]
- Zuber B, Haenni M, Ribeiro T, Minnig K, Lopes F, Moreillon P, Dubochet J. Granular layer in the periplasmic space of gram-positive bacteria and fine structures of Enterococcus gallinarum and Streptococcus gordonii septa revealed by cryo-electron microscopy of vitreous sections. J Bacteriol. 2006;188:6652–6660. doi: 10.1128/JB.00391-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckert WR, Marquis H, Goldfine H. Modulation of enzymatic activity and biological function of Listeria monocytogenes broad-range phospholipase C by amino acid substitutions and by replacement with the Bacillus cereus ortholog. Infect Immun. 1998;66:4823–4831. doi: 10.1128/iai.66.10.4823-4831.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]