Abstract
Nuclear receptors (NRs) are ligand-regulated transcription factors that display canonical domain structure with highly conserved DNA-binding and ligand-binding domains. The identification of the endogenous ligands for several receptors remains elusive or is controversial, thus these receptors are classified as orphans. One such orphan receptor is the retinoic acid receptor-related orphan receptor γ (RORγ). An isoform of RORγ, RORγt, has been shown to be essential for the expression of Interleukin 17 (IL-17) and the differentiation of Th17 cells. Th17 cells have been implicated in the pathology of several autoimmune diseases, including multiple sclerosis (MS) and rheumatoid arthritis (RA). Genetic ablation of RORγ alone or in combination with RORα in mice led to impaired Th17 differentiation and protected the mice from development of experimental autoimmune encephalomyelitis (EAE), a mouse model of MS. Here we describe SR2211, a selective RORγ modulator which potently inhibits production of IL-17 in cells.
Keywords: retinoic acid receptor-related orphan receptor, IL-17, Th17, experimental autoimmune encephalomyelitis, nuclear receptor modulator, autoimmunity
The retinoic acid receptor-related orphan receptor γ (RORγ) has been shown to be essential for Interleukin 17 (IL-17) expression and the differentiation of Th17 cells 1. Th17 cells have been implicated in the pathology of several autoimmune diseases including multiple sclerosis (MS) and rheumatoid arthritis (RA) 2-3 . Genetic ablation of RORγ alone or in combination with RORα in mice led to impaired Th17 cell differentiation and protected the mice from development of experimental autoimmune encephalomyelitis (EAE), a mouse model of MS 1, 4. While the endogenous ligand for RORγ remains controversial, we and others have shown that various oxysterols can bind to RORγ 5-7. This was followed by a study demonstrating that the synthetic LXR agonist T0901317 (T1317) binds to and modulates the activity of RORα and RORγ 6. More recently, we described a synthetic RORα selective inverse agonist, SR33358, and a dual RORα and RORγ inverse agonist, SR1001, which suppresses Th17 cell differentiation and was efficacious at delaying the onset and severity of symptoms in the EAE model 9. Others have described the natural products digoxin and ursolic acid as RORγ selective modulators and these molecules were capable of inhibiting Th17 cell differentiation 10-11. However, their utility as candidates for further development is limiting as digoxin displays significant adverse drug reactions with a narrow therapeutic index and ursolic acid activates the glucocorticoid receptor 12-13. These observations suggest that selective synthetic RORγ modulators that repress IL-17 expression could be potential drug development candidates.
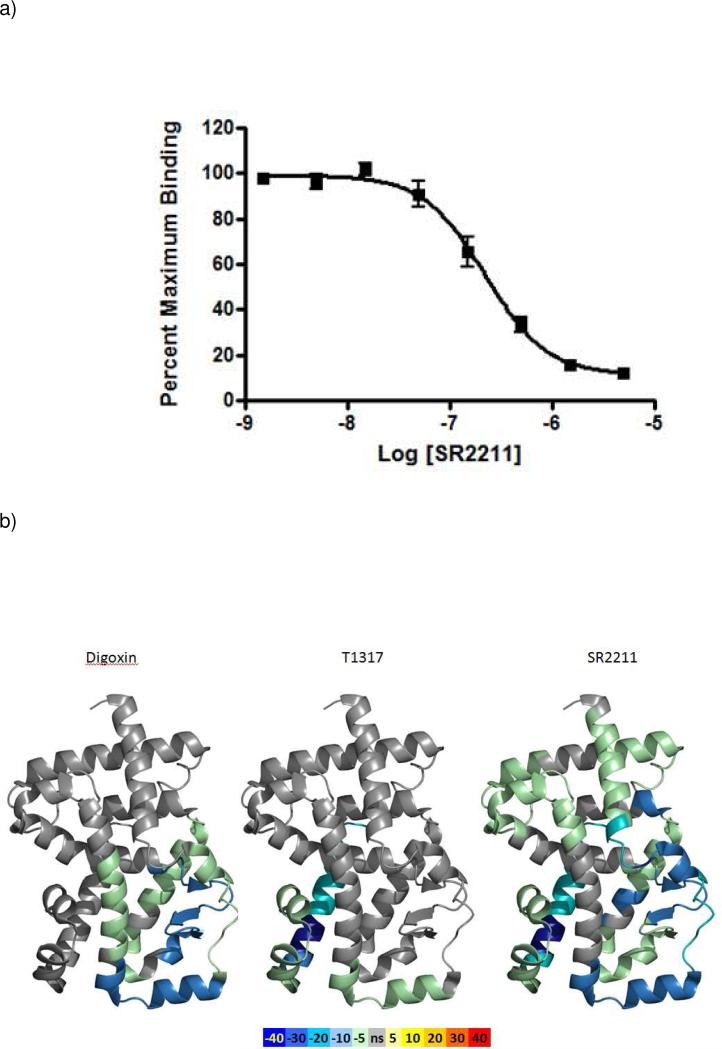
Using a modular chemistry approach, modifications to the SR1001 scaffold were made to develop SAR to diminish RORα activity from the scaffold while maintaining selectivity over LXR. Compounds were profiled using a screening approach based on radioligand binding assay in a Scintillation Proximity Assay (SPA) format. The Kd of [3H]T1317 was ~11.4nM in the SPA assay. The structure of SR2211 is shown in Figure 1. As shown in Fig 2A, the data suggests that SR2211 can bind RORγ and displace radioligand [3H]T1317 in a competition based SPA assay. The calculated Ki value for SR2211 is 105nM. To further evaluate the nature of the interaction of SR2211 with RORγ, we performed differential hydrogen/deuterium exchange (HDX) mass spectrometry analysis of the RORγ LBD in the presence and absence of digoxin, T1317, or SR2211. The differential HDX data is shown in Figure 2B overlaid onto PDB 3KYT where green and blue represent a reduction in HDX as compared with apo receptor. The data shown in Figure 2B suggests that the conformational mobility of the RORγ LBD is significantly reduced in the presence of SR2211. Comparison of the differential HDX data for SR221 with digoxin or T1317 suggests that SR2211 makes significantly more contacts with the receptor.
Figure 1. The structure of SR1001 (N-(5-(N-(4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2- yl)phenyl)sulfamoyl)-4-methylthiazol-2-yl)acetamide) and SR2211 (1,1,1,3,3,3-hexafluoro-2-(2-fluoro-4'-((4-(pyridin-4-ylmethyl)piperazin-1-yl)methyl)-[1,1'-biphenyl]-4-yl)propan-2-ol).
SR2211 was derived from SR1001 after several rounds of SAR. The hexaflurophenyl group was retained while modifying the left-hand portion of the molecule. The sulfonamide residue in SR1001 reduced CNS penetration so efforts were made to replace this with more lipophilic groups.
Figure 2. Demonstration of direct binding of SR2211 to RORγ.
Competition assay was performed to determine IC50 value of SR2211 in a SPA assay (A). Increasing concentrations of SR2211 were incubated with 5nM of [3H]-T1317 and 1ug of GSTRORγ along with Glutathione-YSi beads as detailed in Methods. The percent radioligand bound was calculated at various concentration of SR2211 after 20 hr of incubation. Ki value for SR2211 was calculated to be 105nM using graphpad prism software. Data shown are representative results from two independent experiments performed in triplicates. HDX perturbation results from SR2211 (right) and T1317 (middle) and digoxin (left) with RORγ (B). Negative perturbation values means that the exchange rate is slower for these regions within the protein in the ligand-bound protein.
Previously, we had observed that 25-hydroxy cholesterol strongly binds to RORγ, but we were unable to observe any transcriptional activity 6. To assess the functional transcriptional activity of SR2211, cell based assays using chimeric receptor Gal4 DNA-binding domain (DBD)–NR ligand binding domain cotransfection assay (LBDs of RORα, RORγ, LXRα, FXR and VP-16) were performed. As shown in Figure 3A, SR2211 treatment did not have any impact on the transcriptional activity of RORα, whereas more than 95% inhibition of RORγ activity was observed at 10 M (Figure 3B). Based on the dose response, we calculated the IC50 to be ~320nM. There is a minimal activation of LXRα by SR2211 at the highest concentration tested (Figure 3C) and it is less than 5% as compared to T1317. The activity of SR2211 on LXRα is very weak and EC50 is right shifted by more than 100-fold. Moreover we do not observe any activation of ABCA1 promoter when used in conjuction with full length LXRα (Figure 4E). Additionally, there is no effect on the transcriptional activity of FXR when treated with SR2211 (Figure 3D) where as a significant increase was seen with the positive control GW4064. We also observed no off-target effects/toxicity as there was no change in the luciferase activity of Gal4 DBD-VP16 (Figure 3E). These data clearly demonstrate that we have developed a compound that selectively targets RORγ and is potent and efficacious.
Figure 3. Suppression of constitutive activity of RORγ by SR2211.
293T cells were cotransfected with Gal4-RORa (A), Gal4-RORg (B), Gal4-LXRa (C), Gal4-FXR (D) or Gal4-VP16 (E) along with a UAS-luciferase plasmid. The cells were treated for 20 hr with indicated conc of SR2211 or postice controls SR3335 (A), T1317 (C) and GW4064 (D). Relative change was determined by normalizing to cells treated with vehicle. Each data point was performed in 6 replicates and represented as mean ±SEM, n = 6.
Figure 4. SR2211 modulates full length RORγ in reporter assays.
293T cells were cotransfected with 5X RORE-luc and either empty vector (A) or RORγ (B); IL-17-Luc reporter and either RORα (C) or RORγ (D); ABCA1 luciferase and LXRα (E) followed by treatment with indicated concentration of SR2211 for 20 hr. The luciferase activity was measured. Relative change was determined by normalizing to vehicle treated cells. Each data point was measured in 4-6 replicates and presented as mean ±SEM.
To confirm these results that SR2211 can repress the RORγ transcriptional activity, we used a full length receptor along with a multimerized ROR response element (RORE, five repeats of RORE) driving luciferase gene expression. In the absence of RORγ, there was no change in the luciferase activity of 5X-RORE with the treatment of SR2211 (Figure 4A). SR2211 significantly repressed the 5X-RORE luciferase activity when full length RORγ was added during transfection (Figure 4B), however, there was no effect of SR2211 on RORα co-transfection with 5X-RORE (data not shown). To further to examine the activity of SR2211 in more native promoter based assay, we performed additional cotransfection assays where we transfected cells with full-length RORα or RORγ and a luciferase reporter gene driven by a native promoter derived from a known ROR target gene, Il17. Il17 is a well-characterized ROR target gene that plays a critical role in the inflammatory pathway1. As shown in Figure 4C, in a RORα cotransfection assay, treatment of cells with SR2211 did not alter the transcription driven by the Il17 promoter. We observed a significant, more than 50%, suppression of transcriptional activity of Il17 promoter in a RORγ dependent manner (Figure 4D). As previously mentioned, there was no increase in the full length LXRα target gene, ABCA1, promoter activity (Figure 4F). These results confirm that we have been able to selectively target RORγ.
In order to determine whether SR2211 can inhibit the endogenous Il17 gene expression, we used an EL-4 murine T lymphocyte cell line that has been shown to produce IL-17 in response to phorbol myristate acetate (PMA) and ionomycin treatment. The results shown in Fig 5A demonstrate that pre-treatment of EL-4 cells with 5μM of either SR2211 or digoxin as control followed by stimulation with PMA/ionomycin leads to a significant reduction in the IL-17 gene expression as measured by quantitative real-time PCR. The treatment of EL4 with SR2211 repressed the Il17 gene expression to a greater extent as compared to digoxin. Similarly, the expression of IL-23 receptor, (Il23r) was significantly inhibited by SR2211 and digoxin (Fig 5B) as has been previously reported by Fujita-Sato et al 14. In order to measure the effect of SR2211 on IL-17 production, we determined the intracellular levels of IL-17 using flow cytometry. After the stimulation of EL-4 cells with PMA/ionomycin for 3 hr, the cells were treated with BD GolgiPlug™ (protein transport inhibitor) to allow intracellular accumulation of cytokines. After 2 hr, the cells were fixed and stained to analyze the IL-17 by flow cytometry. As shown in Figure 5C, treatment of EL-4 cells with SR2211 as well as a control digoxin resulted in significant inhibition of IL-17 intracellular staining as compared to vehicle treated cells. These results demonstrate that SR2211 can inhibit the transcriptional activity of RORγ resulting in the suppression of IL-17 production.
Figure 5. SR2211 Modulates the expression of IL 17A and IL-23R in EL-4 cells.
EL-4 cells were pre-treated with Digoxin (5μM) or SR2211 (5μM) or DMSO for 20 hr followed by stimulation with PMA and ionomycin for 5 hr. IL-17A (A) and IL-23R (B) mRNA expression was quantitated and normalized to GAPDH or intracellular IL-17 protein (C) expression was measured as outlined in Methods. The results are shown as mean ±SEM. * p< 0.05, *** p< 0.0005.
In summary, we report the identification of a selective RORγ ligand that functions as an inverse agonist. We show that SR2211 can displace the T1317 in binding assay and does interact with RORγ protein in HDX based experiment to stabilize the protein. In cotransfection assays, SR2211 suppresses transcription activity in both GAL4-RORγ LBD and full-length RORγ contexts. Furthermore, treatment of EL-4 cells with SR2211 results in suppression of gene expression and production of IL-17. These data strongly suggest that SR2211 is a potent and efficacious RORγ modulator and represses its activity. Moreover, SR2211 has the potential utility for the treatment of autoimmune disorders and further experiments are underway to evaluate in vivo actions of SR2211.
METHODS
Synthesis of SR2211 ( 1,1,1,3,3,3-hexafluoro-2-(2-fluoro-4'-((4-(pyridin-4-ylmethyl)piperazin-1-yl)methyl)-[1,1'-biphenyl]-4-yl)propan-2-ol)
Step 1. To 2-fluoroaniline (9.90 mmol) in a pressure vessel was added hexafluoroacetone sesquihydrate (10.9 mmol, 1.1 eq) neat and p-toluenelsuphonic acid (0.990 mmol, 0.1 eq). The vessel was then purged with argon, sealed and heated on an oil bath overnight (12 h) at 90° C. The reaction contents were then diluted with ethyl acetate and washed with NaHCO3 (3 × 100 mL; sat.). The ethyl acetate phase was then washed with brine (100 mL), dried over Na2SO4, and concentrated to a solid residue. The desired product was then isolated by silica gel using hexanes/ethyl acetate and following recrystallization from 10:1 hexanes/ethyl acetate to afford 2-(4-Amino-3-fluorophenyl)-1,1,1,3,3,3-hexafluoropropan-2-ol as white prisms. ESI-MS (m/z): 278 [M+1]+. Step 2. To a solution of 2-(4-Amino-3-fluorophenyl)-1,1,1,3,3,3-hexafluoropropan-2-ol (2.48 mmol) in DMF (2.5 mL) was added sodium nitrite (2.98 mmol, 1.2 eq) in water (1.5 mL) and 6M hydrochloric acid (3 eq), while maintaining the temperature between 0~5 °C. Stirring was continued for 30 min, and then potassium iodide (3.72 mmol,1.5 eq) was added in small portions. The resulting mixture was then allowed to stir overnight at room temperature. The reaction mixture was then diluted with Et2O (200 mL), washed with a saturated sodium thiosulphate (3 x 150 mL) and dried over Na2SO4. The solvent was removed in vacuo leaving a dark crude oil which was separated on silica gel (EtOAc/Hex) to obtain 1,1,1,3,3,3-Hexafluoro-2-(3-fluoro-4-iodophenyl)propan-2-ol. Step 3. To 4-bromomethylphenylboronic acid pinacol ester (1.68 mmol) was added MeCN (5 mL), followed by addition of K2CO3 (5.04 mmol, 3.0 eq), 1-(pyridinyl-4-methyl)-piperazine (2.02 mmol, 1.2 eq), and NaI (2 mole %). The mixture was allowed to stir overnight at rt (~23° C) under an argon balloon. The remaining reaction mixture was then diluted with H2O (50 mL) and extracted with CHCl3 (3 × 100 mL). The organic washes were combined, dried over Na2SO4, concentrated to a solid residue and again extracted with 12:1 hexanes / CH2Cl2 (3 × 100 mL) and concentrated in vacuo to a yellow crystalline. The product 1-(4-pyridinyl-methyl)-piperazine-4-benzyl-para-boronic pinacol ester was isolated by recrystallization from hexanes and used without further purification within the following synthetic step. Step 4. A mixture of 1,1,1,3,3,3-Hexafluoro-2-(3-fluoro-4-iodophenyl)propan-2-ol ( 0.183 mmol), 1-(4-pyridinyl-methyl)-piperazine-4-benzyl-para-boronic pinacol ester (2.20 mmol, 1.2 eq), Pd(PPh3)4 (5 mol%), K2CO3 (0.550 mmol, 3 eq) and 3 : 1 dioxane / H2O (4 mL) in a 20 mL pressure vessel was degassed for 5 min, purged with argon, sealed and heated for 2h at 80°C oil bath. Upon completion, as determined by reverse-phase HPLC, the mixture was allowed to cool and was then extracted with EtOAc (3 × 25 mL). The combine organic layers were washed with saturated NaHCO3 (2 × 25 mL) and dried over Na2SO4. The solvent was removed in vacuo leaving a brown solid crude which was then isolated by flash chromatography on silica gel (CH2Cl2 / MeOH) to obtain the title compound. ESI-MS (m/z): 528 [M+1]+; 1H-NMR (400 MHz, CHCl3 7.26) δ 8.46 (d, J = 5.2 Hz, 2H), 7.62 (s, 1H), 7.59 (s, 1H), 7.53-7.49 (m, 3H), 7.40 (d, J = 8.0 Hz, 2H), 7.30 (d, J = 5.2 Hz, 2H), 3.58 (s, 2H), 3.53 (s, 2H), 2.51 (b, 8H).
Cell Culture and Cotransfections
HEK293 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum at 37 °C under 5% CO2. Reverse transfections were performed in bulk using 1×106 cells in 6 cm plates, 3ug of total DNA in a 1:1 ratio of receptor and reporter and FuGene6 (Roche) in a 1:3 DNA: lipid ratio. Following day, cells re plated in 384 well plates at a density of 10,000 cells/well. After 4 hr, the cells were treated with the compound or DMSO as control. The luciferase levels were assayed following additional 20 hour incubation by one-step addition of BriteLite Plus(Perkin Elmer) and read using an Envision (Perkin Elmer). Data was normalized as fold change over DMSO treated cells.
Radioligand Binding Assay
The assay contains 0.25 mg of beads (Glutathione YSI; PE # RPNQ0033), 1 μg of GST-RORg-LBD, 5 nM of [3H] T1317 as radioligand and varying concentration of SR2211 in the assay buffer (50 mM HEPES, pH 7.4, 0.01% bovine serum albumin, 150 mM NaCl and 5 mM MgCl2, 10% glycerol, 1mM DTT, Complete protease inhibitor from Roche). All the components were gently mixed and incubated for 20 hr and were read in TopCount. The radioligand binding results were analyzed using GraphPad Prism software.
HDX Analysis
Solution-phase amide HDX is performed with a fully automated system as described previously (Chalmers, 2006). Briefly, 4μL of a 10μM protein solution in HDX buffer was diluted to 20μL with D2O-containing HDX buffer, and incubated at 25°C for; 10s, 30s, 60s, 900s, and 3,600s. Following on-exchange, unwanted forward or back exchange is minimized and the protein is denatured by dilution to 50μL with 0.1% TFA in 3M urea (held at 1 °C). Samples are then passed across an immobilized pepsin column (prepared in house) at 50μL min-1 (0.1% TFA, 1°C) and the resulting peptides are trapped onto a C8 trap cartridge (Thermo Fisher, Hypersil Gold). Peptides were then gradient eluted (4% CH3CN to 40% CH3CN, 0.3% formic acid over 5 minutes, 2°C) across a 1mm × 50mm C18 HPLC column (Hypersil Gold, Thermo Fisher) and electrosprayed directly into an Orbitrap mass spectrometer (LTQ Orbitrap with ETD, Thermo Fisher). Data are processed with in-house software and visualized with pyMOL (DeLano Scientific). To measure the difference in exchange rates we calculated the average percentage deuterium uptake for the apo RORγ LBD following 10, 30, 60, 900 and 3600 seconds of on-exchange. From this value, we subtract the average percent deuterium uptake measured for the RORγ LBD + ligand complex. Negative perturbation values means that the exchange rate is slower for these regions within the protein in the ligand-bound protein.
Real-time PCR analysis
One million EL-4 cells were seeded in each well of 6 well plate and incubated with 5 uM of digoxin or SR2211 or DMSO for 20 hrs. Cells were then stimulated with phorbol 12-myristate 13-acetate (PMA) (50ng/ml; Sigma) and Ionomycin (1ug/ml; Sigma) for 5 hours. Then, RNA was extracted with RNeasy midi kit using DNase I (Qiagen), then cDNA was synthesized with high capacity cDNA Reverse Transcription kits (Applied Biosystems). IL17A gene expression was normalized to the expression of GAPDH. IL17A, IL-23R and GAPDH primer sets were as follows: IL17A: CTCCAGAAGGCCCTCAGACTAC (Forward), AGCTTTCCCTCCGCATTGACACAG (Reverse); IL23R: GCC AAGAAGACC ATT CCCGA (Forward), TCA GTG CTA CAA TCT TCT TCA GAG GAC A (Reverse) GAPDH: ACACATTGGGGGTAGGAACA (Forward), AACTTTGGCATTGTGGAAGG (Reverse).
Flow Cytometry
For intracellular cytokine staining, cells were stimulated with PMA (50 ng/Ml) and Ionomycin (1ug/ml) for 5 hrs. After 3 hrs of incubation, BD Golgiplug (BD Bioscience) was added and incubated for 2 hrs. Then, cells were fixed, permeabilized, and stained with PEIL17A Ab (BD Biosciences). Cell sorting was performed using LSRII (BD Biosciences).
Acknowledgement
The efforts of P.R.G. were supported by the National Institutes of Health (NIH) Molecular Library Screening Center Network (MLSCN) grant U54MH074404 (Hugh Rosen, Principal Investigator). This work was also supported by NIH grants DK080201, MH092769 (T.P.B.) and GM084041 (P.R.G).
References
- 1.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 2.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 3.Jetten AM. Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl Recept Signal. 2009;7:e003. doi: 10.1621/nrs.07003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, Watowich SS, Tian Q, Jetten AM, Dong C. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin L, Martynowski D, Zheng S, Wada T, Xie W, Li Y. Structural basis for hydroxycholesterols as natural ligands of orphan nuclear receptor RORgamma. Mol Endocrinol. 2010;24:923–929. doi: 10.1210/me.2009-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar N, Solt LA, Conkright JJ, Wang Y, Istrate MA, Busby SA, Garcia-Ordonez RD, Burris TP, Griffin PR. The benzenesulfoamide T0901317 [N-(2,2,2-trifluoroethyl)-N-[4-[2,2,2-trifluoro-1-hydroxy-1-(trifluorometh yl)ethyl]phenyl]-benzenesulfonamide] is a novel retinoic acid receptor-related orphan receptor-alpha/gamma inverse agonist. Mol Pharmacol. 2010;77:228–236. doi: 10.1124/mol.109.060905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Kumar N, Solt LA, Richardson TI, Helvering LM, Crumbley C, Garcia-Ordonez RD, Stayrook KR, Zhang X, Novick S, Chalmers MJ, Griffin PR, Burris TP. Modulation of retinoic acid receptor-related orphan receptor alpha and gamma activity by 7-oxygenated sterol ligands. J Biol Chem. 2010;285:5013–5025. doi: 10.1074/jbc.M109.080614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar N, Kojetin DJ, Solt LA, Kumar KG, Nuhant P, Duckett DR, Cameron MD, Butler AA, Roush WR, Griffin PR, Burris TP. Identification of SR3335 (ML-176): a synthetic RORalpha selective inverse agonist. ACS Chem Biol. 2011;6:218–222. doi: 10.1021/cb1002762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solt LA, Kumar N, Nuhant P, Wang Y, Lauer JL, Liu J, Istrate MA, Kamenecka TM, Roush WR, Vidovic D, Schurer SC, Xu J, Wagoner G, Drew PD, Griffin PR, Burris TP. Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature. 2011;472:491–494. doi: 10.1038/nature10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huh JR, Leung MW, Huang P, Ryan DA, Krout MR, Malapaka RR, Chow J, Manel N, Ciofani M, Kim SV, Cuesta A, Santori FR, Lafaille JJ, Xu HE, Gin DY, Rastinejad F, Littman DR. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORgammat activity. Nature. 2011;472:486–490. doi: 10.1038/nature09978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu T, Wang X, Zhong B, Nurieva RI, Ding S, Dong C. Ursolic acid suppresses interleukin-17 (IL-17) production by selectively antagonizing the function of RORgamma t protein. J Biol Chem. 2011;286:22707–22710. doi: 10.1074/jbc.C111.250407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kassi E, Sourlingas TG, Spiliotaki M, Papoutsi Z, Pratsinis H, Aligiannis N, Moutsatsou P. Ursolic acid triggers apoptosis and Bcl-2 downregulation in MCF-7 breast cancer cells. Cancer Invest. 2009;27:723–733. doi: 10.1080/07357900802672712. [DOI] [PubMed] [Google Scholar]
- 13.Cha HJ, Park MT, Chung HY, Kim ND, Sato H, Seiki M, Kim KW. Ursolic acid-induced down-regulation of MMP-9 gene is mediated through the nuclear translocation of glucocorticoid receptor in HT1080 human fibrosarcoma cells. Oncogene. 1998;16:771–778. doi: 10.1038/sj.onc.1201587. [DOI] [PubMed] [Google Scholar]
- 14.Fujita-Sato S, Ito S, Isobe T, Ohyama T, Wakabayashi K, Morishita K, Ando O, Isono F. Structural basis of digoxin that antagonizes RORgamma t receptor activity and suppresses Th17 cell differentiation and interleukin (IL)-17 production. J Biol Chem. 2011;286:31409–31417. doi: 10.1074/jbc.M111.254003. [DOI] [PMC free article] [PubMed] [Google Scholar]