Abstract
Individuals with multimorbidity may be at increased risk of hospitalization and death. Comorbidity indexes do not capture severity of illness or healthcare utilization; however, inflammation biomarkers that are not disease-specific may predict hospitalization and death in older adults. We sought to predict hospitalization and mortality of older adults using inflammation biomarkers. From a prospective, observational study, 370 community-dwelling adults 65 years or older from central Alabama participated in an in-home assessment and provided fasting blood samples for inflammation biomarker testing in 2004. We calculated an inflammation summary score (range 0-4), one point each for low albumin, high C-reactive protein, low cholesterol, and high interleukin-6. Utilizing Cox proportional hazards models, inflammation summary scores were used to predicted time to hospitalization and death during a 4-year follow up period. The mean age was 73.7 (+5.9 yrs), and 53 (14%) participants had summary scores of 3 or 4. The rates of dying were significantly increased for participants with inflammation summary scores of 2, 3, or 4 (hazard ratios (HR) 2.22, 2.78, and 7.55, respectively; p<0.05). An inflammation summary score of 4 significantly predicted hospitalization (HR 5.92, p<0.05). Community-dwelling older adults with biomarkers positive for inflammation had increased rates of being hospitalized or dying during the follow up period. Assessment of the individual contribution of particular inflammation biomarkers in the prediction of health outcomes in older populations and the development of validated summary scores to predict morbidity and mortality are needed.
Keywords: inflammation, hospitalization, mortality, community-dwelling older adults
1. INTRODUCTION
Individuals with multimorbidity, the presence of 2 or more chronic conditions, can have increased illness burden and mortality. Summary scores such as the Charlson Comorbidity Index (CCI) have been used to predict mortality based on a list of high-risk diagnoses (Charlson et al., 1987b). Additionally, diagnoses that contribute to frailty or functional decline frequently lead to increased hospital use (Mor et al., 1994). Utilizing comorbidity and functional status data as well as demographic information, tools like the probability of repeated admissions (PRA) predict the risk of hospitalization among older adults (Boult et al., 1993; Boult et al., 1994). However, prediction tools generally lack measures of systemic inflammation, which may offer important information on the illness burden and mortality risk of older adult with multimorbidity.
Elevations in serum biomarkers, such as cytokines, interleukins, and acute phase reactants, reflect the presence of systemic inflammation. Low-level, chronic systemic inflammation has been observed during the aging process (Johnson, 2006; Krabbe et al., 2004; Vasto et al., 2007). Numerous studies have demonstrated the relationship between select inflammation biomarkers and age-related changes such as frailty, decreases in muscle strength and mass, increases in adiposity, osteoporotic fractures, as well as total and cause-specific mortality (Brinkley et al., 2009; Cartier et al., 2009; Cauley et al., 2007; Leng et al., 2007; Newman et al., 2009; Reuben et al., 1999; Reuben et al., 2002b; Schaap et al., 2006; Schalk et al., 2004; Schrager et al., 2007). Furthermore, albumin and cholesterol—both acute phase reactants—are depressed in states of inflammation (Gabay and Kushner, 1999). Low levels of albumin and cholesterols have been associated with increased mortality in prior studies (Reuben et al., 2002a; Reuben et al., 1999; Sullivan, 1994). Therefore, combinations of biomarkers reflect multiple, concurrent pathways of inflammation that may be useful in predicting such outcomes as hospitalization and death.
Of particular note, Reuben et al. assessed the prognostic ability of a summary score of inflammation biomarkers to predict functional decline and death among healthy, high-functioning older adults (Reuben et al., 2002a). Low albumin, high C-reactive protein (CRP), low cholesterol, and high interleukin-6 (IL-6) were assigned one point each to create a summary score for participants at baseline. The components of the summary score predicted mortality better than did functional decline. In their cohort of 870 community-dwelling older adults, participants who had higher summary scores were at increased risk of death at both 3 and 7 year endpoints compared to those with lower scores (Reuben et al., 2002a).
Our aim was to determine whether the inflammation summary score developed by Reuben et al. (Reuben et al., 2002a) could predict hospitalization and mortality in a more diverse population. We hypothesized that in our sample of community-dwelling older adults in Alabama, which included participants with multiple chronic conditions and those with poor functional status, participants with higher indicators of systemic inflammation would be more likely to be hospitalized or die during the follow up period compared to participants with lower scores.
2. MATERIALS AND METHODS
2.1 Study design and setting
This study utilized data available from participants in the University of Alabama at Birmingham (UAB) Study of Aging who were eligible and willing to have an in-home assessment and a blood draw 4 years after their initial enrollment in the study. The UAB Study of Aging is a prospective, observational cohort study of a sample of 1000 community-dwelling individuals recruited between 1999 and 2001. The methods utilized for the initial recruitment have been described in detail elsewhere (Allman et al., 2006). In brief, participants represented a random sample of Medicare beneficiaries age 65 years or older when they were recruited from five counties in central Alabama. The recruitment was stratified by gender, race, and urban/rural residence to assure balanced representation of older African Americans, men, and those from rural communities. Persons were excluded if they lived in a nursing home or were unable to schedule their own appointments. Participants signed informed consents approved by the University of Alabama at Birmingham Institutional Review Board.
During the baseline in-home assessment, demographic information on age, gender, race, rural/urban residence, comorbidities, smoking status and body mass index (BMI) were collected. Participants’ comorbidities were considered verified if participants reported using a prescription medication for the disease, if the primary care physician reported the condition on a standardized questionnaire used for the study, or if the diagnosis was recorded on a discharge summary from a hospitalization within three years of the baseline assessment. Based on the verified comorbidity data, we calculated an unweighted comorbidity count for each participant, assigning one point for each diagnosis in the Charlson Comorbidity Index. (Charlson et al., 1987a).
Four years after the baseline assessment (year 4), 624 participants remained eligible and agreed to participate in a repeat in-home assessment. Following the repeat in-home assessment, 370 (59.3%) of these 624 eligible participants agreed to a fasting blood draw and had data available for all four inflammation biomarkers of interest. These participants are the focus for this paper.
After the year 4 in-home assessment, we ascertained whether participants had been hospitalized or died in telephone interviews that occurred every 6 months in the subsequent four years (years 4 to 8 of study). When hospitalizations or deaths were reported, participants or their alternative contacts were asked the date of the hospital admission or death. If the exact date was unknown by the respondent, the admission date or date of death was defined as the midpoint for the period of time during which the respondent indicated that the event had occurred. For example, if a respondent indicated that a hospital admission occurred during a specific week or month of the previous six-month interval, but the respondent did not recall the specific day, the admission date was defined as the midpoint of the admission week or month, respectively. Mortality was confirmed by the Social Security Death Index. This method permitted the definition of time to first reported hospitalization and survival time.
2.2 Biomarker testing
Fasting blood samples were collected from participants by a home health nurse after the year 4 in-home assessment. These blood draws were scheduled to occur within one month of the in-home assessment. To obtain sera for testing, blood samples were centrifuged at 3200 rpm for 10 minutes at room temperature and frozen at −70°C. All samples were analyzed in duplicate at the University of Alabama at Birmingham University Hospital and Department of Nutrition Science labs. Creatinine was tested using the Beckman Synchron Lx, Jaffe rate method. Normal creatinine was 0.4-1.2 mg/dL for females and 0.7-1.3 mg/dL for males. Albumin was analyzed by Beckman Synchron Lx. CRP was assayed with DPC Immulite 2000, an immunometric assay. CRP concentrations >10 mg/L represent acute inflammation; therefore, values of CRP >10 mg/L (n=2) were excluded from study analyses. Cholesterol was analyzed by Ektachem DTII System. The IL-6 cytokine was assayed using Luminex immunoassay with reagents and materials from Linxco Research.
2.3 Measures
We utilized four biomarkers to define a composite measure of inflammation among study participants as described by Reuben et al: albumin, CRP, cholesterol, and IL-6. Thresholds for each biomarker were set in a manner similar to prior studies (Harris et al., 1999; Reuben et al., 2002a; Reuben et al., 2003; Yaffe et al., 2003). Hypoalbuminemia as a biomarker of inflammation was defined by levels lower than 38 g/L. Based on the bottom decile of the study population, hypocholesterolemia indicating inflammation was designated as less than 142 mg/dL. For CRP and IL-6, we defined inflammation as being in the top tertile levels of IL-6 (>5.6 pg/mL) and CRP (>0.56 mg/L). For each biomarker beyond the inflammation threshold, patients were assigned one point. Then we calculated an inflammation summary score, ranging from 0 (no inflammation based on biomarkers) to 4 points (all biomarkers indicating inflammation).
2.4 Statistical approach
The dependent variables of interest were time to first hospitalization and time to death. Time to first hospitalization was determined as the difference in days between the time of the blood draw for the biomarkers (the year 4 in-home assessment) and the first hospital admission during the subsequent 4 years. For participants who were not hospitalized during this time period, censoring occurred at the time of the last interview for the participant, dropout, or participant death, whichever occurred first. For patients who died, time to death was determined in days from the year 4 assessment to the date of death, and cases without death were censored at the last date of participation in the study.
We performed survival analysis predicting time to first hospitalization and time to death using a Cox proportional hazards model as conducted in SAS Proc PHREG. Covariates in the regression model included age, race, gender, rural/urban residence, comorbidity score, BMI category, and smoking status. Creatinine was also included as a covariate since CRP is elevated in patients with impaired kidney function. Hazard ratios (HR) and 95% confidence intervals (CI) are reported and interpreted for variables in which the 95% CI does not include 1.0. Direct adjusted survival curves for the inflammation summary scores were constructed, based on the methods of Zhang and colleagues (Zhang et al., 2007). In sensitivity analyses, we investigated the effect of excluding patients who had died in the time to hospitalization models. Additionally, we explored the inclusion of comorbidity score in both time to hospitalization and time to death models. All analyses were performed in SAS version 9.2 (Cary, NC).
3. RESULTS
Descriptive statistics for the 370 participants are displayed in Table 1. At the year 4 assessment, the mean age of participants was 73.7 years old (standard deviation + 5.9 years). The 370 participants included in this analysis differed from the 254 persons who did not provide blood samples. Specifically, participants who agreed to provide blood samples were less likely to be African American, female, and/or rural and more likely to have better cognitive function, higher education, and greater income (Allman et al., 2011).
Table 1.
Descriptive statistics of the UAB Study of Aging participants, N = 370
| Variable | N (Percentage) or Mean (SD) |
|---|---|
| Summary inflammation score |
|
| 0 | 118 (31.9) |
| 1 | 122 (33.0) |
| 2 | 77 (20.8) |
| 3 | 44 (11.9) |
| 4 | 9 (2.4) |
| Age, years | 73.7 (5.9) |
| Male | 186 (50.3) |
| African American | 157 (42.4) |
| Rural residence | 159 (43.0) |
| Body Mass Index (BMI), kg/m2 |
|
| <18.5 | 9 (2.4) |
| 18.5-25 | 123 (33.2) |
| 25-30 | 124 (33.5) |
| 30-35 | 70 (18.9) |
| 35+ | 44 (11.9) |
| Creatinine, mg/dL | 1.2 (0.4) |
| Never smoker | 186 (50.3) |
| Former smoker | 159 (43.0) |
| Current smoker | 25 (6.8) |
Overall, 132 (35.7%) participants were hospitalized and 71 (19.2%) died between the year 4 assessment and final endpoint of the study (total observation time=4 years). The median follow up time was 3.7 years. 186 (50.3%) participants were male, 157 (42.4%) were African American, and 159 (43.0%) lived in rural areas. Over half of the participants had inflammation summary scores of 1 or 2, while only 9 (2.4%) participants had a summary score of 4.
The survival analyses results are presented in Table 2. Participants with an inflammation summary score of 4 had significantly higher rates of hospitalization compared to those with a score of 0, the referent group (hazard ratio (HR) 5.92, p<0.01). Likewise, participants with BMI 30-35 had higher rates of hospitalization (HR 1.77, p=0.02). In predicting death there was a significant, gradual increase in rates of death as the inflammation summary score increased. The HRs were 2.22 for score of 2 (p=0.02), 2.78 for score of 3 (p=0.01), and 7.55 for score of 4 (p<0.01).
Table 2.
Survival analyses predicting time to first hospitalization and death
| Time to 1st Hospitalization | Time to Death | |||
|---|---|---|---|---|
| Variable | Hazard Ratio (95% confidence interval)a |
p-value | Hazard Ratio (95% confidence interval)a |
p- value |
| Inflammation summary score 0 |
REFERENT | REFERENT | ||
| 1 | 0.90 (0.58-1.39) | 0.62 | 0.86 (0.41-1.81) | 0.69 |
| 2 | 0.92 (0.55-1.52) | 0.74 | 2.22 (1.11-4.44) | 0.02 |
| 3 | 0.80 (0.42-1.54) | 0.51 | 2.78 (1.29-5.98) | 0.01 |
| 4 | 5.92 (2.21-15.89) | <0.01 | 7.55 (2.42-23.59) | <0.01 |
| BMI <18.5b | 0.94 (0.22-4.03) | 0.94 | 0.84 (0.19-3.74) | 0.81 |
| 18.5-25 | REFERENT | REFERENT | ||
| 25-30 | 0.84 (0.53-1.34) | 0.46 | 0.64 (0.35-1.18) | 0.16 |
| 30-35 | 1.77 (1.10-2.86) | 0.02 | 0.72 (0.34-1.50) | 0.38 |
| 35+ | 1.40 (0.76-2.58) | 0.28 | 1.08 (0.50-2.32) | 0.84 |
| Never smoker | REFERENT | REFERENT | ||
| Quit smoking | 1.14 (0.78-1.67) | 0.51 | 1.30 (0.76-2.24) | 0.34 |
| Current smoker | 1.15 (0.50-2.65) | 0.74 | 2.19 (0.94-5.10) | 0.07 |
Adjusted for age, gender, race, rurality, and creatinine.
Body mass index, kg/m2
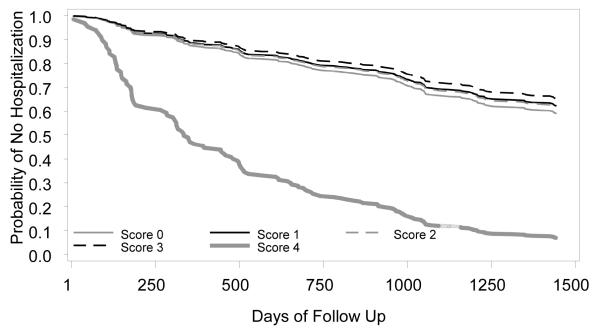
The direct adjusted survival curves for time to hospitalization and death are depicted in Figure 1, panels A and B, respectively. The curves illustrate a steeper decline in survival over time to hospitalization and death for those participants with an inflammation summary score of 4 compared to those with lower scores.
Figure 1.
Panel A. Adjusted time to hospitalization curves predicted by inflammation summary score
When we excluded participants who died in the sensitivity analyses, the results were similar to the main analysis. The rates of hospitalization were significantly higher for participants with an inflammation summary score of 4 compared to those with a score of 0 (data not shown).
We also investigated the effect of multimorbidity (reflected in the comorbidity score) on the relationship between inflammation summary score and time to hospitalization and death. When we controlled for comorbidity score, an inflammation summary score of 4 remained a significant predictor of hospitalization (HR 3.42, p=0.02). Additionally, inflammation summary scores of 3 and 4 were significant predictors of death when we controlled for comorbidity score (HR 2.49, p=0.02 and HR 4.69, p=0.01, respectively). Comorbidity score itself was significantly associated with hospitalization (HR 1.27, p<0.01) and death (HR 1.23, p<0.01), although the magnitude of the association was less than the inflammation summary score.
4. DISCUSSION
In our observational study of community-dwelling older adults, an inflammation summary score predicted hospitalization and death over the 4 year follow up period. Specifically, the rate of death increased as inflammation increased, represented by a composite measure of inflammation including high CRP and IL-6, and low albumin and cholesterol. An increased rate of hospitalization was noted only for participants with a summary score of 4, those with the most systemic inflammation. These results were independent of comorbidity scores.
A plausible explanation for our results is that by measuring inflammation we may be detecting subclinical conditions—conditions not yet identified by patients or health care providers. Subclinical infections such as those caused by Helicobacter pylori or periodontal disease may also be accounting for a portion of inflammatory burden in our older adult cohort. Therefore, using an inflammatory summary score to predict hospitalization and death may indicate an overall inflammatory state not captured by counts of diagnoses.
Why were hospitalization rates significantly higher only for older adults with the highest inflammation summary score (as opposed to mortality where inflammation summary score was predictive in a dose response fashion)? Certainly inflammation may correlate with illness severity, but hospitalization itself may be more of a social construct that is less directly related to inflammation. For example, if symptoms are prominent or worsening, older adults may seek care acutely and be hospitalized. The older adult’s choice to seek care and the healthcare provider’s decision to hospitalize him/her represent a broad range of behaviors. The hospitalization may be prophylactic on one extreme or be too late to rescue the patient at the other extreme. Hence, there are limitations in the ability of physiologic markers to predict hospitalization.
Our results add to the literature supporting the merits of utilizing inflammatory biomarkers as predictors of morbidity and mortality in elderly (De Martinis et al., 2006). One similar population in which inflammatory biomarkers have been linked to these outcomes is the Health, Aging and Body Composition (ABC) study (Cauley et al., 2007). Nearly 3000 high-functioning older adults age 70 to 79 were enrolled in the ABC study. In addition to IL-2 and TNF-α receptors, Cauley et al. found elevated IL-6, TNF-alpha, and CRP were prognostic of osteoporotic fractures, and as the number of elevated biomarkers increased so did the relative risk of fracture. From the same study, higher tertiles of CRP and IL-6 values were associated with functional limitations in adjusted models (Figaro et al., 2006). Additionally, as CRP and TNF-alpha increased in the Health ABC participants, the relative risk of limitations in mobility also increased (Penninx et al., 2004). Similar to the Health ABC study, our findings concur with the prognostic abilities of CRP and IL-6 in predicting morbidity.
Our study augments the inflammation biomarker literature by providing a unique population in which to observe the clinical importance of systemic inflammation over time. The UAB Study of Aging is a balanced sample in terms of gender, race, and urban/rural residence, with oversampling of African American and rural participants. Participants in this study are aging in their communities within Alabama, a state known to have a higher burden of heart disease, stroke, diabetes, and obesity compared to other areas in the U.S.(Center for Disease Control and Prevention, 2008a, b; Roger et al., 2011). Because of the frequent co-occurrence of these and other diseases, measures of cumulative inflammation are more useful clinically than disease-specific ones. Our study did not test specifically test whether comorbidity counts had better predictive value than inflammation biomarkers, nor did we compare inflammation summary score to other predictive tools. Another distinction of our study is that participants were not excluded based on functional status, as they were in the studies by Reuben et al. and Health ABC (Figaro et al., 2006; Reuben et al., 2002a). Therefore, we believe our results are generalizable to community-dwelling older adults regardless of functional status.
One caveat concerning our results must be addressed. Both age and visceral adiposity are associated with increased inflammation. In a cohort of healthy men, the age-related changes in CRP and IL-6 were explained by visceral adipose tissue (Cartier et al., 2009; Schrager et al., 2007), which may explain our finding of increased BMI being linked to hospitalization. BMI, however, is not a direct measure of visceral adiposity, a limitation of our study. Since the average BMI for our study population was in the overweight range, we may be observing the contribution of additional weight to increased inflammation, decreased muscle strength, and resultant functional limitations.
We must consider the study’s limitations. First, the presence of inflammatory biomarkers is an indirect way to measure disease burden, and the summary score may not capture the impact of multimorbidity accurately. Additionally, we could not determine the time course between the onset of increased inflammation and timing of hospitalization or occurrence of death. Also we did not perform psychometric evaluation of the individual inflammation summary score components; however, all four biomarkers used in our study do have predictive value (Reuben et al., 2002a). Of note, we dichotomized the biomarkers based on population-derived yet clinically meaningful thresholds, rather than analyzing the biomarkers in a continuous fashion; therefore, the generalizability of the results may be limited. In addition, the number of participants with summary score of 4 was small, and the effects on our two outcomes of interest were driven primarily by this group. Finally, in this analysis, we did not evaluate measures of social support or access to medical care, which can influence whether elders seek medical care in a timely manner.
5. CONCLUSIONS
Our study demonstrates the prognostic ability of a composite measure of systemic inflammation including four biomarkers in predicting hospitalization and death over the 4 year follow up period in a cohort of community-dwelling older adults. Further studies are needed to determine the individual contribution of particular biomarkers in the prediction of health outcomes in older populations and the development of validated summary scores to predict morbidity and mortality.
Acknowledgments
Role of the funding source: This work was supported by the National Institute on Aging [grant numbers R01-AG015062, P30AG031054] to RMA; the National Institute on Aging [grant number 1K07AG31779] to CSR; and the Department of Veterans Affairs Health Services Research & Development Service, Research Career Development Award [grant number E6326W] to CJB; and the National Institutes of Health grant award 1UL 1RR025777 partially supported RMA. Blood analyses supported by GCRC Grant M01 RR-00032 from the National Center for Research Resources. The funding sources had no role in the study design, in the collection, analysis and interpretation of data; in the writing of the manuscript; nor in the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest:
CJB: member of the editorial board for JAGS.
DLR, JLL, RMA: grant funding from National Institute on Aging (NIA).
JLL: grant funding from American Cancer Society.
CSR: employment at UAB, Birmingham VA Medical Center; grants/funds from AHRQ, NIH, HRSA, Donald W. Reynolds Foundation; honoraria from Center to Advance Palliative Care, Macon State College, & American Board of Internal Medicine; consultant for University of Puerto Rico; royalties from UpToDate; member of American Academy of Hospice & Palliative Medicine Board of Directors
REFERENCES
- Allman R, Sawyer P, Crowther M, Strothers H, III, Turner T, Fouad M. Predictors of 4-year retention among African American and white community dwelling participants in the UAB Study of Aging. The Gerontologist. 2011 doi: 10.1093/geront/gnr024. accepted by. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allman R, Sawyer P, Roseman J. The UAB Study of Aging: Background and insights into life-space mobility among older Americans in rural and urban settings. Aging Health. 2006;2:417–429. [Google Scholar]
- Boult C, Dowd B, McCaffrey D, Boult L, Hernandez R, Krulewitch H. Screening elders for risk of hospital admission. J Am Geriatr Soc. 1993;41:811–817. doi: 10.1111/j.1532-5415.1993.tb06175.x. [DOI] [PubMed] [Google Scholar]
- Boult L, Boult C, Pirie P, Pacala JT. Test-retest reliability of a questionnaire that identifies elders at risk for hospital admission. J Am Geriatr Soc. 1994;42:707–711. doi: 10.1111/j.1532-5415.1994.tb06528.x. [DOI] [PubMed] [Google Scholar]
- Brinkley TE, Leng X, Miller ME, Kitzman DW, Pahor M, Berry MJ, Marsh AP, Kritchevsky SB, Nicklas BJ. Chronic inflammation is associated with low physical function in older adults across multiple comorbidities. J Gerontol A Biol Sci Med Sci. 2009;64:455–461. doi: 10.1093/gerona/gln038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier A, Cote M, Lemieux I, Perusse L, Tremblay A, Bouchard C, Despres JP. Age-related differences in inflammatory markers in men: contribution of visceral adiposity. Metabolism. 2009;58:1452–1458. doi: 10.1016/j.metabol.2009.04.025. [DOI] [PubMed] [Google Scholar]
- Cauley JA, Danielson ME, Boudreau RM, Forrest KY, Zmuda JM, Pahor M, Tylavsky FA, Cummings SR, Harris TB, Newman AB. Inflammatory markers and incident fracture risk in older men and women: the Health Aging and Body Composition Study. J Bone Miner Res. 2007;22:1088–1095. doi: 10.1359/jbmr.070409. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention . Age-adjusted estimates of the percentage of adults who are obese. National Diabetes Surveillance System; 2008a. [Google Scholar]
- Center for Disease Control and Prevention . Age-adjusted estimates of the percentage of adults with diagnosed diabetes. National Diabetes Surveillance System; 2008b. [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987a;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, McKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of Chronic Diseases. 1987b;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflammation markers predicting frailty and mortality in the elderly. Exp Mol Pathol. 2006;80:219–227. doi: 10.1016/j.yexmp.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Figaro MK, Kritchevsky SB, Resnick HE, Shorr RI, Butler J, Shintani A, Penninx BW, Simonsick EM, Goodpaster BH, Newman AB, Schwartz AV, Harris TB. Diabetes, inflammation, and functional decline in older adults: findings from the Health, Aging and Body Composition (ABC) study. Diabetes Care. 2006;29:2039–2045. doi: 10.2337/dc06-0245. [DOI] [PubMed] [Google Scholar]
- Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr., Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Johnson TE. Recent results: biomarkers of aging. Exp Gerontol. 2006;41:1243–1246. doi: 10.1016/j.exger.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. 2004;39:687–699. doi: 10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Leng SX, Xue QL, Tian J, Walston JD, Fried LP. Inflammation and frailty in older women. J Am Geriatr Soc. 2007;55:864–871. doi: 10.1111/j.1532-5415.2007.01186.x. [DOI] [PubMed] [Google Scholar]
- Mor V, Wilcox V, Rakowski W, Hiris J. Functional transitions among the elderly: patterns, predictors, and related hospital use. Am J Public Health. 1994;84:1274–1280. doi: 10.2105/ajph.84.8.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AB, Sachs MC, Arnold AM, Fried LP, Kronmal R, Cushman M, Psaty BM, Harris TB, Robbins JA, Burke GL, Kuller LH, Lumley T. Total and cause-specific mortality in the cardiovascular health study. J Gerontol A Biol Sci Med Sci. 2009;64:1251–1261. doi: 10.1093/gerona/glp127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninx BW, Kritchevsky SB, Newman AB, Nicklas BJ, Simonsick EM, Rubin S, Nevitt M, Visser M, Harris T, Pahor M. Inflammatory markers and incident mobility limitation in the elderly. J Am Geriatr Soc. 2004;52:1105–1113. doi: 10.1111/j.1532-5415.2004.52308.x. [DOI] [PubMed] [Google Scholar]
- Reuben DB, Cheh AI, Harris TB, Ferrucci L, Rowe JW, Tracy RP, Seeman TE. Peripheral blood markers of inflammation predict mortality and functional decline in high-functioning community-dwelling older persons. J Am Geriatr Soc. 2002a;50:638–644. doi: 10.1046/j.1532-5415.2002.50157.x. [DOI] [PubMed] [Google Scholar]
- Reuben DB, Ix JH, Greendale GA, Seeman TE. The predictive value of combined hypoalbuminemia and hypocholesterolemia in high functioning community-dwelling older persons: MacArthur Studies of Successful Aging. J Am Geriatr Soc. 1999;47:402–406. doi: 10.1111/j.1532-5415.1999.tb07230.x. [DOI] [PubMed] [Google Scholar]
- Reuben DB, Judd-Hamilton L, Harris TB, Seeman TE. The associations between physical activity and inflammatory markers in high-functioning older persons: MacArthur Studies of Successful Aging. J Am Geriatr Soc. 2003;51:1125–1130. doi: 10.1046/j.1532-5415.2003.51380.x. [DOI] [PubMed] [Google Scholar]
- Reuben DB, Keeler E, Seeman TE, Sewall A, Hirsch SH, Guralnik JM. Development of a method to identify seniors at high risk for high hospital utilization. Med Care. 2002b;40:782–793. doi: 10.1097/00005650-200209000-00008. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaap LA, Pluijm SM, Deeg DJ, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006;119:526, e529–517. doi: 10.1016/j.amjmed.2005.10.049. [DOI] [PubMed] [Google Scholar]
- Schalk BW, Visser M, Deeg DJ, Bouter LM. Lower levels of serum albumin and total cholesterol and future decline in functional performance in older persons: the Longitudinal Aging Study Amsterdam. Age Ageing. 2004;33:266–272. doi: 10.1093/ageing/afh073. [DOI] [PubMed] [Google Scholar]
- Schrager MA, Metter EJ, Simonsick E, Ble A, Bandinelli S, Lauretani F, Ferrucci L. Sarcopenic obesity and inflammation in the InCHIANTI study. J Appl Physiol. 2007;102:919–925. doi: 10.1152/japplphysiol.00627.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan D. Cholesterol and non-cardiovascular disease: basic science. Aust N Z J Med. 1994;24:92–97. doi: 10.1111/j.1445-5994.1994.tb04443.x. [DOI] [PubMed] [Google Scholar]
- Vasto S, Candore G, Balistreri CR, Caruso M, Colonna-Romano G, Grimaldi MP, Listi F, Nuzzo D, Lio D, Caruso C. Inflammatory networks in ageing, age-related diseases and longevity. Mech Ageing Dev. 2007;128:83–91. doi: 10.1016/j.mad.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Lindquist K, Penninx BW, Simonsick EM, Pahor M, Kritchevsky S, Launer L, Kuller L, Rubin S, Harris T. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 2003;61:76–80. doi: 10.1212/01.wnl.0000073620.42047.d7. [DOI] [PubMed] [Google Scholar]
- Zhang X, Loberiza FR, Klein JP, Zhang MJ. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed. 2007;88:95–101. doi: 10.1016/j.cmpb.2007.07.010. [DOI] [PubMed] [Google Scholar]