Abstract
Yellow fever virus (YFV), a member of the genus Flavivirus, is a mosquito-borne virus found in tropical regions of Africa and South America that causes severe hepatic disease and death in humans. Despite the availability of effective vaccines, YFV is responsible for an estimated 200,000 cases and 30,000 deaths annually. There are currently no prophylactic or therapeutic strategies approved for use in human YFV infections. Furthermore, implementation of YFV17D-204 vaccination campaigns has become problematic due to an increase in reported post-vaccinal adverse events. We have created human/murine chimeric MAbs of a YFV-reactive murine monoclonal antibody (mMAb), 2C9, that was previously shown to protect mice from lethal YFV infection and to have therapeutic activity. The new chimeric (c)MAbs were constructed by fusion of the m2C9 IgG gene variable regions with the constant regions of human IgG and IgM and expressed in Sp2 murine myelomas. The 2C9 cMAbs (2C9-cIgG and 2C9-cIgM) reacted with 17D-204 vaccine strain in an enzyme-linked immunosorbent assay and neutralized virus in vitro similarly to the parent m2C9. Both m2C9 and 2C9-cIgG when administered prophylactically 24 hours prior to infection protected AG129 mice from peripheral 17D-204 challenge at antibody concentrations ≥ 1.27 μg/mouse; however, the 2C9-cIgM did not protect even at a dose of 127 μg/mouse. The 17D-204 infection of AG129 mice is otherwise uniformly lethal. While the m2C9 was shown previously to be therapeutically effective in YFV-infected BALB/c mice at day 4 post-infection, the m2C9 and 2C9-cIgG demonstrated therapeutic activity only when administered 1 day post-infection in 17D-204-infected AG129 mice.
Keywords: yellow fever, human monoclonal antibodies, treatment, prophylaxis
1. INTRODUCTION
Yellow fever virus (YFV), a member of the genus Flavivirus, family Flaviviridae, is found in tropical regions of Africa and South America, and is transmitted to primates by mosquitoes. Despite the availability of effective vaccines, YF is still a matter of public health concern - responsible for an estimated 200,000 cases and 30,000 deaths annually - and is considered a re-emerging disease. This is due to the reinfestation of countries with the Aedes aegypti vector, lapses in implementation of preventative vaccination programs in endemic regions such as sub-Saharan Africa, and the lack of compliance by at-risk populations (Barnett, 2007; Monath, 2006). Given the increase in travel and commerce originating from once isolated, YFV-endemic regions, there is a growing concern regarding the potential of YFV to cause urban epidemics if introduced into geographical regions containing the Ae. aegypti mosquito vector and large concentrations of susceptible humans (e.g., the southern U.S.) (Gardner and Ryman, 2010; Gubler, 2001, 2002).
For those with access to vaccination, the use of the live-attenuated YF vaccine is contraindicated in a number of individuals. It is advised that infants under 6 months of age not be given the YF vaccine due to a risk of viral encephalitis developing in the child (Cetron et al., 2002; Staples et al., 2010). Also at risk are those individuals who suffer from hypersensitivity to eggs since the YF vaccine is prepared in embryonated eggs. The YF vaccine is not suitable for those who are immunocompromised due to AIDS or HIV infection, or whose immune system has been altered by either diseases such as leukemia and lymphoma or through drugs and radiation (Cetron et al., 2002; Staples et al., 2010). Studies have shown that persons aged ≥ 65 years are particularly susceptible to systemic adverse events following immunization with 17D-204 (Khromava et al., 2005; Martin et al., 2001; Massad et al., 2005; Monath et al., 2005). Additionally, recent reports suggest an increase in post-vaccinal severe adverse events during YF vaccination campaigns (Barrett et al., 2008; Barrett and Teuwen, 2009; Doblas et al., 2006; Engel et al., 2006; Ferguson et al., 2010).
Treatment options are limited for YFV-infected individuals (Monath, 2008). There is currently no specific treatment for YF, a disease that is estimated to have a case-fatality rate of approximately 20%. A small number of antivirals, such as ribavirin or recombinant interferon (IFN), have shown efficacy in reducing viremia and/or prolonging time to death in animal models of YF if administered early in the disease. However, the effectiveness of these therapies is dramatically reduced if given after the onset of clinical symptoms (Julander et al., 2007; Monath, 2008; Sbrana et al., 2004). The study by Sbrana et al., showed that early treatment with ribavirin appears to reduce liver damage associated with YFV infection (Sbrana et al., 2004); however, for the most part treatment of YFV-infected patients is limited to supportive therapy and is directed towards the organ systems involved.
Treatment of YFV infection with murine monoclonal antibodies (mMAbs) provides in vivo protection in a mouse model. Brandriss et al. showed that neutralizing mMAbs directed against the envelope (E) glycoprotein of the 17D-204 vaccine strain protected mice from lethal encephalitis if administered either 1 day prior to or 3 to 5 days after viral intracerebral (i.c.) challenge with 17D-204 vaccine (Brandriss et al., 1986; Schlesinger et al., 1983; Schlesinger et al., 1984). A similar study by Schlesinger et al. demonstrated that prophylactic administration of non-neutralising mMAbs directed against the 17D non-structural glycoprotein, NS1, also protected mice from lethal YF encephalitis (Schlesinger et al., 1986). Unfortunately, human treatment with mMAbs can be compromised by the human anti-mouse antibody response.
Advances in antibody engineering have now made it possible to produce human-murine chimeric (cMAbs) or fully human MAbs. Both cMAbs and hMAbs retain the specificity, avidity and neutralizing activity of the mMAbs they are derived from; however, cMAbs and hMAbs reduce the human anti-mouse antibody (HAMA) response in humans, and are more effective therapeutics than mMabs. In addition, MAbs provide an infinite source of antibodies that are homogeneous in both specificity and affinity, thus making them an attractive substitute for human polyclonal sera for use in human therapeutics. A number of cMAbs have been approved by the FDA for treatment or prophylaxis of various disorders including non-Hodgkin’s lymphoma, renal allograft rejection, and rheumatoid arthritis (Gaffo et al., 2006; Leget and Czuczman, 1998; Lupo et al., 2008). Fully human and humanized MAbs have been successfully used to protect against West Nile virus (WNV) and Venezuelan equine encephalomyelitis virus (VEEV) in both prophylactic and therapeutic animal models of infection (Hunt et al., 2011; Hunt et al., 2006; Hunt et al., 2010; Morrey et al., 2006; Morrey et al., 2007; Morrey et al., 2008; Nybakken et al., 2005; Oliphant et al., 2005). Clinical trials are currently being conducted to evaluate the safety and efficacy of humanized E16 antibody (MGAWN1) for use in WNV infections (Beigel et al., 2010). In this study, we report creation of human/murine chimeric cIgG and cIgM from a protective and therapeutic YFV-reactive mMAb, 2C9, that is specific for the envelope (E) protein for either wild-type or vaccine YF stains (Brandriss et al., 1986; Schlesinger et al., 1983). Sequencing of the E protein of 2C9 neutralization resistant 17D-204 virus identified amino acids 71 and 72 as being involved in 2C9 binding, therefore this MAb recognizes an E protein Domain II epitope (Lobigs et al., 1987). Both the mMAb and the cMAb 2C9-cIgG were shown to be effective prophylactically and therapeutically in a new YF mouse model of infection that utilizes 17D-204 vaccine peripherally-challenged, interferon receptor-deficient AG129 mice (Lee and Lobigs, 2008; Meier et al., 2009).
2. MATERIALS AND METHODS
2.1. Mice
The 129/Sv/Ev mice deficient for IFN-α/β and -γ receptors in combination (strain AG129) obtained from B & K Universal (Hull, United Kingdom) and housed in the Division of Vector-Borne Disease (DVBD) animal care facilities at CDC were used for all animal studies (Johnson and Roehrig, 1999). Mice were euthanized with isoflurane followed by cervical dislocation when signs of illness became obvious as indicated by reduced activity and increased huddling during normal activity hours, lack of appetite, and the development of neurologic signs such as hind leg paralysis or weakness. The use of animals for research purposes complied with all relevant federal guidelines and specific protocols were approved by the DVBD Institutional Animal Care and Use Committee.
2.2. Cells and virus
The Sp2/0-Ag14 (Sp2) murine myeloma and 2C9 murine hybridoma (Roehrig et al., 1980; Schlesinger et al., 1983) were propagated in hybridoma growth medium (HGM) unless noted otherwise. The YFV vaccine strain (17D-204) was obtained from the DVBD reference collection (Fort Collins, CO). A single pool containing 2.0 × 107 plaque-forming units per ml (PFU/ml) of 17D-204 grown in Vero cells with minimum essential medium (MEM; D-MEM supplemented with 10% FBS, 2 mM L-glutamine, 0.15% sodium bicarbonate, 100 U/ml penicillin G sodium, and 100 μg/ml streptomycin sulfate) was used for all inoculations.
2.3. Assembly of human-murine chimeric (c)2C9 IgG and IgM plasmid constructs
The heavy and light variable regions (VH and VK) of the IgG gene were amplified from the m2C9 hybridoma and subsequently modified by PCR for insertion into the human IgG expression vector pdHL2 or the human IgM expression vector pJH2-24-95B1, referred to as pJH2 (Abbott Laboratories, Abbott Park, IL) as previously described (Hackett et al., 1998; Thibodeaux et al., 2010; Thibodeaux and Roehrig, 2009). Degenerate primers used for VH and VK amplification and primers used to add partial 5′ leader sequences, 3′ splice donor junctions, and appropriate restriction sites to the variable regions are listed in Table 1. The VH and VK regions of m2C9 IgG were incorporated separately into pdHL2 or pJH2 by ligation as previously described (Hackett et al., 1998) generating plasmids pdHL2-2C9 and pJH2-2C9. Plasmids pdHL2-2C9 and pJH2-2C9 were used to transform E. coli DH5αE (Invitrogen) by electroporation following the manufacturer’s protocol.
Table 1.
Oligonucleotide primers for amplification and modification of antibody V-regions
| Primer Name | Sequencea |
|---|---|
| First Round Primers | |
| M-IgG2a | 5′ ATTCGGATAGATCTAGTGGATAGACCGATGG 3′ |
| MK-REV | 5′ ATTCGGATAGATCTTGGATGGTGGGAAGATG 3′ |
| MHV-5 | 5′ ACTAGTCGACATGGACTCCAGGCTCAAT TTAGTTTTCCTT 3′ |
| MKV-4 | 5′ ACTAGTCGACATGAGGGCCCCTGCTCACTTTTTTGGCTTCTTG 3′ |
| Second Round Primers | |
| VK-5′ | 5′ TCACGAAGTCTAGACTGGACATGAGGGCCCCTGCTCACTTTTTTGG 3′ |
| VK-3′ | 5′ GAATCTATGGATCCTGACACACTTACGCTTTATTTCCAACTTTGTCCCCGA 3′ |
| VH-5′ | 5′ ACACTATACTCGAGCTCACGATGAACTTTGGGCTCAGCTTGATTT 3′ |
| VH-3′ | 5′ TTCAGATCAAGCTTGACACACTTACCTGAGGAGACGGTGACTGAGGTTC 3′ |
Restriction endonuclease sites are underlined
2.4. Sequencing
V regions were sequenced in triplicate to ensure sequence fidelity after initial amplification and again after PCR modification. Sequencing reactions were performed using the BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA) and sequence data analyzed using the ABI 3130xl Genetic Analyzer (Applied Biosystems).
2.5. Transfection of cells with cIg plasmid constructs
Sp2 cells were prepared and transfected with plasmids pdHL2-2C9 or pJH2-2C9 as previously described (Thibodeaux et al., 2010). Transfected cells were expanded and screened for human IgG or IgM production by ELISA.
2.6. ELISA for detection of YFV-specific cIg
To detect the presence of human YFV-specific cIgG antibody in samples of cell culture supernatants or titrate purified cIgG, a modification of the indirect IgG-ELISA protocol originally described by Johnson et al. was used (Johnson et al., 2000; Thibodeaux et al., 2010). The flavivirus cross-reactive 6GF4 cIgG and the alphavirus cross-reactive 1GD5 cIgG were used as positive and negative control antibodies in the ELISA (Thibodeaux and Roehrig, 2009). To detect the presence of cIgM, IgM antibody-capture (MAC) ELISA was used as previously described (Thibodeaux et al., 2011; Thibodeaux and Roehrig, 2009). The flavivirus cross-reactive 6ME2 cIgM and the alphavirus cross-reactive 1MD11 cIgM were used as positive and negative control antibodies in the ELISA (Thibodeaux and Roehrig, 2009). All ELISAs were performed in duplicate.
2.7. Antibody purification
Transfected Sp2 cells that tested positive for production of cIgG or cIgM were expanded and passaged three times in HGM with methotrexate followed by one additional passage in HGM without methotrexate. Supernatants from each passage were tested for human anti-YFV activity using the indirect IgG ELISA described above. Stably transformed cell lines that exhibited consistently high levels of human anti-YFV cMAb -- 2C9-H4 (cIgG) or 2C9-G9b (cIgM) -- were used for commercial ascites fluid production and antibody purification (QED Bioscience Inc., San Diego, CA). Ascitic fluid and purified IgG were also made from the parent m2C9 hybridoma by QED Biosciences, Inc. Antibodies that were purified from ascitic fluid and eluted in PBS were at final concentrations of 0.55 mg/ml (2C9-cIgG), 0.51 mg/ml (2C9 mMAb) and 0.92 mg/ml (2C9-cIgM).
2.8. YFV plaque reduction neutralization test (PRNT)
The methods for determining flaviviral neutralizing antibody titers have been published previously (Roehrig et al., 2008). Briefly antibody diluted in PBS was mixed with an equal volume of 17D-204 seed (1.0 × 103 PFU/ml) diluted in MEM. Antibody-virus mixtures were allowed to incubate at 37°C for 1 h prior to being dispensed onto 6-well Vero plates in aliquots of 200μl/well. Plates were overlaid with a mixture of agarose and growth medium and incubated at 37°C for 4 days, after which a second overlay containing 0.0053% neutral red (Sigma) was added. Plates were incubated at 37°C for an additional 24 h before plaque formation was recorded. The PRNT50 of each antibody was defined as the titre of antibody required to reduce total PFU/well by 50% compared to wells inoculated with 17D-204 alone. Starting antibody concentrations were standardized to 0.51 mg/ml. The humanized alphavirus cross-reactive 1GD5 cIgG was used as a negative control antibody (Thibodeaux et al., 2010).
2.9. Complementarity determining region (CDR) analysis
CDR determination was performed using the method described by Kabat (Johnson and Wu, 2000).
2.10. Nucleotide sequence accession numbers
The 2C9 V region sequences have been submitted to GenBank and assigned the following accession numbers: 2C9 VH, GU724339: 2C9 Vκ, GU724340.
2.11. Animal studies
All animal studies were performed under a DVBD/CDC IACUC-approved protocol. Mice used in all challenge experiments were between 6 and 8 weeks of age. All mice challenged with 17D-204 received a single intraperitoneal (i.p.) injection of 100 μl containing 2×105 PFU/mouse. Mice treated prophylactically or therapeutically with purified antibody received a single dose of antibody in a volume of 250 μl delivered by i.p. injection. Beginning at day 12 post-infection (p.i.) which coincided with the appearance of morbidity in untreated challenged mice, mice were euthanized upon exhibiting signs of morbidity; tissues were collected at time of sacrifice. Blood was collected via post-mortem cardiac puncture; serum was separated using microtainer tubes (Becton-Dickinson, Franklin Lakes, NJ). Serum samples from euthanized mice were analyzed for de novo production of murine anti-YFV IgM antibodies by MAC-ELISA as previously described to confirm viral infection (Thibodeaux and Roehrig, 2009).
Brain and liver samples were also collected and were manually chopped into small sections using a scalpel and rinsed in PBS prior to being placed into pre-weighed MagNA Lyser Green Bead tubes (Roche Diagnostics GmbH, Mannheim, Germany) containing 500 μl MEM (0% FBS) and stored at −70°C; serum samples separated from blood were stored at −70°C. These tissue specimens were used in qRT-PCR to determine genome copy levels (see Viral Quantitation below).
2.11.1. Protective and therapeutic efficacy
AG129 mice challenged with 2 × 105 PFU/mouse 17D-204 via i.p. injection were administered a single i.p. injection of 127 μg m2C9 IgG, 2C9-cIgG, 2C9-cIgM, or PBS 1 day prior to infection, or one of the IgG MAbs at 1, 3, or 5 days p.i. Individual mice were euthanized upon demonstrating signs of morbidity as described above. Brain samples were collected post-mortem and processed as described. Antibody-treated mice that failed to exhibit signs of morbidity were monitored for an additional 7 days following the sacrifice of the last untreated mouse before being euthanized for tissue collection (day 31). Additionally, prophylactic dose-response analysis was performed using diminishing concentrations of m2C9 and 2C9-cIgG.
2.11.2. Viral Quantitation
2.11.2.1. RNA Extraction from Tissues
For determination of viral genome content of mouse tissues, frozen samples of brain were thawed and homogenized for two 30 s cycles at 4,000 rpm using a Roche MagNA Lyser (Roche Diagnostics GmbH). Homogenized samples were centrifuged for 5 min at 10,000 rpm at room temperature (RT) and total RNA was purified from 50 μl of clarified homogenate using an RNeasy 96 Universal Kit (Qiagen, Valencia, CA) following the manufacturer’s protocol. Viral RNA was extracted from 140 μl serum samples using a QIAamp® Viral RNA Mini Kit (Qiagen) following the manufacturer’s protocol. Viral RNA was purified from ten-fold serially-diluted 17D-204 in parallel with tissue samples using the RNeasy 96 Universal Kit for generation of a standard curve. The 17D-204 used for RNA standards was also titrated by plaque assay to correlate the viral genome equivalents with PFU/ml.
2.11.1.2. Quantitative Reverse-Transcription Polymerase Chain Reaction (qRT-PCR)
Primers and probe used for qRT-PCR were obtained from the CDC Biotechnology Core Facility (Atlanta, GA) and are detailed in Table 1. Each reaction mixture contained 5 μl of purified RNA; primers and probes were used at final concentrations of 1 μM for 8280F and 8354R and 0.2 μM for the 8308 probe. Amplifications were performed in an iQ™5 Real-Time PCR Detection System (Bio-Rad, Hercules, CA) using a QuantiTect® Probe RT-PCR Kit (Qiagen) under the following conditions: 50°C for 30 min, 95°C for 12 min 30 s, followed by 45 cycles of 94°C for 15 s, 55°C for 1 min with continuous fluorescence data collection. Each RNA sample was tested in duplicate (growth curve) or triplicate (protection study) and virus genome equivalents were determined by extrapolation from the standard curves generated within each experiment.
3. RESULTS
3.1. Development of cIgG and cIgM for YFV
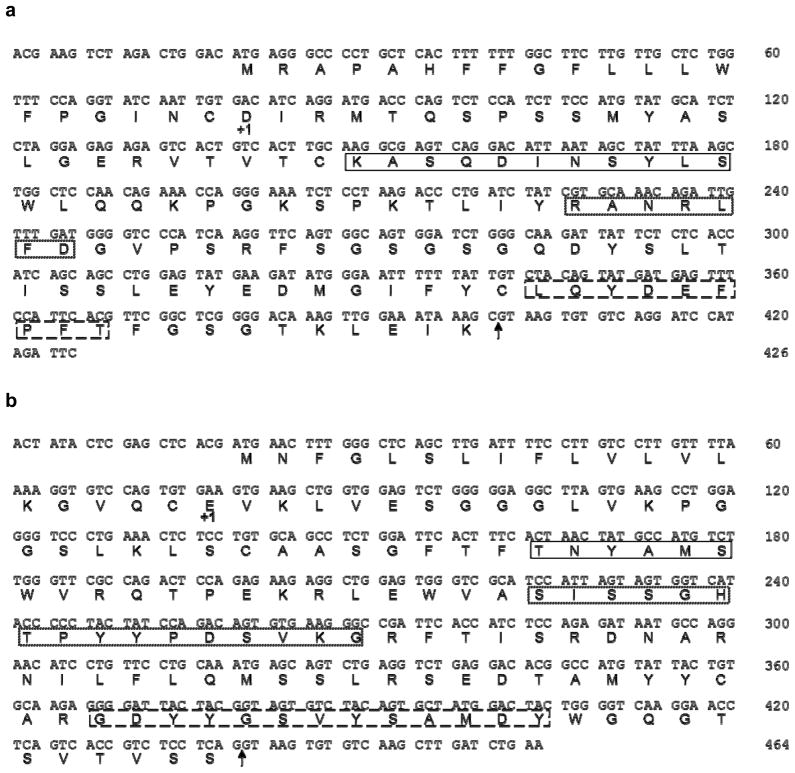
We have shown previously that m2C9 was capable of protecting mice from lethal i.c. challenge with 17D-204 when administered prophylactically or therapeutically to BALB/c or CD-1 mice (Brandriss et al., 1986). Using the pdHL2 expression vector, which contains genomic clones of both the kappa (Cκ) and IgG1 constant (Cγ1) region genes of human immunoglobulin, we constructed a 2C9 cIgG antibody that maintained the specificity and protective capacities of the m2C9 parent. Additionally, to determine the effect that a YFV-neutralizing IgM containing the same antigen binding site as the YFV 2C9-cIgG would have on infection, we also constructed a 2C9-cIgM antibody. Multiple clones of each V gene amplicon were sequenced to ensure against possible DNA polymerase-induced errors, Fig. 1.
Fig. 1.
Nucleotide and deduced amino acid sequences of m2C9 variable regions. (Panel a), Variable region of the κ-chain. (Panel b), Variable region of the γ-chain. +1 denotes start of mature protein; solid line box, CDR 1; dotted box, CDR 2; dashed box, CDR 3; the splice points of Jκ2 to Cκ (A) and of JH2 to the C region gene (B) are indicated by the arrows.
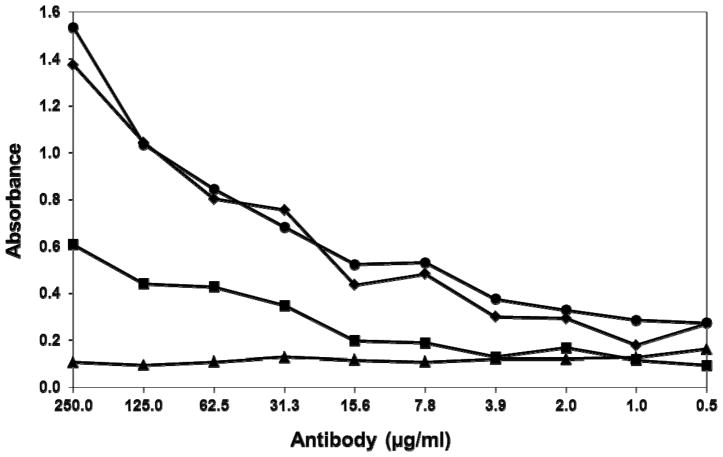
Cell-free supernatants of Sp2 cells transfected with pdHL2-2C9 or pJH2-2C9 were analyzed by ELISA for the presence of human-murine chimeric Ig approximately two weeks after transfection. A total of three wells, H4, F6, and G9, for cIgG and three wells, G-9, G-9b, and H-11, for cIgM of transfected Sp2 cells tested positive for YFV-specific antibody by ELISA. Single stably transformed cell lines, 2C9-H4 (cIgG) and 2C9-G9 (cIgM) that consistently secreted high levels of anti-YFV antibody over multiple passages were sent to a commercial company for production and purification of the antibodies used in this study. The reactivities of the purified 2C9-cIgG and m2C9 were similar by ELISA against YFV17D-204 virus (data not shown). More interestingly, the reactivities of the 2C9-cIgG and 2C9-cIgM with YFV-17D were verified by ELISA and also shown to be identical to one another, Fig. 2. The similar binding curves of purified and standardized 2C9-cIgG and 2C9-cIgM suggests similar binding avidities with 17D-204. This result was a bit unexpected given the difference in size between IgG and IgM.
Fig. 2.
End-point titration of purified cMAbs. Symbols: 2C9-cIgG (●); 2C9-cIgM (◆); cIgG 1GD5 (alphavirus cross-reactive) (■); cIgM 1MD11 (alphavirus cross-reactive) (▲). IgM cMAbs were titrated in MAC-ELISA and the absorbance was measured at 450nm; IgG cMAbs were titrated in indirect ELISA and absorbance was measured at 405nm and titration results were superimposed. Insert: YFV neutralization end-point titres of MAbs m2C9, 2C9-cIgG, 2C9-cIgM, and cIgG 1GD5, expressed as PRNT50 reciprocal end-points.
3.2. 2C9-H4 and 2C9-G9 Neutralizing Activity in vitro
The YFV-neutralizing titers of purified 2C9-cIgG and 2C9-cIgM were compared to that of the parent m2C9 by PRNT in Vero cell cultures, Fig. 2, inset. The PRNT50 end-point reciprocal titre for the 2C9-cIgG (128) was two-fold less than the parent m2C9 MAb (256) and two-fold more than the 2C9-cIgM (68). The alphavirus cIgG 1GD5 was used as a negative control antibody and did not neutralize YFV.
3.3. Prophylaxis and therapy of YFV infections using 2C9-cIgG and 2C9-cIgM
The 2C9-cIgG, 2C9-cIgM, and their m2C9 parent were assayed for their efficacy in prophylaxis and therapy of 17D-204 infection of AG129 mice. Prior to testing, 127 μg/mouse of 2C9-cIgG was administered via i.p. injection to small groups of AG129 mice to ensure that no adverse reactions resulted and no adverse reactions were observed (data not shown).
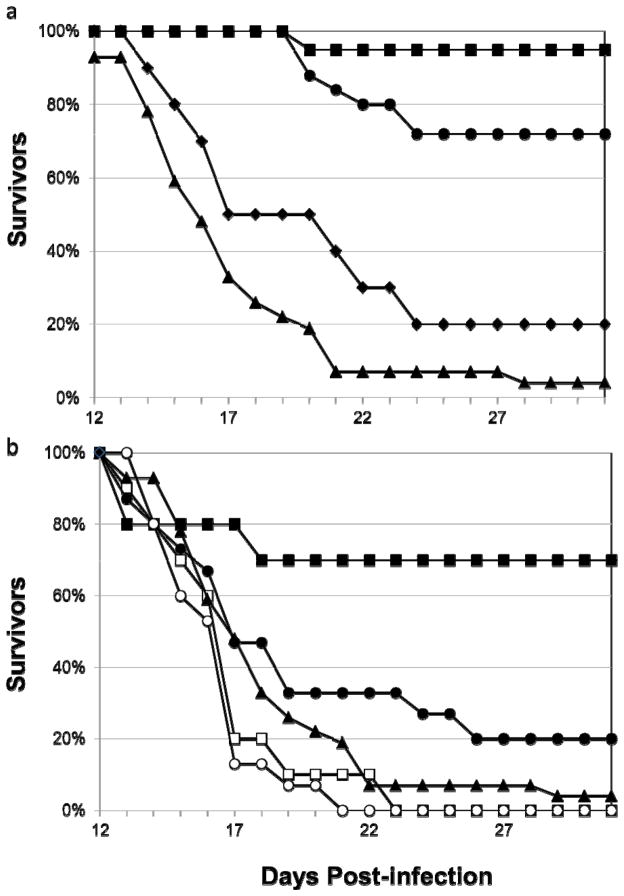
Using the AG129/17D-204 peripheral challenge model, the 2C9-cIgG and m2C9 parent MAbs were first tested for their ability to limit YF disease when administered 24 h prior to virus challenge. Protection was defined as increased average survival time (AST) and decreased morbidity of treated mice compared to non-treated mice. The aggregate results of 2–4 independent experiments are summarized in Fig. 3a and Table 2. Mice receiving either 2C9-cIgG or m2C9 24 h prior to infection with 17D-204 showed a significant increase in AST (m2C9 AST=30.4±2.5 days; 2C9-cIgG AST=28.3±4.5 days) compared to infected mice receiving PBS alone (AST=17.3±4.3 days). Additionally, 95% of animals receiving m2C9 and 72% of animals receiving 2C9-cIgG survived viral challenge. Interestingly, the 2C9-cIgM failed to protect significant numbers of mice (2/10) from viral challenge and did not significantly increase ASTs (20.6±5.9 days vs. 17.3±4.3 days for PBS control animals) even though it contained the same antigen-combining site as 2C9-cIgG and had the same virus-neutralizing activity.
Fig. 3.
Survival curves for antibody-treated mice. Panel a, survival of AG129 mice that were passively transferred intraperitoneally 127 μg of m2C9 (■), 2C9-cIgG (●), 2C9-cIgM (◆), or PBS (▲), 24 hours prior to i.p. challenge with 17D-204. Clinical signs began after 11 days p.i. so graphs show surviving animals from days 12 to 31, after which experiments were terminated. Panel b, survival of AG129 mice challenged i.p. with 17D-204 and administered i.p. 127 μg of m2C9 (■) or 2C9-cIgG (●) at 24 h or m2C9 (□) or 2C9-cIgG (○) 72 h p.i. A survival curve for PBS control mice is included for comparison (▲). Mice were monitored as in Panel a.
Table 2.
Prophylactic and therapeutic activity of m2C9, 2C9-H4 cIgG, and 2C9-H4 cIgM in 17D-204-challenged AG129 mice.
| Treatmenta | Survivors/Total Mice (% Survivors) | Brain Viral Loadb | ASTc | |
|---|---|---|---|---|
| Survivors | Dead | |||
| PBS Controls | 1/27 (4) | n.a. | 6.9 (0.6) | 17.3 (4.3) |
| Prophylaxis Day -1 | ||||
| m2C9 IgG | 18/19 (95)d | 1.2 (0.8)e | 7.0 | 30.4 (2.5)d |
| 2C9-H4 IgG | 18/25 (72)d | 0.7 (0.4)e | 6.7 | 28.3 (4.5)d |
| 2C9-H4 IgM | 2/10 (20) | 6.0 (0.1)f | 7.7 (0.3) | 20.6 (5.9) |
| Treatment Day 1 | ||||
| m2C9 IgG | 7/10 (70)d | 2.0 (2.0)e | 7.4 (3.0) | 25.8 (8.8)d |
| 2C9- H4 IgG | 3/15 (20) | 1.7d | 6.7 (0.5) | 19.4 (7.0) |
| Treatment Day 3 | ||||
| m2C9 IgG | 0/10 (0) | n.a. | 6.6 (0.3) | 15.8 (2.8) |
| 2C9-H4 IgG | 0/15 (0) | n.a. | 6.7 4) | 15.3 (2.0) |
Mice were inoculated i.p. with 127 μg purified MAb 24 hours pre-infection or 24 or 72 hours post-infection by an i.p. viral challenge.
Expressed as mean (s.d.) genomic copies (log10)/g tissue.
Experiments were terminated at 31 days post-infection. Average survival time (AST) in days (s.d.).
ρ <0.05 by two-tailed t-test statistic vs. PBS control mice.
ρ <0.05 by two-tailed t-test statistic vs. paired PBS control mice or paired dead mice.
ρ <0.05 by two-tailed t-test statistic vs. paired dead mice only.
Mice that were treated with m2C9 one day after viral challenge demonstrated a significantly increased AST (25.8±8.5 days) compared to untreated mice (AST=17.3±4.3 days) Table 2 and Fig. 3b. Mice receiving 2C9-cIgG at one day p.i. demonstrated a slightly increased AST (19.4±7.0). Seventy percent of animals receiving m2C9 and 20% of animals receiving 2C9-cIgG 1 day after infection survived viral challenge. Survival curves are shown in Fig. 3b. Infected mice receiving either m2C9 or 2C9-cIgG at days 3 or 5 p.i. (not shown) did not show an increase in AST compared to PBS treated mice. ASTs were slightly shorter for mice administered MAb at 3 days p.i. compared to PBS control animals (15.8 and 15.3 days vs. 17.3 days, Table 2), however these differences were not statistically significant (t-tailed t-test, ρ≤0.05). Because of its low level of prophylactic capacity, the therapeutic capacity of the 2C9-cIgM was not tested.
Dose-response titrations of the protective capacity of m2C9 and 2C9-cIgG indicated that m2C9 was more effective in protecting mice than was 2C9-cIgG. At a dose of 1.27 μg/mouse, m2C9 protected 60% of challenged mice while 2C9-cIgG protected 20% of mice, Table 3. Both antibodies protected efficiently at the higher doses of 127 or 12.7 μg/mouse.
Table 3.
Prophylactic dose-response comparison of m2C9 and 2C9-H4 IgG in 17D-204 challenged AG129 mice.
| Group | μg Antibodya | Survivors/Total Mice (% Survivors) | Brain Viral Loadb | ASTc | |
|---|---|---|---|---|---|
| Survivors | Dead | ||||
| PBS Controls | n/a | 0/5 (0) | n.a. | 6.9 (0.6) | 18.8 (5.9) |
| 2C9-H4 IgG | 127 | 4/5 (80)d | 2.2 (2.5)d,e | 6.74 | 28.8 (4.9)d |
| 12.7 | 10/10 (100)d | 1.0 (0.4)d | n.a. | 31 (0)d | |
| 1.27 | 2/10 (20) | 5.1 (0.7)d,e | 6.7 (0.3) | 19.2 (6.9) | |
| m2C9 IgG | 127 | 5/5 (100)d | 0.9 (0.3)d | n.a. | 31 (0)d |
| 12.7 | 8/10 (80)d | 1.5 (1.3)d,e | 6.5 (0.1) | 27.0 (6.9)d | |
| 1.27 | 6/10 (60)d | 3.9 (2.4)d,e | 6.7 0.5) | 26.1 (6.8)d | |
Mice were inoculated i.p. with varying doses of purified MAb 24 hours prior to i.p. virus challenge. Experiments were terminated at 31 days post-infection.
Expressed as mean (s.d.) of the genomic copies (log10)/g tissue.
Average survival time (AST) in days (s.d.).
ρ <0.05 by two-tailed t-test statistic vs. paired PBS control mice.
ρ <0.05 by two-tailed t-test statistic vs. paired dead mice.
There were no significant differences in viral load in brain tissue between mice showing morbidity in either antibody-treated or untreated groups, both demonstrating 6.6 to 6.9 log10 genomic equivalents/g of brain tissue, Table 3. Variation within groups was high, however, with 17D-204 vRNA below detectable levels in some mice. Mice surviving viral challenge demonstrated significantly reduced viral load compared to non-surviving mice given the same treatment. In fact, most surviving mice, regardless of IgG treatment, were essentially virus free at the end of the experiment. Interestingly, the two surviving mice treated prophylactically with 2C9-cIgM were not virus free, but they did have significantly reduced viral loads in the brain when compared to the mice that did not survive challenge (6.0 vs. 7.0 log10 genomic equivalents/g of brain tissue, Table 3). Serum samples taken from all mice were positive for murine anti-YFV IgM as determined by MAC-ELISA, indicating that all mice were infected and mounted an antibody response (data not shown).
4. DISCUSSION
In this study, use of the AG129/17D-204 model permitted us to analyze the protective capacity of our MAbs in a neurotropic model of infection that resembles the original i.c. BALB/c 17D-204 infection model used previously (Brandriss et al., 1986). In this case, however, we were able to use a peripheral route of viral challenge. These earlier studies determined that m2C9 could protect 100% of mice (40 μg m2C9/mouse) or 60% of mice (4 μg m2C9/mouse) subsequently challenged i.c. with 17D-204. Additionally, m2C9 cured 90% of 17D-204-infected mice when 40 μg 2C9/mouse were administered by day 4 p.i. The use of specific antibody for prophylaxis or therapy of viral infection has been explored for a number of other flaviviruses with varying degrees of success. Numerous studies have illustrated that treatment of flavivirus infections with immune serum or mMAbs (both neutralizing and non-neutralizing) can result in protection in rodents. However, evidence also suggests that successful treatment depends on the dosage and timing of antibody administration. In the case of encephalitic flaviviruses, passive mMAb therapy is usually only effective if administered before viral replication in the brain (Mathews and Roehrig, 1984; Roehrig et al., 2001). Other factors to consider in the use of passive antibody therapy for flaviviruses include the possibility of antibody dependent enhancement (ADE) of infection arising from the presence of sub-neutralizing concentrations of antibody as is seen during secondary DENV infection.
Although ADE has not been described for YFV, a similar phenomenon, referred to as antibody-dependent early death (ADED), has been described in models of YFV infection involving passive antibody therapy of encephalitic disease in rodents. We did not observe ADED when using lower, partially-protective doses of antibody (1.27 μg/mouse), as compared to PBS control animals (Table 3). This syndrome results from specific reactions between virus and antibody in the infected brain and is not mediated through Fc- and complement receptor-bearing macrophages (Barrett and Gould, 1986; Gould et al., 1987). ADED has been reported only with selected virus/antibody combinations, usually with antibodies demonstrating little or no YFV-neutralizing activity (Barrett and Gould, 1986). A study by Gould et al. suggested that neutralizing antibodies normally capable of protecting mice from virus challenge enhanced neurovirulence only if they were given at a time after infection when infection in the mouse brain was established (Gould and Buckley, 1989).
For this study, the m2C9 MAb chosen for humanization is one of only a handful of YFV type-specific mMAbs currently in existence and is 1 of only 3 mMAbs capable of neutralizing both the wt YF-Asibi and 17D-204 vaccine strains in vitro and demonstrating protective activity against both strains in vivo. The growth curve of 17D-204 in AG129 brain tissue indicates that viral dissemination to and replication in the brain occurs as early as day 3 p.i. (Lee and Lobigs, 2008; Meier et al., 2009). Administration of 2C9 MAbs at either days 3 or 5 p.i. did not provide significant protection in this model, Table 2 and Fig. 3b.
The observation that m2C9 (and to a lesser extent 2C9-cIgG) only provided protection to AG129 mice when administered 1 day prior to or 1 day after infection is in contrast to our previous studies (Brandriss et al., 1986). Although m2C9 and 2C9-cIgG shared similar 17D-204 neutralizing capacity in vitro, it is conceivable that the humanization of 2C9 may have reduced the protective capacity of this antibody in murine models of YF disease. A study by Schlesinger and Chapman reported that the loss or absence of the murine IgG Fc region resulted in a loss in the protective capacity of 2E10 mMAb in mice (Schlesinger and Chapman, 1995). The inability of 2C9-cIgM to protect animals from viral challenge even though its antigen recombining site was identical to that of 2C9-cIgG supports the involvement of the IgG Fc portion in protection. Considering that mouse IgG2a and human IgG1 are structurally similar and analogous in that both are able to fix complement and bind to human Fcγ-RIA (CD64) with very high affinity, it is likely that human IgG1 binds tightly to mouse CD64. An alternative explanation for the observed decrease in protective activity could also be attributable to the different mouse strains used in the protection assays.
Alternatively, the AG129 mice used in this protection study have defects in their type I and II IFN responses, unlike the BALB/c or CD-1 mice used in the initial characterization of m2C9. It is likely that the early protection afforded mice by the mouse IFN response also increases the protective capacity of the 2C9 MAbs. To address this question we will analyze the protective and therapeutic activities of these MAbs in the hamster model of YFV infection. This model uses the wt YFV-Asibi strain as the challenge virus (Julander et al., 2007; Sbrana et al., 2006; Tesh et al., 2001; Xiao et al., 2001)
If the reduced protective activity of 2C9-cIgG in AG129 mice is due to incompatibility between the cMAb and murine immune system or is a result of the immune deficiencies in the mouse strain used, then it is likely that the 2C9-cIgG will be more effective in a human host as is intended. In any event, we are currently investigating these possibilities and are also humanizing additional YFV-protective mMAbs for combination therapy with 2C9-cIgG in order to reduce the risk of selection for YFV neutralization-escape variants in response to treatment with a single MAb.
The involvement of flaviviral IgM in protection and recovery from viral infection has not been well studied. In a previous study with YFV it was noted that a 400 μg of a highly neutralizing YFV mMAb, 8A3, was not able to passively protect mice from 17D-204 challenge (Brandriss et al., 1986). Additionally, the IgM fraction of immune sera cannot facilitate immune-enhancement through Fc-receptor binding to cells (Schlesinger and Brandriss, 1981). A subsequent study investigated the role of IgM in protecting WNV-challenged mice defective in their immune response (Diamond et al., 2003). This study suggested that IgM was critical in limiting early viral replication and could protect animals from virus challenge. Not unexpectedly, passive transfer of a virus-reactive, but non-neutralizing IgM MAb had no effect on viral replication (Diamond et al., 2003). Because we had the ability to re-engineer this mMAbs, we created an IgM with the same Fab as the paired IgG. This cIgM retained the virus-neutralizing and virus binding activity of 2C9-cIgG, Fig. 2.
While treatment with this 2C9-cIgM reduced viral loads in surviving mice, it was at least 100-fold less protective than 2C9-cIgG. It is well known that both IgG and IgM enter the blood stream following i.p. inoculation (Maitta et al., 2004; Faria-Neto et al., 2006; Dadchova et al., 2007; Nakouzi et al., 2008; and Han, 2010) and have pharmacological activity. Therefore, the lack of in vivo protective capacity of the 2C9-cIgM could be due to either its inability to cross the blood-brain barrier (unlike IgG), or its lack of a free Fc-region, while maintaining its in vitro virus-neutralizing capacity. In this regard, we have shown previously that monoclonal IgG antibody with high in vitro virus-neutralizing activity, loses its capacity to protect mice from a challenge with Venezuelan equine encephalitis – a neurotropic alphavirus – if the Fc portion of the antibody is removed prior to i.p. inoculation (Mathews et al., 1985).
In this study we have demonstrated that the AG129/17D-204 model of YF is an effective model for evaluating the protective activity of a MAbs. The 2C9-cIgG shows promise as a possible prophylactic or therapeutic agent for YFV infection and may be useful in individuals for whom vaccination with 17D-204 is contraindicated or for unvaccinated individuals who contract YFV infections. Furthermore, both the 2C9-cIgG and 2C9-cIgM may have utility as YFV-specific human immunoglobulins for use in serodiagnosis of YFV infection.
There are no specific treatments for human yellow fever (YF) infection.
We have created human/murine chimeric IgG (cIgG) and cIgMantibodies for YF virus.
The cIgG prophylactically protects AG129 mice from peripheral 17D-204 challenge.
The cIgGis therapeutically active at 1 day post-infection with 17D-204.
The cIgM has no antiviral activity in vivo.
Acknowledgments
This research was supported by NIH/NIAID grant U54AI-065357 to the Rocky Mountain Regional Center of Excellence for Biodefense and Emerging Infectious Disease Research (http://www.rmrce.colostate.edu/), the U.S. Centers for Disease Control and Prevention.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barnett ED. Yellow Fever: Epidemiology and Prevention. Clinical Infectious Diseases. 2007;44:850–856. doi: 10.1086/511869. [DOI] [PubMed] [Google Scholar]
- Barrett AD, Gould EA. Antibody-mediated early death in vivo after infection with yellow fever virus. J Gen Virol. 1986;67 (Pt 11):2539–2542. doi: 10.1099/0022-1317-67-11-2539. [DOI] [PubMed] [Google Scholar]
- Barrett AD, Niedrig M, Teuwen DE. International laboratory network for yellow fever vaccine-associated adverse events. Vaccine. 2008;26:5441–5442. doi: 10.1016/j.vaccine.2008.06.025. [DOI] [PubMed] [Google Scholar]
- Barrett AD, Teuwen DE. Yellow fever vaccine - how does it work and why do rare cases of serious adverse events take place? Curr Opin Immunol. 2009;21:308–313. doi: 10.1016/j.coi.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Beigel JH, Nordstrom JL, Pillemer SR, Roncal C, Goldwater DR, Li H, Holland PC, Johnson S, Stein K, Koenig S. Safety and pharmacokinetics of single intravenous dose of MGAWN1, a novel monoclonal antibody to West Nile virus. Antimicrob Agents Chemother. 2010;54:2431–2436. doi: 10.1128/AAC.01178-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandriss MW, Schlesinger JJ, Walsh EE, Briselli M. Lethal 17D yellow fever encephalitis in mice. I. Passive protection by monoclonal antibodies to the envelope proteins of 17D yellow fever and dengue 2 viruses. J Gen Virol. 1986;67 (Pt 2):229–234. doi: 10.1099/0022-1317-67-2-229. [DOI] [PubMed] [Google Scholar]
- Cetron MS, Marfin AA, Julian KG, Gubler DJ, Sharp DJ, Barwick RS, Weld LH, Chen R, Clover RD, Deseda-Tous J, Marchessault V, Offit PA, Monath TP. Yellow fever vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2002. MMWR Recomm Rep. 2002;51:1–11. quiz CE11–14. [PubMed] [Google Scholar]
- Diamond MS, Sitati EM, Friend LD, Higgs S, Shrestha B, Engle M. A critical role for induced IgM in the protection against West Nile virus infection. J Exp Med. 2003;198:1853–1862. doi: 10.1084/jem.20031223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doblas A, Domingo C, Bae HG, Bohorquez CL, de Ory F, Niedrig M, Mora D, Carrasco FJ, Tenorio A. Yellow fever vaccine-associated viscerotropic disease and death in Spain. J Clin Virol. 2006;36:156–158. doi: 10.1016/j.jcv.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Engel AR, Vasconcelos PF, McArthur MA, Barrett AD. Characterization of a viscerotropic yellow fever vaccine variant from a patient in Brazil. Vaccine. 2006;24:2803–2809. doi: 10.1016/j.vaccine.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Ferguson M, Shin J, Knezevic I, Minor P, Barrett A. WHO Working Group on Technical Specifications for Manufacture and Evaluation of Yellow Fever Vaccines, Geneva, Switzerland, 13–14 May 2009. Vaccine. 2010;28:8236–8245. doi: 10.1016/j.vaccine.2010.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffo A, Saag KG, Curtis JR. Treatment of rheumatoid arthritis. Am J Health Syst Pharm. 2006;63:2451–2465. doi: 10.2146/ajhp050514. [DOI] [PubMed] [Google Scholar]
- Gardner CL, Ryman KD. Yellow fever: a reemerging threat. Clin Lab Med. 2010;30:237–260. doi: 10.1016/j.cll.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould EA, Buckley A. Antibody-dependent enhancement of yellow fever and Japanese encephalitis virus neurovirulence. J Gen Virol. 1989;70 (Pt 6):1605–1608. doi: 10.1099/0022-1317-70-6-1605. [DOI] [PubMed] [Google Scholar]
- Gould EA, Buckley A, Groeger BK, Cane PA, Doenhoff M. Immune enhancement of yellow fever virus neurovirulence for mice: studies of mechanisms involved. J Gen Virol. 1987;68 (Pt 12):3105–3112. doi: 10.1099/0022-1317-68-12-3105. [DOI] [PubMed] [Google Scholar]
- Gubler DJ. Human arbovirus infections worldwide. Ann N Y Acad Sci. 2001;951:13–24. doi: 10.1111/j.1749-6632.2001.tb02681.x. [DOI] [PubMed] [Google Scholar]
- Gubler DJ. The global emergence/resurgence of arboviral diseases as public health problems. Arch Med Res. 2002;33:330–342. doi: 10.1016/s0188-4409(02)00378-8. [DOI] [PubMed] [Google Scholar]
- Hackett J, Jr, Hoff-Velk J, Golden A, Brashear J, Robinson J, Rapp M, Klass M, Ostrow DH, Mandecki W. Recombinant mouse-human chimeric antibodies as calibrators in immunoassays that measure antibodies to Toxoplasma gondii. J Clin Microbiol. 1998;36:1277–1284. doi: 10.1128/jcm.36.5.1277-1284.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt AR, Bowen RA, Frederickson S, Maruyama T, Roehrig JT, Blair CD. Treatment of mice with human monoclonal antibody 24h after lethal aerosol challenge with virulent Venezuelan equine encephalitis virus prevents disease but not infection. Virology. 2011;414:146–152. doi: 10.1016/j.virol.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt AR, Frederickson S, Hinkel C, Bowdish KS, Roehrig JT. A humanized murine monoclonal antibody protects mice either before or after challenge with virulent Venezuelan equine encephalomyelitis virus. J Gen Virol. 2006;87:2467–2476. doi: 10.1099/vir.0.81925-0. [DOI] [PubMed] [Google Scholar]
- Hunt AR, Frederickson S, Maruyama T, Roehrig JT, Blair CD. The first human epitope map of the alphaviral E1 and E2 proteins reveals a new E2 epitope with significant virus neutralizing activity. PLoS neglected tropical diseases. 2010;4:e739. doi: 10.1371/journal.pntd.0000739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AJ, Martin DA, Karabatsos N, Roehrig JT. Detection of anti-arboviral immunoglobulin G by using a monoclonal antibody-based capture enzyme-linked immunosorbent assay. J Clin Microbiol. 2000;38:1827–1831. doi: 10.1128/jcm.38.5.1827-1831.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AJ, Roehrig JT. New mouse model for dengue virus vaccine testing. J Virol. 1999;73:783–786. doi: 10.1128/jvi.73.1.783-786.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G, Wu TT. Kabat database and its applications: 30 years after the first variability plot. Nucleic Acids Res. 2000;28:214–218. doi: 10.1093/nar/28.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julander JG, Morrey JD, Blatt LM, Shafer K, Sidwell RW. Comparison of the inhibitory effects of interferon alfacon-1 and ribavirin on yellow fever virus infection in a hamster model. Antiviral Res. 2007;73:140–146. doi: 10.1016/j.antiviral.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khromava AY, Eidex RB, Weld LH, Kohl KS, Bradshaw RD, Chen RT, Cetron MS. Yellow fever vaccine: an updated assessment of advanced age as a risk factor for serious adverse events. Vaccine. 2005;23:3256–3263. doi: 10.1016/j.vaccine.2005.01.089. [DOI] [PubMed] [Google Scholar]
- Lee E, Lobigs M. E protein domain III determinants of yellow fever virus 17D vaccine strain enhance binding to glycosaminoglycans, impede virus spread, and attenuate virulence. J Virol. 2008;82:6024–6033. doi: 10.1128/JVI.02509-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leget GA, Czuczman MS. Use of rituximab, the new FDA-approved antibody. Curr Opin Oncol. 1998;10:548–551. doi: 10.1097/00001622-199811000-00012. [DOI] [PubMed] [Google Scholar]
- Lobigs M, Dalgarno L, Schlesinger JJ, Weir RC. Location of a neutralization determinant in the E protein of yellow fever virus (17D vaccine strain) Virology. 1987;161:474–478. doi: 10.1016/0042-6822(87)90141-3. [DOI] [PubMed] [Google Scholar]
- Lupo L, Panzera P, Tandoi F, Carbotta G, Giannelli G, Santantonio T, Rendina M, Gentile A, Memeo V. Basiliximab versus steroids in double therapy immunosuppression in liver transplantation: a prospective randomized clinical trial. Transplantation. 2008;86:925–931. doi: 10.1097/TP.0b013e318186b8a3. [DOI] [PubMed] [Google Scholar]
- Martin M, Weld LH, Tsai TF, Mootrey GT, Chen RT, Niu M, Cetron MS. Advanced age a risk factor for illness temporally associated with yellow fever vaccination. Emerg Infect Dis. 2001;7:945–951. doi: 10.3201/eid0706.010605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massad E, Coutinho FA, Burattini MN, Lopez LF, Struchiner CJ. Yellow fever vaccination: how much is enough? Vaccine. 2005;23:3908–3914. doi: 10.1016/j.vaccine.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Mathews JH, Roehrig JT. Elucidation of the topography and determination of the protective epitopes on the E glycoprotein of Saint Louis encephalitis virus by passive transfer with monoclonal antibodies. J Immunol. 1984;132:1533–1537. [PubMed] [Google Scholar]
- Meier KC, Gardner CL, Khoretonenko MV, Klimstra WB, Ryman KD. A mouse model for studying viscerotropic disease caused by yellow fever virus infection. PLoS Pathog. 2009;5:e1000614. doi: 10.1371/journal.ppat.1000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monath TP. Yellow fever as an endemic/epidemic disease and priorities for vaccination. Bull Soc Pathol Exot. 2006;99:341–347. [PubMed] [Google Scholar]
- Monath TP. Treatment of yellow fever. Antiviral Res. 2008;78:116–124. doi: 10.1016/j.antiviral.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Monath TP, Cetron MS, McCarthy K, Nichols R, Archambault WT, Weld L, Bedford P. Yellow fever 17D vaccine safety and immunogenicity in the elderly. Hum Vaccin. 2005;1:207–214. doi: 10.4161/hv.1.5.2221. [DOI] [PubMed] [Google Scholar]
- Morrey JD, Siddharthan V, Olsen AL, Roper GY, Wang H, Baldwin TJ, Koenig S, Johnson S, Nordstrom JL, Diamond MS. Humanized Monoclonal Antibody against West Nile Virus Envelope Protein Administered after Neuronal Infection Protects against Lethal Encephalitis in Hamsters. J Infect Dis. 2006;194:1300–1308. doi: 10.1086/508293. [DOI] [PubMed] [Google Scholar]
- Morrey JD, Siddharthan V, Olsen AL, Wang H, Julander JG, Hall JO, Li H, Nordstrom JL, Koenig S, Johnson S, Diamond MS. Defining limits of treatment with humanized neutralizing monoclonal antibody for West Nile virus neurological infection in a hamster model. Antimicrob Agents Chemother. 2007;51:2396–2402. doi: 10.1128/AAC.00147-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrey JD, Siddharthan V, Wang H, Hall JO, Skirpstunas RT, Olsen AL, Nordstrom JL, Koenig S, Johnson S, Diamond MS. West Nile virus-induced acute flaccid paralysis is prevented by monoclonal antibody treatment when administered after infection of spinal cord neurons. J Neurovirol. 2008;14:152–163. doi: 10.1080/13550280801958930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nybakken GE, Oliphant T, Johnson S, Burke S, Diamond MS, Fremont DH. Structural basis of West Nile virus neutralization by a therapeutic antibody. Nature. 2005;437:764–769. doi: 10.1038/nature03956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliphant T, Engle M, Nybakken GE, Doane C, Johnson S, Huang L, Gorlatov S, Mehlhop E, Marri A, Chung KM, Ebel GD, Kramer LD, Fremont DH, Diamond MS. Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nat Med. 2005;11:522–530. doi: 10.1038/nm1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehrig JT, Corser JA, Schlesinger MJ. Isolation and characterization of hybrid cell lines producing monoclonal antibodies directed against the structural proteins of Sindbis virus. Virology. 1980;101:41–49. doi: 10.1016/0042-6822(80)90481-x. [DOI] [PubMed] [Google Scholar]
- Roehrig JT, Hombach J, Barrett AD. Guidelines for Plaque-Reduction Neutralization Testing of Human Antibodies to Dengue Viruses. Viral Immunol. 2008 doi: 10.1089/vim.2008.0007. [DOI] [PubMed] [Google Scholar]
- Roehrig JT, Staudinger LA, Hunt AR, Mathews JH, Blair CD. Antibody prophylaxis and therapy for flavivirus encephalitis infections. Ann N Y Acad Sci. 2001;951:286–297. doi: 10.1111/j.1749-6632.2001.tb02704.x. [DOI] [PubMed] [Google Scholar]
- Sbrana E, Xiao SY, Guzman H, Ye M, Travassos da Rosa AP, Tesh RB. Efficacy of post-exposure treatment of yellow fever with ribavirin in a hamster model of the disease. Am J Trop Med Hyg. 2004;71:306–312. [PubMed] [Google Scholar]
- Sbrana E, Xiao SY, Popov VL, Newman PC, Tesh RB. Experimental yellow fever virus infection in the golden hamster (Mesocricetus auratus) III. Clinical laboratory values. Am J Trop Med Hyg. 2006;74:1084–1089. [PubMed] [Google Scholar]
- Schlesinger JJ, Brandriss MW. Antibody-mediated infection of macrophages and macrophage-like cell lines with 17D-yellow fever virus. J Med Virol. 1981;8:103–117. doi: 10.1002/jmv.1890080204. [DOI] [PubMed] [Google Scholar]
- Schlesinger JJ, Brandriss MW, Cropp CB, Monath TP. Protection against yellow fever in monkeys by immunization with yellow fever virus nonstructural protein NS1. J Virol. 1986;60:1153–1155. doi: 10.1128/jvi.60.3.1153-1155.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger JJ, Brandriss MW, Monath TP. Monoclonal antibodies distinguish between wild and vaccine strains of yellow fever virus by neutralization, hemagglutination inhibition, and immune precipitation of the virus envelope protein. Virology. 1983;125:8–17. doi: 10.1016/0042-6822(83)90059-4. [DOI] [PubMed] [Google Scholar]
- Schlesinger JJ, Chapman S. Neutralizing F(ab′)2 fragments of protective monoclonal antibodies to yellow fever virus (YF) envelope protein fail to protect mice against lethal YF encephalitis. J Gen Virol. 1995;76 (Pt 1):217–220. doi: 10.1099/0022-1317-76-1-217. [DOI] [PubMed] [Google Scholar]
- Schlesinger JJ, Walsh EE, Brandriss MW. Analysis of 17D yellow fever virus envelope protein epitopes using monoclonal antibodies. J Gen Virol. 1984;65 (Pt 10):1637–1644. doi: 10.1099/0022-1317-65-10-1637. [DOI] [PubMed] [Google Scholar]
- Staples JE, Gershman M, Fischer M. Yellow fever vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2010;59:1–27. [PubMed] [Google Scholar]
- Tesh RB, Guzman H, da Rosa AP, Vasconcelos PF, Dias LB, Bunnell JE, Zhang H, Xiao SY. Experimental yellow fever virus infection in the Golden Hamster (Mesocricetus auratus). I. Virologic, biochemical, and immunologic studies. J Infect Dis. 2001;183:1431–1436. doi: 10.1086/320199. [DOI] [PubMed] [Google Scholar]
- Thibodeaux BA, Liss NM, Panella AN, Roehrig JT. Development of a human-murine chimeric immunoglobulin M for use in the serological detection of human alphavirus antibodies. Clin Vaccine Immunol. 2011;18:2181–2182. doi: 10.1128/CVI.05269-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeaux BA, Panella AN, Roehrig JT. Development of human-murine chimeric immunoglobulin G for use in the serological detection of human flavivirus and alphavirus antibodies. Clin Vaccine Immunol. 2010;17:1617–1623. doi: 10.1128/CVI.00097-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeaux BA, Roehrig JT. Development of a human-murine chimeric immunoglobulin M antibody for use in the serological detection of human flavivirus antibodies. Clin Vaccine Immunol. 2009;16:679–685. doi: 10.1128/CVI.00354-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao SY, Zhang H, Guzman H, Tesh RB. Experimental yellow fever virus infection in the Golden hamster (Mesocricetus auratus). II. Pathology. J Infect Dis. 2001;183:1437–1444. doi: 10.1086/320200. [DOI] [PubMed] [Google Scholar]