Abstract
Aims
Topiramate has shown efficacy at facilitating abstinence from alcohol and cocaine abuse. This double-blind, placebo-controlled outpatient trial tested topiramate for treating methamphetamine addiction.
Design
Participants (N=140) were randomized to receive topiramate or placebo (13 weeks) in escalating doses from 50 mg/day to the target maintenance of 200 mg/day in weeks 6–12 (tapered in week 13). Medication was combined with weekly brief behavioral compliance enhancement treatment.
Setting
The trial was conducted at eight medical centers in the United States.
Participants
One hundred forty methamphetamine-dependent adults took part in the trial.
Measurements
The primary outcome was abstinence from methamphetamine during weeks 6 – 12. Secondary outcomes included use reduction versus baseline, as well as psychosocial variables.
Findings
In the intent-to-treat analysis, topiramate did not increase abstinence from methamphetamine during weeks 6–12. For secondary outcomes, topiramate reduced weekly median urine methamphetamine levels and observer-rated severity of dependence scores significantly. Subjects with negative urine before randomization (N=26) had significantly greater abstinence on topiramate versus placebo during study weeks 6–12. Topiramate was safe and well tolerated.
Conclusions
Topiramate does not appear to promote abstinence in methamphetamine users but can reduce the amount taken and reduce relapse rates in those who are already abstinent.
Keywords: topiramate, methamphetamine abuse, abstinence facilitation, treatment
Introduction
After a positive finding in a proof-of-concept study of topiramate in alcoholics [1] and a successful multi-site, placebo-controlled, randomized trial of topiramate for alcohol dependence [2], topiramate was considered an appropriate candidate for treating stimulant abuse. In a placebo-controlled pilot study, topiramate was effective at reducing cocaine use after the full dose of topiramate was achieved in week 8 [3].
Two potential mechanisms of action are relevant to the treatment of stimulant abuse. Topiramate facilitates GABAergic function through a non-benzodiazepine site on the gamma-aminobutyric acid-A (GABAA) receptor, depressing cortico-mesolimbic dopaminergic activity. Pharmacologically increasing GABA concentration has been shown to block cocaine self-administration in an animal model [4]. Topiramate also antagonizes glutaminergic activity through an effect at kainate/alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors [5]. Experimentally, blocking glutamate through the kainate receptor reduced reinstatement of drug-seeking behavior [6]. Anticonvulsants with GABAergic properties have been shown to treat craving for alcohol [1], nicotine [7], and cocaine [3], and eating disorders [8].
The potential of topiramate for facilitating abstinence from methamphetamine is less clear. A study in rats of conditioned place preference for methamphetamine demonstrated no topiramate effect [9], but other models of drug-seeking behavior have not been tested. In a clinical case, however, topiramate treatment aided successfully the abstinence of a 3,4-methylenedioxymethamphetamine abuser and blocked his euphoria [10].
The need to expand upon previous clinical trials and findings on topiramate’s mechanisms of action led us to conduct a multi-site clinical trial investigating topiramate’s potential to facilitate abstinence from methamphetamine.
Methods
This study was a placebo-controlled randomized trial of daily oral topiramate in methamphetamine-dependent adults. Under an inter-agency agreement between the National Institute on Drug Abuse and the Veterans Affairs (VA) Cooperative Studies Program, eight medical centers participated: University of Virginia (Charlottesville), UCLA (Los Angeles), START Research and Treatment (Kansas City), University of Hawaii (Honolulu), South Bay Treatment Center (San Diego), Iowa Lutheran Hospital (Des Moines), Matrix Institute (West Los Angeles), and Salt Lake City Health Care System, Department of Veterans Affairs (Salt Lake City). The sites’ Institutional Review Boards and the VA Human Rights Committee approved the protocol and conduct of the study. The principal investigator was Prof. Bankole Johnson.
Study Design
One hundred forty Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) [11]–diagnosed methamphetamine-dependent individuals ≥18 years of age were randomized into two treatment groups: topiramate (N=69) and placebo (N=71). After providing written informed consent, they were screened for up to 14 days, and if meeting the eligibility criteria, they began 14 days of baseline assessment. Exclusion criteria included serious medical illness, psychiatric conditions requiring ongoing medication, pregnancy or lactation, nephrolithiasis or renal impairment, and court-mandated drug abuse treatment. Screening and baseline assessments were completed during the 28 days before randomization. Subjects had to provide ≥1 methamphetamine-positive urine specimen (>500 ng/ml) during screening and ≥4 urine specimens during completion of other baseline assessments. All candidates had a Structured Clinical Interview for Axis I disorders according to DSM-IV criteria [12], Montgomery-Asberg Depression Rating Scale assessment [13], physical examination, 12-lead electrocardiogram, electrolytes and liver enzymes, complete blood count, urinalysis, urine pregnancy test of female subjects, and tuberculin (purified protein derivative) skin test or chest X-ray completed during screening.
Study medication was randomized in a 1:1 ratio of daily oral topiramate or matched placebo. Adaptive randomization was balanced on investigational site and positive or negative methamphetamine use within 7 days before randomization, according to self-report and/or urine sample.
Once during screening and once per week during the treatment phase, all subjects received brief behavioral compliance enhancement treatment (BBCET), a manual-driven, low-intensity supportive program to promote compliance with the study medication and continuation in the study. BBCET’s use was based on compliance with treatment in alcoholics, where historical comparison to more cognitive-intensive behavioral therapy suggested comparable efficacy [14].
Treatment was conducted over 13 weeks. Commercially available topiramate (Topamax®; Ortho-McNeil Neurologics, Titusville, NJ) and matched placebo were film-coated and distributed from a central pharmacy (VA Cooperative Studies Program, Albuquerque, NM). At randomization, oral topiramate or placebo was initiated at 25 mg/day and escalated over the first 35 days of the study until 200 mg/day or the subject’s maximum tolerated dose was achieved. Over weeks 6–12, this dose was maintained. However, if a subject was intolerant of side effects, the investigator could reduce the daily dose once during maintenance, to the highest previously tolerated dose. The subject had to take ≥50 mg/day to remain in the study. Over the last week of treatment (week 13), the dose was tapered to 100 mg/day for 3 days, 50 mg/day for 2 days, and then 25 mg/day for 2 days. Medication compliance was measured by pill count. A final follow-up assessment was conducted approximately 28 days after treatment completion.
Prior and concomitant medication use, self-report of substances used, and a urine drug screen for substances of abuse were assessed during screening, baseline, and treatment. Urine samples were screened for methamphetamine and amphetamines in a central laboratory by radioimmunoassay with a minimal sensitivity of 300 ng/ml. Positive samples were assayed by gas chromatography/mass spectrometry with a methamphetamine quantification of ≥78 ng/ml.
Data Analysis
The primary outcome assessment was negative “methamphetamine use weeks” during study weeks 6–12. Urine samples were collected from subjects three times per week. Methamphetamine use was based on the qualitative urine screen performed at a central laboratory for methamphetamine and amphetamines. “Use week” was defined as each 7-day period starting with the first day of topiramate administration. A positive use week was any week in which ≥1 qualitative urine drug screen for methamphetamine was positive. A negative use week was any week in which all qualitative urine drug screens for methamphetamine were negative, even if only 1 or 2 urine samples were collected and tested. If and only if no drug screening results were available, the data for that week were considered missing.
A generalized estimating equations (GEE) model was used to analyze the primary outcome for study weeks 6–12. The GEE model included methamphetamine use week as the dependent variable (1=use week, 0=non-use week), treatment group, study week as the time variable, and the first-order interaction term between treatment and study week.
Per the protocol, secondary analyses of use reduction were conducted on the intent-to-treat population, including measures of weekly methamphetamine use by urine assays or self-report, abstinence for 21 days anytime during the treatment period, relapse rate for subjects with negative urine at randomization, overall methamphetamine use reduction during treatment compared with each individual’s historical self-report during screening, and quantitative reductions in amount of methamphetamine measured in weekly urine samples. GEE models, Fisher’s exact tests, and Cox proportional hazards models were used, where appropriate, for the secondary analyses. For each variable, the GEE model was the same as that applied for the primary outcome variable. Psychological effects of treatment were assessed using the Clinical Global Impression Scale-Observer and -Self (CGI-O and CGI-S), Brief Substance Craving Scale (BSCS), and Addiction Severity Index-Lite (ASI-Lite). Study retention from randomization to last study visit was compared between groups by log-rank test. Exploratory analyses were conducted to examine the influence of alcohol dependence, severity of methamphetamine use at entry, and dose of medication achieved.
Results
Subjects
Of 338 subjects screened, 193 were ineligible. Major reasons for exclusion included inability to comply with study requirements, failure to provide one positive urine sample during the screening period, failure to provide four urine samples for testing during the baseline period, dependence on other psychoactive substances besides methamphetamine, nicotine, or marijuana, significant psychiatric history or other medical problems, and failure to return to complete screening assessments. Five of the 145 eligible subjects declined to participate, leaving 140 who were randomized equally between treatment groups (Figure 1).
Figure 1.
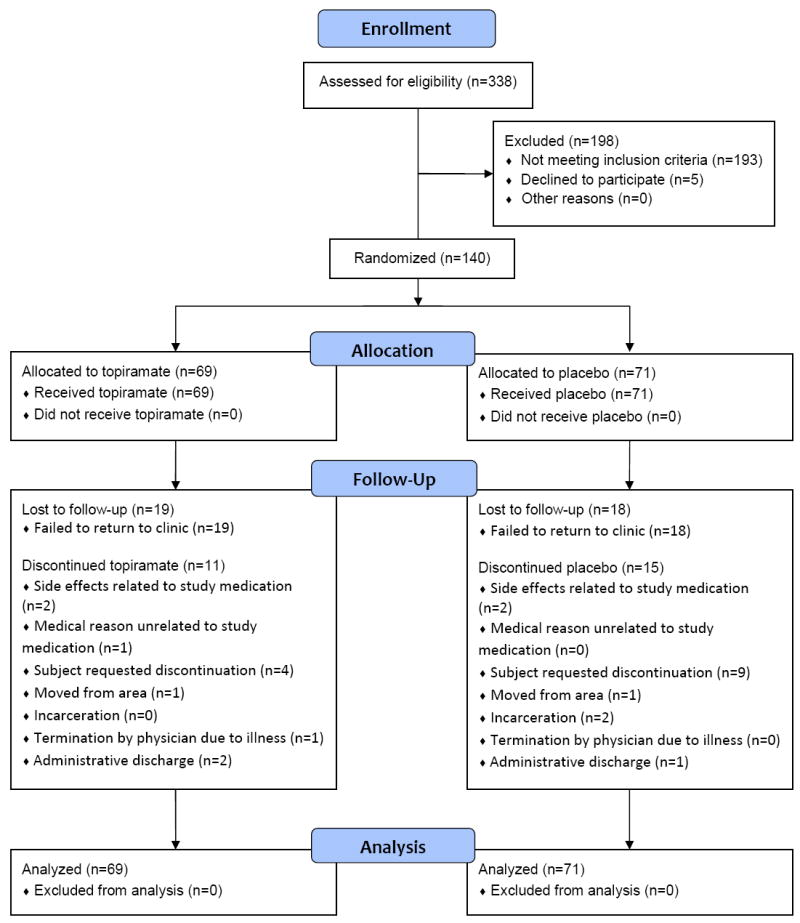
Trial flow diagram.
The two groups were well matched demographically (Table 1). The topiramate group had a slightly higher number of women, but the difference from the placebo group was not statistically significant. No statistically significant difference existed between treatment groups in mean number of days of methamphetamine use during the 30 days before informed consent—21.2±8.98 (mean±SD) days and 21.4±7.84 days for topiramate and placebo, respectively.
Table 1.
Demographics of randomized subjects.
| Topiramate | Placebo | Total | |
|---|---|---|---|
| Gender: N (%) | |||
| Male | 41 (59.4) | 48 (67.6) | 89 (63.6) |
| Female | 28 (40.6) | 23 (32.4) | 51 (36.4) |
| Age (yr) | |||
| Mean (SD) | 38.4 (8.83) | 37.5 (8.47) | 38.0 (8.62) |
| Median | 39 | 37 | 38 |
| Range | 19–59 | 18–58 | 18–59 |
| Race: N (%) | |||
| White | 59 (85.5) | 57 (80.3) | 116 (82.9) |
| Black/African-American | 0 (0.0) | 3 (1.2) | 3 (2.1) |
| Asian | 1 (1.5) | 1 (1.4) | 2 (1.4) |
| Hawaiian/Native-American/Other | 8 (13.2) | 10 (14.1) | 18 (12.9) |
| Unknown | 1 (1.5) | 0 (0.0) | 1 (0.7) |
| Employment: N (%) | |||
| Full-time | 32 (46.4) | 29 (40.9) | 61 (43.6) |
| Part-time | 20 (29) | 15 (21.1) | 35 (25.0) |
| Student | 0 (0) | 2 (2.8) | 2 (1.4) |
| Retired/disability | 1 (1.5) | 4 (5.6) | 5 (3.6) |
| Unemployed | 16 (23.2) | 18 (25.4) | 34 (24.3) |
| Other | 0 (0) | 3 (4.2) | 3 (2.1) |
| Marital status: N (%) | |||
| Married/cohabiting | 20 (29.0) | 13 (18.3) | 33 (23.6) |
| Widowed/separated/divorced | 22 (31.9) | 23 (32.4) | 45 (32.1) |
| Never married | 27 (39.1) | 35 (49.3) | 62 (44.3) |
| Education (yr) | |||
| Mean years (SD) | 12.8 (1.74) | 13.1 (1.95) | 12.9 (1.85) |
| Median | 12.0 | 13.0 | 12.0 |
| Range | 8–16 | 8–18 | 8–18 |
| Self-report of methamphetamine use in last 30 days | |||
| Mean days (SD) | 21.2 (8.98) | 21.4 (7.84) | 21.3 (8.39) |
| Median | 25.0 | 23.0 | 23.5 |
| Range | 1–30 | 4–30 | 1–30 |
Study Retention
Seventy-seven of 140 randomized subjects completed 12 weeks and had ≥1 visit in week 13 (Figure 2). Of these, 39 were topiramate recipients and 38 received placebo. Attrition among placebo recipients was greater until around week 4, after which dropout among topiramate recipients was greater, causing retention in both groups to be similar by the end of the study. At week 6, retention was 70%; this decreased weekly to 55% by week 12. The most common reason for dropout was failure to return to the clinic (19 topiramate, 18 placebo). Two subjects in each group dropped out because of reported side effects or toxicity related to study medication. Differences between groups in total dropout rate were not statistically significant (log-rank p-value=0.72).
Figure 2.
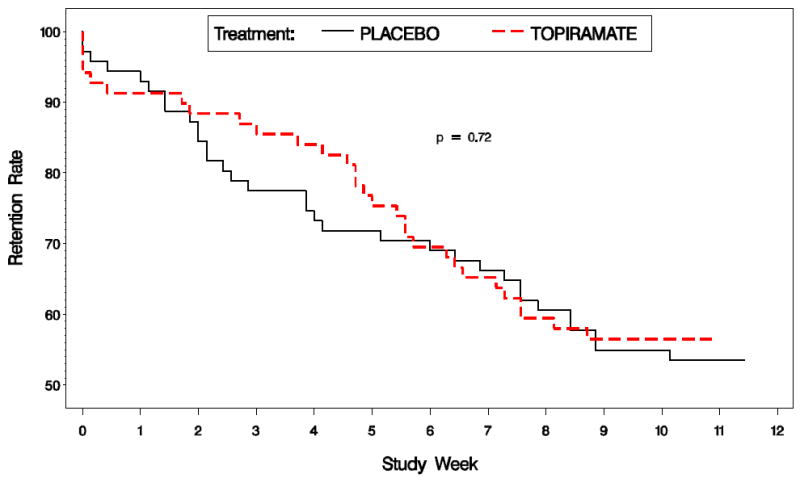
Study retention for the topiramate and placebo groups.
Compliance
Medication compliance rate was the total dose (mg) dispensed minus total dose returned divided by recommended dose, multiplied by 100. Mean compliance rate was 69.8%±40.8 for topiramate and 67.4%±43.2 for placebo, with no significant difference between groups.
Outcomes
Figure 3 represents the primary outcome variable, percentage of subjects with a negative methamphetamine use week during study weeks 6–12 by treatment group. The GEE model used to analyze the primary outcome for study weeks 6–12 included methamphetamine use week as the dependent variable, treatment group, study week as the time variable, and the first-order interaction term between treatment and study week. No significant difference existed between treatment groups over weeks 6–12 (p=0.13). A secondary analysis of the primary outcome variable was adjusted for additional covariates. Abstinence or use at baseline (p=0.001), age (p=0.032), and race (p=0.008) were significant and remained in the model; however, despite adjustment for these covariates, the difference remained non-significant between groups. When the outcome was expanded to include study weeks 1–12, the percentage of topiramate recipients with a negative use week rose from 20% at week 1 to 40% at week 12, but this change remained non-significant compared with placebo.
Figure 3.
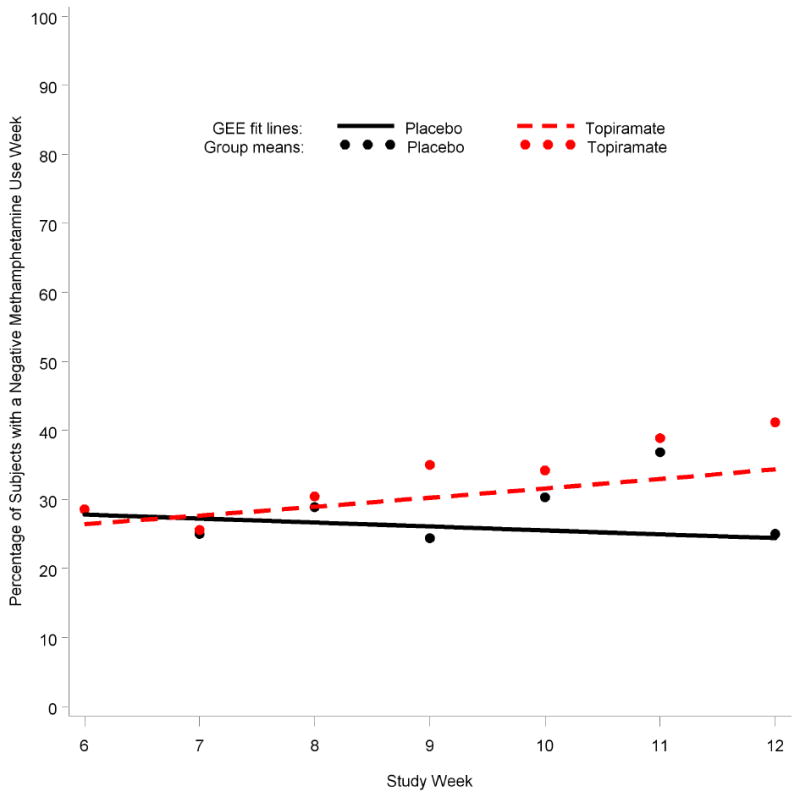
Percentage of subjects with a negative methamphetamine use week during weeks 6–12 for topiramate (N=69) and placebo (N=71). Generalized estimating equations (GEE) result for treatment over weeks 6–12 (p=0.13).
Some secondary measures of use reduction in the whole population supported a topiramate treatment effect. When use reduction was measured by weekly median quantitative methamphetamine urine level, significantly more topiramate versus placebo recipients (64.2% vs. 42.3%; Fisher’s exact test, p=0.03) reduced their use by ≥25% of the baseline rate during weeks 6–12. For weeks 1–12, a trend in favor of topiramate versus placebo (53.6% vs. 36.6%; p=0.06) was seen for a reduction by ≥25% of urine baseline concentrations and was significant for a reduction to ≤50% of baseline (42.0% vs. 25.4%; p=0.05). A ≥50% reduction of use by self-report also favored a significant topiramate treatment effect versus placebo for both the entire treatment period (37.9% vs. 14.3%; p=0.003) and weeks 6–12 (49.1% vs. 26.9%; p=0.027).
The evaluable population comprised randomized subjects who contributed ≥6 usable on-study urine samples and took ≥50 mg/day of topiramate (or equivalent placebo) for 21 days. Of the 140 randomized subjects, 111 (79.3%) were evaluable (58 topiramate, 53 placebo). The placebo group was 71% male, versus 57% of topiramate recipients. No other demographic differences existed between groups. Results in the evaluable population were similar to those in the intent-to-treat analysis for weeks 6–12 and 1–12; no difference existed between placebo and topiramate in percentage of subjects with a negative use week.
Psychometrics
Topiramate recipients experienced an improvement in observer-rated global severity of dependence, measured by CGI-O (p=0.03). For self-rated global severity of dependence (CGI-S), a difference was observed at baseline but not over time between treatment groups. Topiramate showed a trend toward decreasing craving over time, measured by BSCS (p=0.09). There were no significant changes in any categories of ASI-Lite between treatment groups and no treatment effect on Montgomery-Asberg Depression Rating Scale scores or HIV Risk-Taking Behavior Scale scores.
Exploratory Analysis
Several post hoc exploratory analyses of the data were conducted. We investigated the possible influences of underlying factors such as alcohol dependence, severity of methamphetamine use at entry, and dose of medication achieved. History of alcohol dependence at baseline had no effect on primary outcome. Treatment group differences were analyzed based on self-reported frequency of use during the 30 days before informed consent, and on whether the last urine obtained before randomization was positive or negative, which is a good proxy for severity of dependence [15].
When primary outcome was examined based on whether the last urine obtained before randomization was positive or negative, subjects in both groups were more successful during weeks 6–12 if their last urine before randomization was negative (Figure 4). Additionally, while subjects in both groups who were negative at randomization were not different at 6 weeks, a sustained treatment effect for topiramate was seen in weeks 6–12 (p=0.02).
Figure 4.
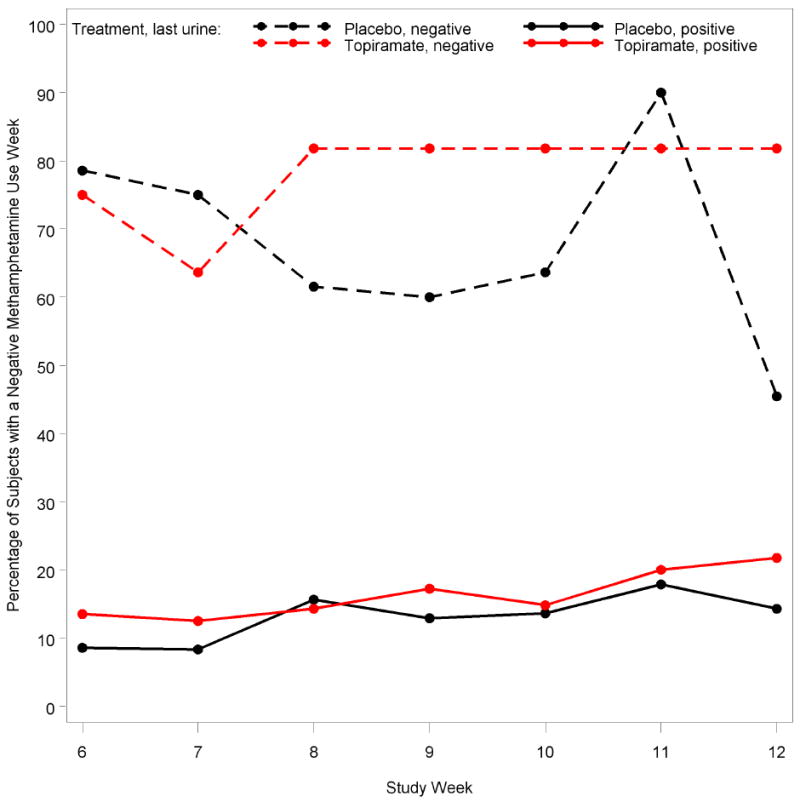
Treatment group and last urine result prior to randomization for the percentage of subjects with a negative methamphetamine use week in study weeks 6–12. At week 6, the total sample size of subjects with a negative baseline urine test for methamphetamine use was 26, with 13 in the placebo group and 13 in the topiramate group. At week 6, the total sample size of subjects with a positive baseline urine test for methamphetamine use was 78, with 38 in the placebo group and 40 in the topiramate group.
Because this protocol allowed adjustment of the total medication dose, we quantified whether subjects reached and maintained the target dose (200 mg/day). The majority reached a mean maintenance dose of 150 mg/day during weeks 6–12. Only six subjects achieved and maintained the target dose of topiramate or placebo equivalent during all 49 days of treatment in weeks 6–12.
Safety
Sixty-three topiramate recipients (91%) and 64 placebo recipients (90%) experienced adverse events during the trial. The most frequently reported complaint was headache, in 48% of topiramate subjects and 42% of placebo subjects. Other common complaints were: fatigue, reported in 29% of topiramate subjects and 20% of placebo subjects; paresthesia, in 26% and 6%, respectively; cough, in 23% and 15%; attention disturbance, in 17% and 15%; nausea, in 16% and 13%; diarrhea, in 16% and 11%; dizziness, in 13% and 7%, and dysgeusia, in 16% and 4%, respectively. Of all adverse events, only the differences between groups for paresthesia and dysgeusia were statistically significant. Eye-related complaints and visual disturbances were infrequent in both groups, with eye and vision disorders experienced by 25% of topiramate and 15% of placebo recipients (difference not statistically significant). Thirteen serious adverse events occurred and were resolved by the conclusion of the trial. Only one serious adverse event was categorized as “possibly” and one as “probably” related to the study medication; both were in placebo recipients.
Discussion
Topiramate was not different from placebo in achieving the primary efficacy goal of abstinence from methamphetamine use in those who remained in the study during weeks 6–12. Positive findings on secondary variables showed that topiramate versus placebo reduced methamphetamine use over time.
Topiramate showed efficacy at reducing methamphetamine use in those who were abstinent at the trial’s outset. In this secondary analysis, 26 subjects (evenly divided between the placebo and topiramate groups) were abstinent at the trial’s outset. With the caveat that these findings were observed in a subset of the total cohort, it is reasonable to propose that topiramate should be considered for relapse prevention rather than simply being targeted to decrease methamphetamine use in current users. In clinical practice, this has the strong rationale of asking patients to be abstinent briefly before commencing medication treatment. Another advantage is that subjects who can manage this short period of abstinence before starting treatment might also be more likely to comply with the medication regimen and maintain higher doses.
Topiramate recipients also were significantly more likely to achieve a 50% or 25% reduction in baseline level of methamphetamine use, suggesting that even when topiramate treatment did not lead to abstinence, it was associated with a significant decrease in risk of harm from methamphetamine use. This was underscored by the observer-rated clinical impression of a global reduction in the severity of methamphetamine dependence in topiramate recipients.
Our study had four important limitations. First, similar to previous pharmacotherapy studies with methamphetamine, attrition after the first 6 weeks of treatment was relatively high, thereby limiting our potential to identify differences between active treatment and placebo. We view this as characteristic of the target population, who are often migratory or whose disease state perhaps is associated with greater dissociation from society than those who abuse other psychostimulants or alcohol. Nevertheless, the compliance enhancement treatment ensured that attrition was no different for topiramate versus placebo despite the higher level of adverse events with topiramate.
Second, unexpectedly, few subjects achieved the maximum target dose of 200 mg/day. Indeed, the majority of subjects took ≤150 mg daily, which might have reduced our ability to demonstrate stronger therapeutic effects. Probably, this occurred because the study protocol allowed liberal downward dose titration of topiramate, thereby inadvertently encouraging the use of the lower topiramate doses. Since alcoholics have tolerated and responded to topiramate at 300 mg/day, the question arises as to whether the final dose that we chose was sufficient. The target dose of 200 mg/day was the same dose that Kampman et al. used in their pilot study of cocaine abstinence [3]; Johnson et al. later studied it as the maximum dose for safety with methamphetamine [16]. An additional consideration is the unintended heterogeneity of doses that the subjects—received a common problem in medication studies. Although the medication compliance rate of 70% is higher than that achieved in previous pharmacotherapy studies with methamphetamine [17-19], the use of pill count to monitor compliance is fraught with inaccuracy. Medication studies must incorporate specific markers of medication compliance, including blood level monitoring and riboflavin (for both active and placebo medication), to avoid inconclusive results.
The third limitation concerns the effect of topiramate, a mild carbonic anhydrase inhibitor, on the metabolism and excretion of methamphetamine. Urinary filtration of methamphetamine is enhanced by acidification of urine and impeded by alkalinization. This pharmacokinetic mechanism has been demonstrated experimentally by intravenous administration of either an alkalinizing or acidifying agent [20]. However, carbonic anhydrase inhibition simultaneously acidifies blood and alkalinizes urine. A rise of urine pH should reduce methamphetamine elimination, causing higher blood levels of methamphetamine to be sustained, but the downward pH shift in the plasma should also accelerate methamphetamine’s metabolism. Johnson et al.’s interaction study [16] suggests that methamphetamine plasma concentrations increase slightly in topiramate’s presence, possibly a net effect of reduced urinary excretion. Due to topiramate’s mild effect on carbonic anhydrase, its net effect on methamphetamine’s metabolism and elimination kinetics may not be significant; on the other hand, the net effect of a pH shift on methamphetamine’s concentration gradient across the blood–brain barrier is unknown. Whether topiramate exerts an unusual pharmacokinetic effect on methamphetamine has an important implication for chronic therapy because although users are consuming less methamphetamine, urine sampling might underestimate the extent of this reduction.
Fourth, from the small therapeutic effect evidenced by this study, a double-blind controlled trial of ampler sample size, testing higher doses, is needed for more definitive conclusions.
In conclusion, topiramate at doses up to 200 mg/day was safely tolerated by currently using methamphetamine-dependent individuals in this study, but its efficacy in helping them achieve abstinence was not supported. Nonetheless, some use reduction variables were indicative of an effect by topiramate. As seen in some other medication studies, a subgroup of “light” users are more responsive to topiramate, possibly because they are more likely to adhere to study requirements, or perhaps because we administered a barely therapeutic dose. Topiramate’s utility in preventing relapse in those who have ceased methamphetamine use should be explored further.
Acknowledgments
We are grateful to the National Institute on Drug Abuse for its generous support through the Department of Veterans Affairs Cooperative Studies Program (Interagency Agreement No. Y1-DA4006). We also thank Robert H. Cormier, Jr., B.A., for his assistance with manuscript preparation.
Footnotes
Declaration of interest: E. Yu serves as a member of the scientific advisory board for US WorldMeds, LLC. C. Gorodetzky serves as a consultant to Catalyst Pharmaceutical Partners, Inc., Helicon Therapeutics, Inc., and US WorldMeds, LLC. M. D. Li serves as a scientific advisor to ADial Pharmaceuticals LLC. B. A. Johnson has served as a consultant to Johnson & Johnson (Ortho-McNeil Janssen Scientific Affairs, LLC), Transcept Pharmaceuticals, Inc., D&A Pharma, Organon, ADial Pharmaceuticals LLC, Psychological Education Publishing Company (PEPCo LLC), and Eli Lilly and Company. A. Elkashef, R. Kahn, E. Iturriaga, S.-H. Li, A. Anderson, N. Chiang, N. Ait-Daoud, D. Weiss, F. McSherry, T. Serpi, R. Rawson, M. Hrymoc, D. Weis, M. McCann, T. Pham, C. Stock, R. Dickinson, J. Campbell, W. Haning, B. Carlton, and J. Mawhinney report no competing interests.
References
- 1.Johnson BA, Ait-Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361:1677–1685. doi: 10.1016/S0140-6736(03)13370-3. [DOI] [PubMed] [Google Scholar]
- 2.Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, et al. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007;298:1641–1651. doi: 10.1001/jama.298.14.1641. [DOI] [PubMed] [Google Scholar]
- 3.Kampman KM, Pettinati H, Lynch KG, Dackis C, Sparkman T, Weigley C, et al. A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Depend. 2004;75:233–240. doi: 10.1016/j.drugalcdep.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Kushner SA, Dewey SL, Kornetsky C. The irreversible gamma-aminobutyric acid (GABA) transaminase inhibitor gamma-vinyl-GABA blocks cocaine self-administration in rats. J Pharmacol Exp Ther. 1999;290:797–802. [PubMed] [Google Scholar]
- 5.Gryder DS, Rogawski MA. Selective antagonism of GluR5 kainate-receptor-mediated synaptic currents by topiramate in rat basolateral amygdala neurons. J Neurosci. 2003;23:7069–7074. doi: 10.1523/JNEUROSCI.23-18-07069.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.García Campayo J, Sobradiel N, Alda M, Mas A, Andrés E, Magallon R, et al. Effectiveness of topiramate for tobacco dependence in patients with depression; a randomised, controlled trial. BMC Fam Pract. 2008;9:28. doi: 10.1186/1471-2296-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leombruni P, Lavagnino L, Fassino S. Treatment of obese patients with binge eating disorder using topiramate: a review. Neuropsychiatr Dis Treat. 2009;5:385–392. doi: 10.2147/ndt.s3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tatsuta T, Kitanaka N, Kitanaka J, Morita Y, Takemura M. Lack of effect of anticonvulsant topiramate on methamphetamine-induced stereotypy and rewarding property in mice. Pharmacol Biochem Behav. 2007;87:48–55. doi: 10.1016/j.pbb.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 10.Akhondzadeh S, Hampa AD. Topiramate prevents ecstasy consumption: a case report. Fundam Clin Pharmacol. 2005;19:601–602. doi: 10.1111/j.1472-8206.2005.00355.x. [DOI] [PubMed] [Google Scholar]
- 11.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, D.C.: American Psychiatric Association; 1994. [Google Scholar]
- 12.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders — Patient Edition (SCID-I/P, Version 2.0) New York: New York State Psychiatric Institute, Biometrics Research Department; 1994. [Google Scholar]
- 13.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 14.Johnson BA, DiClemente CC, Ait-Daoud N, Stoks SM. Brief Behavioral Compliance Enhancement Treatment (BBCET) manual. In: Johnson BA, Ruiz P, Galanter M, editors. Handbook of Clinical Alcoholism Treatment. Baltimore, MD: Lippincott Williams & Wilkins; 2003. pp. 282–301. [Google Scholar]
- 15.Kampman KM, Volpicelli JR, Mulvaney F, Rukstalis M, Alterman AI, Pettinati H, et al. Cocaine withdrawal severity and urine toxicology results from treatment entry predict outcome in medication trials for cocaine dependence. Addict Behav. 2002;27:251–260. doi: 10.1016/s0306-4603(01)00171-x. [DOI] [PubMed] [Google Scholar]
- 16.Johnson BA, Wells LT, Roache JD, Wallace CL, Ait-Daoud N, Dawes MA, et al. Kinetic and cardiovascular effects of acute topiramate dosing among non-treatment-seeking, methamphetamine-dependent individuals. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:455–461. doi: 10.1016/j.pnpbp.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson BA, Ait-Daoud N, Elkashef AM, Smith EV, Kahn R, Vocci F, et al. A preliminary randomized, double-blind, placebo-controlled study of the safety and efficacy of ondansetron in the treatment of methamphetamine dependence. Int J Neuropsychopharmacol. 2008;11:1–14. doi: 10.1017/S1461145707007778. [DOI] [PubMed] [Google Scholar]
- 18.Shearer J, Darke S, Rodgers C, Slade T, van Beek I, Lewis J, et al. A double-blind, placebo-controlled trial of modafinil (200 mg/day) for methamphetamine dependence. Addiction. 2009;104:224–233. doi: 10.1111/j.1360-0443.2008.02437.x. [DOI] [PubMed] [Google Scholar]
- 19.Elkashef AM, Rawson RA, Anderson AL, Li SH, Holmes T, Smith EV, et al. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33:1162–1170. doi: 10.1038/sj.npp.1301481. [DOI] [PubMed] [Google Scholar]
- 20.Davis JM, Kopin IJ, Lemberger L, Axelrod J. Effects of urinary pH on amphetamine metabolism. Ann N Y Acad Sci. 1971;179:493–501. doi: 10.1111/j.1749-6632.1971.tb46926.x. [DOI] [PubMed] [Google Scholar]


