Abstract
Activation of renal dopamine D1 (D1R) and angiotensin II type 1 receptors (AT1Rs) influences the activity of proximal tubular sodium transporter Na,K-ATPase and maintains sodium homeostasis and blood pressure. We reported recently that diminished D1R and exaggerated AT1R functions are associated with hypertension in old Fischer 344 × Brown Norway F1 (FBN) rats, and oxidative stress plays a central role in this phenomenon. Here we studied the mechanisms of age-associated increase in oxidative stress on diminished D1R and exaggerated AT1R functions in the renal proximal tubules of control and antioxidant Tempol-treated adult and old FBN rats. Although D1R numbers and D1R agonist SKF38393-mediated stimulation of [35S]-GTPγS binding (index of D1R activation) were lower, G protein– coupled receptor kinase 4 (kinase that uncouples D1R) levels were higher in old FBN rats. Tempol treatment restored D1R numbers and G protein coupling and reduced G protein– coupled receptor kinase 4 levels in old FBN rats. Angiotensin II–mediated stimulation of [35S]-GTPγS binding and Na,K-ATPase activity were higher in old FBN rats, which were also restored with Tempol treatment. We also measured renal AT1R function in adult and old Fischer 344 (F344) rats, which, despite exhibiting an age-related increase in oxidative stress and diminished renal D1R function, are normotensive. We found that diuretic and natriuretic responses to candesartan (indices of AT1R function) were similar in F344 rats, a likely explanation for the absence of age-associated hypertension in these rats. Perhaps, alterations in both D1R (diminished) and AT1R (exaggerated) functions are necessary for the development of age-associated hypertension, as seen in old FBN rats.
Keywords: aging, oxidative stress, sodium, dopamine, angiotensin, blood pressure
The counterregulatory modulation of sodium excretion by natriuretic dopamine and antinatriuretic angiotensin II (Ang II) plays a vital role in regulating blood pressure.1 Dopamine exerts its natriuretic action primarily via activation of D1-like receptors (composed of D1R and D5R subtypes) and contributes to ≈60% of sodium excretion during increased sodium intake.2–4 In contrast, Ang II mediates its antinatriuretic effects via Ang II type 1 receptors (AT1Rs), causing an increase in sodium reabsorption.5–7 Although impairments in natriuretic and diuretic responses to D1-like receptor agonists are linked to hypertension in humans and rodents,8–14 altered functioning of AT1R has also been linked to various forms of hypertension.15,16
Blood pressure increases with advancing age17–19 and may account for various cardiovascular disorders in the aging population.18 A host of structural and functional changes in the kidney accompanies the aging process, which is often associated with an inability of the kidney to regulate sodium balance.20,21 Aging is also associated with increase in oxidative stress22 and alterations in renal D1R23,24 and AT1R25 functions. In hypertensive animal models, like spontaneously hypertensive and obese Zucker rats, which also exhibit increased oxidative stress, altered renal D1R and AT1R functions have been reported.9,26–29
We recently reported a causal role of oxidative stress in diminished D1R-mediated natriuretic response, exaggerated AT1R-mediated antinatriuretic response, and high blood pressure in old Fischer 344 × Brown Norway F1 (FBN) rats.30 However, the underlying mechanisms for these oxidative stress-mediated effects on D1R and AT1R functions in old FBN rats are not known. We hypothesized that age-associated increase in oxidative stress impairs D1R and AT1R signaling in the renal proximal tubules (RPTs), thereby causing diminished D1R and exaggerated AT1R functions contributing to high blood pressure in old FBN rats. To test this hypothesis, we treated adult (3-month) and old (21-month) FBN rats without (control) and with antioxidant Tempol and studied D1R and AT1R signaling cascade in the RPTs of these rats using radioligand, Western blotting, and various biochemical methodologies.
We also investigated age-related changes in renal AT1R function in response to candesartan (AT1R antagonist) in adult (6-month) and old (24-month) Fischer 344 (F344) rats, another rat aging model. This experiment was warranted to test our hypothesis, because, despite exhibiting age-associated increase in oxidative stress and impaired renal D1R function, these rats do not develop high blood pressure with aging.
Methods
Animals
Adult (3-month) and old (21-month) male FBN rats raised by Harlan Laboratories (Indianapolis, IN) were purchased from the National Institute on Aging (Bethesda, MD). Adult (6-month) and old (24-month) male F344 rats raised by Taconic Farms, Inc (Hudson, NY) were also purchased from the National Institute on Aging. The rats were housed in plastic cages in the University of Houston animal care facility and were used as per the National Institutes of Health guidelines and approved protocols by the Institutional Animal Care and Use Committee. Animals had free access to standard rodent chow and drinking water.
Tempol Supplementation in FBN Rats
FBN rats were randomly divided into 4 groups, namely, control and Tempol-treated adult and old rats (adult control, adult Tempol, old control, and old Tempol). In the treated groups, Tempol (Sigma-Aldrich; 1 mmol/L) was supplemented in drinking water for a period of 3 to 4 weeks, whereas control rats received drinking water devoid of Tempol. Water supplemented with Tempol was changed twice a day to minimize its oxidation.
In Vitro Biochemical Studies in FBN Rats
Preparation of RPTs
An in situ enzyme digestion procedure was used to prepare RPTs, as described previously.31
Preparation of Renal Proximal Tubular Membranes
RPTs were homogenized using Wheaton homogenizer, and the membranes were prepared using a differential centrifugation method, as described previously.31
Measurement of Dopamine D1R Numbers and D1R-G-Protein Coupling
Radioligand [3H] SCH-23390, a selective D1 receptor antagonist, was used to determine D1R numbers. Binding of D1R with G proteins was determined in response to the D1R agonist SKF-38393. Both D1R numbers and D1R-G-protein coupling were measured on renal proximal tubular membranes and have been described in detail previously.32
Measurement of Protein Kinase C Activity
Protein kinase C (PKC) activity was measured in the RPTs (20 μg) using a commercially available kit (Enzo Life Sciences, Inc, Butler Pike Plymouth Meeting, PA) according to the manufacturer’s instructions.
Measurement of D1R, G Protein–Coupled Receptor Kinases, Gα Subunits, and AT1R Proteins in RPTs by Western Blotting
Specific antidopamine receptor D1 (1:500; Millipore, Billerica, MA), anti–G protein– coupled receptor kinase (GRK; GRK-2; 1:500; Santa Cruz Biotechnology, Santa Cruz, CA), anti–GRK-4 (1:250; Santa Cruz Biotechnology), anti-Gαq/11 (1:1000; EMD, Darmstadt, Germany), anti–Gαi-1 (1:1000; EMD), and antiangiotensin AT1R (1:200; Santa Cruz Biotechnology) antibodies were used to detect respective proteins using standard Western blotting protocol. In renal proximal tubular homogenates, β-actin (loading control) was detected using a specific anti–β-actin (1:1000) antibody (Santa Cruz Biotechnology).
Measurement of Angiotensin AT1R Coupling With G Proteins
Binding of angiotensin AT1R with G proteins in response to Ang II was determined on renal proximal tubular membranes using the [35S]-GTPγS binding protocol described previously.33
Measurement of Ang II–Mediated Stimulation of Na,K-ATPase Activity
Ang II–mediated stimulation of Na,K-ATPase (NKA) activity was determined in freshly isolated RPTs, as described previously.34
Measurement of Protein in Urine
Bladder urine samples were diluted (1:100) with deionized water, and protein levels were measured by the bicinchoninic acid method using protein assay kit (Pierce, Rockford, IL) reagents and BSA as protein standards.
In Vivo Studies in F344 Rats
Blood pressure and renal AT1R function in response to candesartan in F344 rats were measured using the same experimental protocol as in FBN rats and have been described previously.30
Data Analysis
Data are presented as mean ± SEM. One-way ANOVA, followed by Newman-Keuls post hoc test, was used to compare variations among the groups. Repeated-measures ANOVA, followed by Dunnett post hoc test, was used to compare variations (from control) within the group. Student t test was used wherever appropriate. Statistical analysis was carried out using a software program (GraphPad Prism version 5; GraphPad Software, San Diego, CA). The minimum level of significance was considered at P < 0.05.
Results
Effect of Aging on Abundance of D1R and AT1R in RPTs of FBN Rats
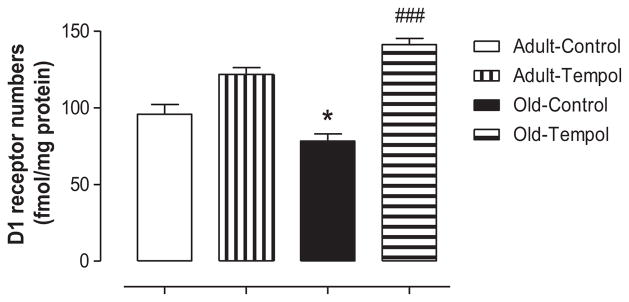
There were no differences in the levels of D1R protein in renal proximal tubular homogenates between control adult and control old rats, and they remained unchanged with Tempol treatment (Table). There was an age-related decline in D1R numbers on the renal proximal tubular membranes in control old compared with control adult rats. Treatment with Tempol restored D1R numbers in old rats (Figure 1). There were no age-associated changes in the levels of AT1R protein in both homogenates and membranes of RPTs, which also remained unchanged with Tempol treatment (Table).
Table.
Levels of Urinary Protein and Various Components in AT1R and D1R Signaling Cascades in Adult and Old FBN Rats Treated Without/With Antioxidant Tempol
| Parameters | Adult Control | Adult Tempol | Old Control | Old Tempol |
|---|---|---|---|---|
| D1R protein, density units | 39.02 ± 3.55 | 36.6 ± 4.31 | 39.59 ± 4.63 | 39.08 ± 4.36 |
| AT1R protein in homogenates, density units | 0.53 ± 0.08 | 0.65 ± 0.2 | 0.61 ± 0.1 | 0.57 ± 0.06 |
| AT1R protein in membranes, density units | 1397 ± 48.95 | 1389 ± 70.12 | 1304 ± 85.3 | 1281 ± 75.08 |
| PKC activity, relative kinase activity per mg of protein | 3.79 ± 0.11 | 3.94 ± 0.59 | 4.1 ± 0.16 | 4.1 ± 0.67 |
| GRK-2 protein, density units | 42.29 ± 1.22 | 38.22 ± 1.85 | 42.25 ± 3.5 | 42.77 ± 3.63 |
| Gαi-1 protein, density units | 1690 ± 31.18 | 1720 ± 75.36 | 1491 ± 46.2* | 1556 ± 61.66 |
| Gαq/11 protein, density units | 1657 ± 45.93 | 1592 ± 116 | 1516 ± 133 | 1582 ± 71.23 |
| Protein in urine, μg/μL | 15.63 ± 4.36 | 9.44 ± 1.03 | 29.81 ± 8.61* | 7.32 ± 0.88† |
Results are mean ± SEM. AT1R indicates angiotensin II type 1 receptor; D1R, dopamine D1 receptor; FBN, Fischer 344 × Brown Norway F1; PKC, protein kinase C; GRK, G protein– coupled receptor kinase.
Data are significantly different from adult control rats at P < 0.05.
Data are significantly different from old control rats at P < 0.05 (1-way ANOVA followed by Newman-Keuls post hoc test).
Figure 1.
Antioxidant Tempol restores age-associated decline in dopamine D1 receptor (D1R) numbers in old Fischer 344 × Brown Norway F1 (FBN) rats. Dopamine D1R numbers were measured on the renal proximal tubular membranes using radio-ligand binding assay (details in the Methods section). Results are mean ± SEM (n = 3–5 rats in each group). *Significantly different from control adult rats at P < 0.05. ###Significantly different from control old rats at P < 0.001 (1-way ANOVA followed by Newman-Keuls post hoc test).
Effect of Aging on Coupling of D1R and AT1R With G Proteins in Renal Proximal Tubular Membranes of FBN Rats
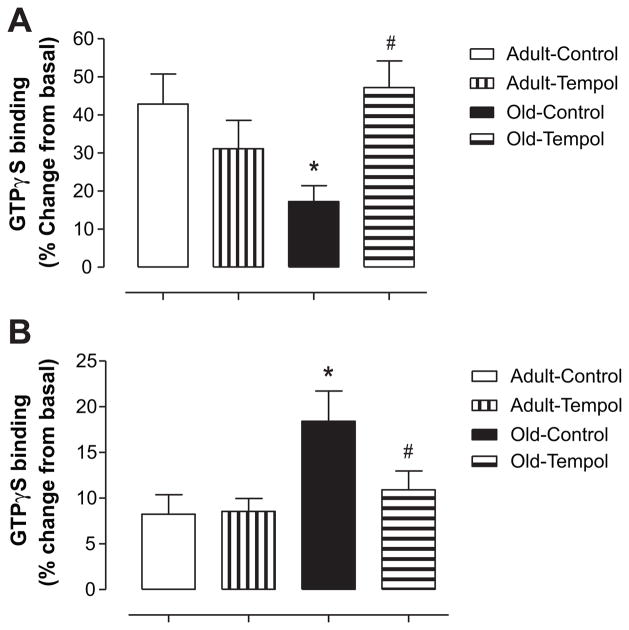
Treatment of renal proximal tubular membranes with SKF-38393 increased [35S]-GTPγS binding in control and Tempol-treated adult rats. However, the ability of SKF-38393 to stimulate [35S]-GTPγS binding was attenuated in control old rats. Tempol treatment in old rats restored SKF-38393–mediated [35S]-GTPγS binding (Figure 2A). Basal [35S]-GTPγS binding was not different between control and Tempol-treated adult and old rats (in fmol/μg: adult control, 0.26 ± 0.01; adult Tempol, 0.25 ± 0.01; old control, 0.23 ± 0.01; old Tempol, 0.24 ± 0.01). [35S]-GTPγS binding in response to Ang II increased in both control adult and old rats, however, with a much higher degree in control old rats. Tempol treatment in old rats reduced Ang II–mediated increase in [35S]-GTPγS binding to the levels seen in adult rats (Figure 2B). Basal [35S]-GTPγS binding was not different between control and Tempol-treated adult and old rats (in fmol/μg: adult control, 0.71 ± 0.06; adult Tempol, 0.61 ± 0.08; old control, 0.51 ± 0.04; old Tempol, 0.51 ± 0.06).
Figure 2.
A, Antioxidant Tempol restores age-related decline in SKF-38393–mediated [35S]-GTPγS binding (an index of D1 receptor [D1R] G-protein coupling) in old Fischer 344 × Brown Norway F1 (FBN) rats. [35S]-GTPγS binding in the absence and presence of D1R agonist SKF-38393 (10−6 mol/L) was measured on the renal proximal tubular membranes, as described in the Methods section. Results are mean ± SEM (n = 8 –9 rats in each group). *Significantly different from adult control rats at P < 0.05. #Significantly different from old control rats at P < 0.05 (1-way ANOVA followed by Newman-Keuls post hoc test). B, Antioxidant Tempol restores age-associated elevated angiotensin II (Ang II)–mediated [35S]-GTPγS binding (an index of Ang II type 1 receptor [AT1R] G-protein coupling] in old FBN rats. [35S]-GTPγS binding in the absence and presence of Ang II (10−6 mol/L) was measured on the renal proximal tubular membranes, as described in the Methods section. Results are mean ± SEM (n = 6–7 rats in each group). *Significantly different from adult control rats at P < 0.05. #Significantly different from old control rats at P < 0.05 (1-way ANOVA followed by Newman-Keuls post hoc test).
Effect of Aging on PKC Activity in RPTs of FBN Rats
The PKC activity was not different between adult and old rats and remained unchanged with Tempol treatment (Table).
Effect of Aging on Abundance of GRK-2 and GRK-4 Proteins in Renal Proximal Tubular Homogenate of FBN Rats
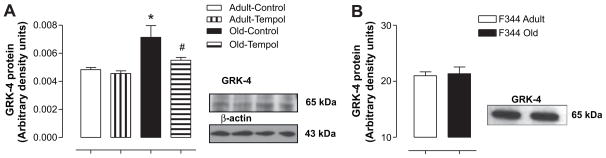
The levels of GRK-2 protein were not different between adult and old rats and remained unchanged with Tempol treatment (Table). However, whereas the levels of GRK-4 protein increased in an age-dependent manner, treatment with Tempol reduced GRK4 levels in old rats to the levels seen in adult rats (Figure 3A).
Figure 3.
A, Aging increases the levels of G protein– coupled receptor kinase (GRK) 4 protein in Fischer 344 × Brown Norway F1 (FBN) rats. Tempol treatment decreases the age-related increase in GRK-4 protein levels in old FBN rats. GRK-4 protein levels were measured in the renal proximal tubules (RPTs) using standard Western blotting techniques (details in the Methods section). The same blot was stripped off and probed for β-actin. Right, Representative blots of GRK-4 and β-actin. In lanes from left to right are adult control, adult Tempol, old control, and old Tempol. Left, Bars are ratios of the densities between GRK-4 and β-actin protein bands. Results are mean ± SEM (n = 4 –5 rats in each group). *Significantly different from adult control rats at P < 0.05. #Significantly different from old control rats at P < 0.05 (1-way ANOVA followed by Newman-Keuls post hoc test). B, There are no age-related changes in the levels of GRK-4 protein in F344 rats. GRK-4 protein levels were measured in RPTs using standard Western blotting techniques (details in the Methods section). Right, Representative blot of GRK-4 (left lane, adult; right lane, old). Left, Bars represent the densities of the GRK-4 band in adult and old rats. Results are mean ± SEM (n = 3 rats in each group).
Effect of Aging on Abundance of Gα Subunits in Renal Proximal Tubular Membranes of FBN Rats
With respect to Gαi-1 protein, there was a moderate but significant decrease in control old compared with control adult rats, which remained unchanged with Tempol treatment (Table). However, there were no age-associated changes in Gαq/11 protein levels between control adult and control old rats, which remained unchanged with Tempol treatment (Table).
Effect of Aging on Ang II–Mediated NKA Activity in RPTs of FBN Rats
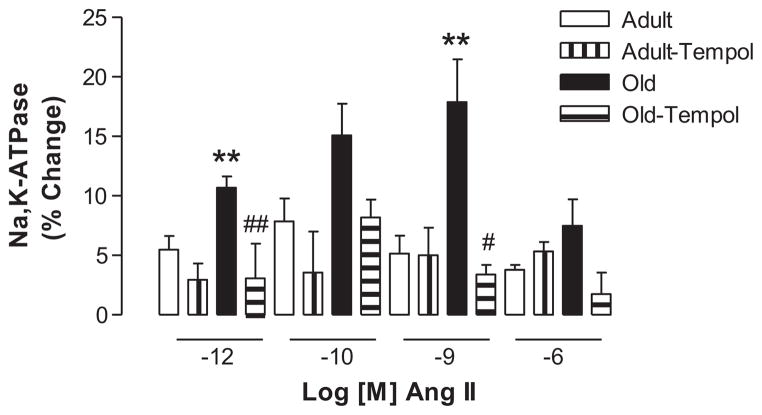
Ang II (10−12 and 10−9 mol/L) stimulated NKA activity to a greater extent in control old compared with control adult rats. Tempol treatment in old rats normalized Ang II–mediated stimulation of NKA to levels seen in adult rats. Tempol treatment had no effect on Ang II–induced stimulation of NKA in adult rats (Figure 4). The basal NKA activity (in nanomoles of Pi per milligram per minute) was not different between control adult (323.90 ± 20.33) and control old (364.70 ± 31.96) rats.
Figure 4.
Antioxidant Tempol restores age-associated exaggerated angiotensin II (Ang II)–mediated response on Na,K-ATPase (NKA) in old Fischer 344 × Brown Norway F1 (FBN) rats. NKA activity was measured in the freshly isolated renal proximal tubules (RPTs) by a colorimetric assay as described in the Methods section. Results are mean ± SEM (n = 4 –10 rats in each group). **Significantly different from adult control rats using 1-way ANOVA followed by Newman-Keuls post hoc test at P < 0.01. Significantly different from old control rats using 1-way ANOVA followed by Newman-Keuls post hoc test (#P < 0.05, ##P < 0.01).
Effect of Aging on Urinary Protein Excretion in FBN Rats
Urinary protein levels increase with age and are considered general markers of kidney dysfunction. There was an age-related increase in the levels of urinary proteins, which decreased with Tempol treatment in old rats. However, Tempol treatment did not affect the levels of urinary proteins in adult rats (Table).
Effect of Aging on Blood Pressure and Renal AT1R Function in F344 Rats
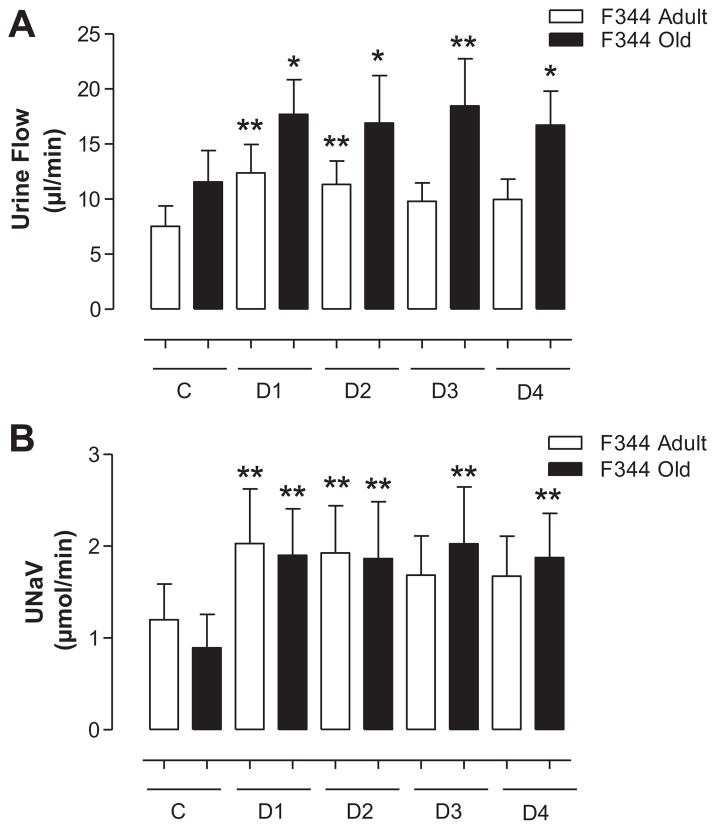
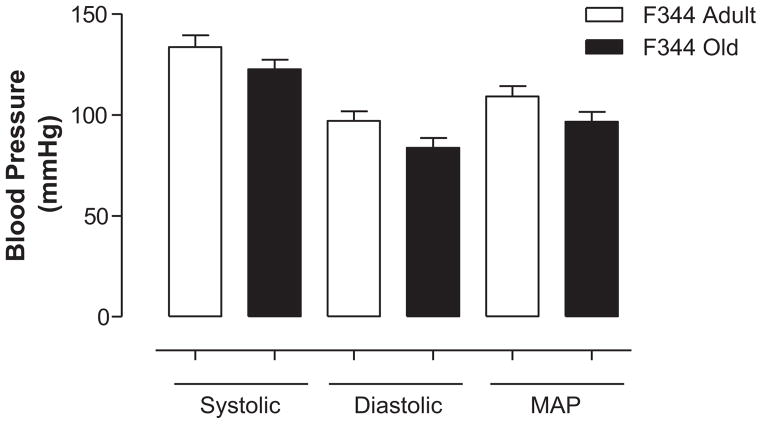
Administration of candesartan significantly increased urine flow (Figure 5A) and sodium excretion (Figure 5B) compared with basal activity in both adult and old F344 rats. However, there were no significant differences in diuretic and natriuretic responses to candesartan between adult and old rats (Figure 5A and 5B). Heart rate (in bpm: adult, 318.30 ± 7.57; old, 276.60 ± 22.67) and systolic, diastolic, and mean arterial pressures were also not different between adult and old F344 rats (Figure 6).
Figure 5.
A, There are no age-associated changes in diuretic response (urine flow) to candesartan in F344 rats. Urine flow in response to angiotensin II type 1 receptor (AT1R) antagonist candesartan (10 μg/kg IV bolus) was determined in adult and old F344 rats, as described in the Methods section. C represents the average of two 30-minute control periods where saline only was infused. D1 through D4 represent drug infusion periods (each 30 minutes) after candesartan was administered as a bolus dose. Results are mean ± SEM (n = 5–7 rats). Significantly different from C (basal) using repeated-measures ANOVA followed by the Dunnett post hoc test (**P < 0.01 and *P < 0.05). B, There are no age-associated changes in natriuretic response to candesartan in F344 rats. Urinary sodium excretion (UNaV) in response to AT1R antagonist candesartan (10 μg/kg IV bolus) was determined in adult and old F344 rats, as described in the Methods section. C represents the average of two 30-minute control periods where saline only was infused. D1 through D4 represent drug infusion periods (each 30 minutes) after candesartan was administered as a bolus dose. Results are mean ± SEM (n = 5–7 rats). Significantly different from C (basal) using repeated-measures ANOVA followed by the Dunnett post hoc test (**P < 0.01).
Figure 6.
There are no age-associated changes in blood pressure in F344 rats. Blood pressure (systolic, diastolic, and mean arterial) was measured in anesthetized adult and old F344 rats, as described in the Methods section. Results are mean ± SEM (n = 6–7 rats).
Discussion
In FBN rats, we have reported recently an age-related increase in oxidative stress and blood pressure. Although natriuretic response to D1R activation was diminished (impaired renal D1R function), increased natriuresis (exaggerated renal AT1R function) was observed in response to candesartan in old FBN rats.30 We showed a causal link between oxidative stress and age-associated changes in blood pressure and renal AT1R function, because antioxidant Tempol treatment reduced blood pressure and restored AT1R function in old FBN rats.30 Oxidative stress-mediated decline in renal D1R function with aging has also been reported in normotensive old F344 rats, another widely used rat aging model.32 The objective of the current study was to investigate biochemical mechanisms by which oxidative stress may alter renal D1R and AT1R functions, leading to the development of age-associated hypertension in FBN rats.
To examine the mechanism(s) of oxidative stress in age-related hypertension in FBN rats, we treated adult and old rats with antioxidant Tempol in drinking water. Tempol intake was slightly but significantly higher in old compared with adult FBN rats (in mL per rat per day: adult Tempol, 26.3 ± 1.12; old Tempol, 30.99 ± 1.33). Tempol is a known antioxidant with superoxide dismutase mimetic properties and has been used previously to reduce oxidative stress and high blood pressure in spontaneously hypertensive rats.35 Recently, we have shown that activation of D1R failed to increase sodium excretion in old FBN rats, suggesting an age-associated defect in renal D1R function in these animals.30 To investigate the mechanism(s) for impaired renal D1R function with aging in FBN rats, we measured the levels of D1R and its associated signaling molecules in the RPTs of control and Tempol-treated adult and old FBN rats. We found reduced D1R numbers on the renal proximal tubular membranes of old FBN rats. The levels of D1R protein in RPTs, however, were not different between adult and old FBN rats. These are interesting findings, because similar observations have been reported in hypertensive obese Zucker rats, which also have increased oxidative stress.28
G-protein coupling in response to D1R activation was reduced in old FBN rats. Antioxidant Tempol treatment in old FBN rats restored D1R G-protein coupling. The uncoupling of D1R from its G protein effector complex involves activation of GRKs.36–40 GRKs 2 to 6 have been reported to cause D1R desensitization in heterologous expression systems.38–41 Previously, we have reported that age-associated increase in oxidative stress via PKC activates proximal tubular GRK-2, resulting in D1R-G-protein uncoupling in normotensive old F344 rats.32 However, in these hypertensive old FBN rats, we did not find any changes in the levels of either PKC or GRK-2, but GRK-4 levels were elevated in RPTs of old FBN rats. Interestingly, we did not find any increase in GRK-4 levels in the RPTs of normotensive old F344 rats (Figure 3B) despite an age-related increase in oxidative stress.32 We speculate that this could be because of strain-specific differences, as well as differences in the degree of oxidative stress between these 2 rat aging models. Although PKC and GRK-2 levels remained unchanged with Tempol treatment, GRK-4 levels were reduced in RPTs of old FBN rats, suggesting oxidative stress-mediated changes in GRK-4 in these rats.
GRK-4 is considered more important than other GRKs (including GRK-2) in the desensitization of D1R in RPTs.42 GRK-4 gene variants are also associated with salt-sensitive hypertension43,44; however, we are not aware of any such report in FBN rats. GRK-4 is reported to cause D1R G-protein uncoupling, resulting in reduced D1R responsiveness.43 The cause for higher GRK-4 levels in RPTs of old FBN rats is not known. We speculate that an age-associated increase in oxidative stress may have either a direct effect on GRK-4 or via some protein interaction, other than PKC, activates GRK-4 in RPTs of these rats, which warrants future investigation.
Furthermore, reduction of oxidative stress with Tempol in old FBN rats restored age-related decline in D1R numbers and D1R G-protein coupling (an index of D1R activation), suggesting a role for age-associated oxidative stress in impaired renal D1R function in old FBN rats. These results in FBN rats are in agreement with our previous findings in normotensive old F344 rats, where Tempol, while reducing oxidative stress, also restored age-associated decline in D1R numbers and G-protein coupling.32
To understand the underlying mechanism of exaggerated renal AT1R function in hypertensive old FBN rats,30 we measured levels of angiotensin AT1R protein and its signaling components in the RPTs of these rats. The levels of AT1R protein did not change in these animals. We attempted to determine AT1R numbers in RPTs of adult and old FBN rats; however, we were not successful in carrying out this experiment despite making several attempts and applying various protocols. Previously, increases in Gαi and Gαq/11 protein expression have been attributed to increased renal AT1R responses in hypertensive obese Zucker rats.16,27 However, we did not find any increases in the levels of these G proteins in the RPTs of adult and old FBN rats. Rather, there was a modest decrease in Gαi-1 protein levels, ruling out its involvement in higher AT1R responses in old FBN rats. We found an increase in Ang II–mediated stimulation of AT1R-G-protein coupling, as well as NKA activity in the RPTs of old FBN rats. Antioxidant Tempol treatment in old FBN rats normalized the exaggerated response of Ang II on AT1R-G-protein coupling and NKA activity to the levels seen in adult FBN rats, suggesting a role for oxidative stress in this phenomenon.
Similar to D1R, AT1R is also desensitized by GRKs. As discussed above, GRK-4, and not GRK-2, plays a major role in D1R desensitization, whereas the reverse is true for AT1R desensitization.45 We found an increase in GRK-4 but not GRK-2 levels in the RPTs of old FBN rats, which explains why only D1Rs and not AT1Rs are desensitized in the RPTs of old FBN rats. The mechanism by which oxidative stress increases AT1R-G-protein coupling in old FBN rats, how ever, remains to be established. It is likely that an age-associated increase in oxidative stress modifies AT1R protein conformation, thereby increasing the affinity of the receptor for Ang II, causing an increase in AT1R-G-protein coupling, as seen in old FBN rats. Indirect evidence for this statement comes from studies that demonstrate that sulfhydryl containing antioxidants N-acetylcysteine and dithiothreitol cause rapid and concentration-dependent decreases in Ang II radio-ligand binding to AT1R and AT1R-mediated signaling response in cultured vascular smooth muscle cells.46,47 Furthermore, in a similar study in HEK293 cells, antioxidant ascorbic acid decreases the binding affinity of the AT1R for Ang II.48
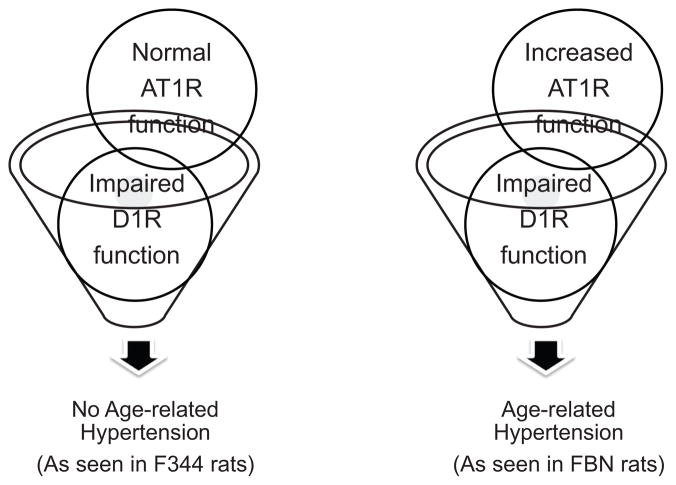
We have always asked this question: why are old Fischer 344 rats normotensive despite exhibiting increased oxidative stress and diminished renal D1R function? Perhaps the answer lies in our findings in hypertensive old FBN rats exhibiting exaggerated AT1R and diminished D1R functions (Reference 30 and the present study). It seems that exaggerated AT1R and diminished D1R functions are akin to hypertensive phenotype. To test this, we undertook a renal AT1R function study, in terms of the ability of candesartan to produce diuresis and natriuresis, and measured blood pressure in adult and old F344 rats exactly the same way as we did in FBN rats.30 Although candesartan-mediated natriuresis is considered an index of renal AT1R function, the role of other receptor systems (eg, renal AT2R) in mediating this effect cannot be ruled out.49–52 To our knowledge, a renal AT1R function study in F344 rats has not been investigated before. We found that diuretic and natriuretic responses to candesartan, as well as blood pressure, were not different between adult and old F344 rats. This finding is in contrast to our recent report in FBN rats in which candesartan produced exaggerated natriuresis.30 Taken together, these studies in old F344 and old FBN rats suggest that perhaps impairment in both AT1R (exaggerated) and D1R (diminished) functions is necessary for hypertensive phenotype in aging (Figure 7). This notion is supported by studies in young rats and mice, as mentioned below.
Figure 7.
Alterations in both renal D1 receptor (D1R) (diminished) and renal angiotensin II type 1 receptor (AT1R) (exaggerated) functions are required for the development of age-related hypertension.
In various hypertensive rat models, such as spontaneously hypertensive and obese Zucker rats, the hypertensive phenotype is associated with both exaggerated renal AT1R and diminished renal D1R functions.9,26–29 Genetic studies have also demonstrated that, in mice, D1-like receptor (D1 and D5) gene knockout increases AT1R expression and causes a hypertensive phenotype of mice.1,53 Conversely, ablation of the AT1R gene increases D1-like receptor (D5) expression but results in lowering of blood pressure in mice.1,53 Thus, it appears that the presence of both exaggerated AT1R and diminished D1R functions results in a hypertensive phenotype, as seen in old FBN rats. The reason for normal AT1R function in old F344 rats, while exhibiting diminished D1R function, is not known. F344 rats may have developed some compensatory mechanisms so as to balance AT1R function during the course of the aging process.
In addition to AT1R and D1R functions, we also measured general kidney function by measuring urinary protein levels in adult and old FBN rats. Proteinuria is an index of kidney dysfunction and a prognostic indicator of progressive kidney disease and poor cardiovascular outcomes. Its role as an independent risk factor for loss of renal function is well recognized.54 We found that there was an age-related increase in urinary protein levels, which decreased with antioxidant Tempol treatment in old FBN rats, suggesting that reduction of oxidative stress also improves general function of aging kidneys.
The present study provides a mechanism for impairment in renal D1R and AT1R functions, which may have been responsible for a hypertensive phenotype observed in old FBN rats. Age-associated oxidative stress in FBN rats may play a central role in this phenomenon.
Perspectives
It is estimated that, by 2030, ≈70 million people in the United states would be ≥65 years of age. Therefore, the number of elderly with high blood pressure and associated diseases would rise, thereby increasing the economic burden associated with health care for this population group in the United States. Hence, studies are needed to better understand the etiology of hypertension so as to develop superior therapeutic strategies to control high blood pressure in aging. The present study delineates the mechanism by which an age-associated increase in oxidative stress alters the functioning of D1R and AT1R, 2 key renal receptors involved in sodium homeostasis and blood pressure regulation. Therapeutic targeting of these receptors, separately, is in use in the clinic to control blood pressure in hypertensive patients. Our findings from the present and previous30 studies in FBN rats are clinically relevant as targeting both D1R and AT1R; preferably using antioxidants could result in a better management of age-related high blood pressure in the elderly.
Acknowledgments
We acknowledge AstraZeneca UK Limited for providing candesartan for renal function studies.
Sources of Funding
Financial assistance from National Institutes of Health/National Institute on Aging (AG039836, AG029904, and AG025056) grants is acknowledged.
Footnotes
Disclosures
None.
References
- 1.Gildea JJ. Dopamine and angiotensin as renal counterregulatory systems controlling sodium balance. Curr Opin Nephrol Hypertens. 2009;18:28–32. doi: 10.1097/MNH.0b013e32831a9e0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carey RM. Theodore Cooper Lecture: renal dopamine system–paracrine regulator of sodium homeostasis and blood pressure. Hypertension. 2001;38:297–302. doi: 10.1161/hy0901.096422. [DOI] [PubMed] [Google Scholar]
- 3.Hussain T, Lokhandwala MF. Renal dopamine receptors and hypertension. Exp Biol Med (Maywood) 2003;228:134–142. doi: 10.1177/153537020322800202. [DOI] [PubMed] [Google Scholar]
- 4.Jose PA, Eisner GM, Felder RA. Renal dopamine and sodium homeostasis. Curr Hypertens Rep. 2000;2:174–183. doi: 10.1007/s11906-000-0079-y. [DOI] [PubMed] [Google Scholar]
- 5.Kaschina E, Unger T. Angiotensin AT1/AT2 receptors: regulation, signalling and function. Blood Press. 2003;12:70–88. doi: 10.1080/08037050310001057. [DOI] [PubMed] [Google Scholar]
- 6.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 7.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 8.Albrecht FE, Drago J, Felder RA, Printz MP, Eisner GM, Robillard JE, Sibley DR, Westphal HJ, Jose PA. Role of the D1A dopamine receptor in the pathogenesis of genetic hypertension. J Clin Invest. 1996;97:2283–2288. doi: 10.1172/JCI118670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C, Beach RE, Lokhandwala MF. Dopamine fails to inhibit renal tubular sodium pump in hypertensive rats. Hypertension. 1993;21:364–372. doi: 10.1161/01.hyp.21.3.364. [DOI] [PubMed] [Google Scholar]
- 10.Chen CJ, Lokhandwala MF. An impairment of renal tubular DA-1 receptor function as the causative factor for diminished natriuresis to volume expansion in spontaneously hypertensive rats. Clin Exp Hypertens A. 1992;14:615–628. doi: 10.3109/10641969209036211. [DOI] [PubMed] [Google Scholar]
- 11.Damasceno A, Santos A, Serrao P, Caupers P, Soares-da-Silva P, Polonia J. Deficiency of renal dopaminergic-dependent natriuretic response to acute sodium load in black salt-sensitive subjects in contrast to salt-resistant subjects. J Hypertens. 1999;17:1995–2001. doi: 10.1097/00004872-199917121-00033. [DOI] [PubMed] [Google Scholar]
- 12.Felder RA, Seikaly MG, Cody P, Eisner GM, Jose PA. Attenuated renal response to dopaminergic drugs in spontaneously hypertensive rats. Hypertension. 1990;15:560–569. doi: 10.1161/01.hyp.15.6.560. [DOI] [PubMed] [Google Scholar]
- 13.Jose PA, Eisner GM, Felder RA. Renal dopamine receptors in health and hypertension. Pharmacol Ther. 1998;80:149–182. doi: 10.1016/s0163-7258(98)00027-8. [DOI] [PubMed] [Google Scholar]
- 14.Zeng C, Eisner GM, Felder RA, Jose PA. Dopamine receptor and hypertension. Curr Med Chem Cardiovasc Hematol Agents. 2005;3:69–77. doi: 10.2174/1568016052773289. [DOI] [PubMed] [Google Scholar]
- 15.Banday AA, Lokhandwala MF. Oxidative stress-induced renal angiotensin AT1 receptor upregulation causes increased stimulation of sodium transporters and hypertension. Am J Physiol Renal Physiol. 2008;295:F698–F706. doi: 10.1152/ajprenal.90308.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah S, Hussain T. Enhanced angiotensin II-induced activation of Na+, K+-ATPase in the proximal tubules of obese Zucker rats. Clin Exp Hypertens. 2006;28:29–40. doi: 10.1080/10641960500386650. [DOI] [PubMed] [Google Scholar]
- 17.Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension. 2005;46:454–462. doi: 10.1161/01.HYP.0000177474.06749.98. [DOI] [PubMed] [Google Scholar]
- 18.Pestana M. Hypertension in the elderly. Int Urol Nephrol. 2001;33:563–569. doi: 10.1023/a:1019552602793. [DOI] [PubMed] [Google Scholar]
- 19.Vasan RS, Beiser A, Seshadri S, Larson MG, Kannel WB, D’Agostino RB, Levy D. Residual lifetime risk for developing hypertension in middle-aged women and men: the Framingham Heart Study. JAMA. 2002;287:1003–1010. doi: 10.1001/jama.287.8.1003. [DOI] [PubMed] [Google Scholar]
- 20.Epstein M. Aging and the kidney. J Am Soc Nephrol. 1996;7:1106–1122. doi: 10.1681/ASN.V781106. [DOI] [PubMed] [Google Scholar]
- 21.Percy CJ, Power D, Gobe GC. Renal ageing: changes in the cellular mechanism of energy metabolism and oxidant handling. Nephrology (Carlton) 2008;13:147–152. doi: 10.1111/j.1440-1797.2008.00924.x. [DOI] [PubMed] [Google Scholar]
- 22.Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beheray S, Kansra V, Hussain T, Lokhandwala MF. Diminished natriuretic response to dopamine in old rats is due to an impaired D1-like receptor-signaling pathway. Kidney Int. 2000;58:712–720. doi: 10.1046/j.1523-1755.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- 24.Zemel MB, Sowers JR. Salt sensitivity and systemic hypertension in the elderly. Am J Cardiol. 1988;61:7H–12H. doi: 10.1016/0002-9149(88)91098-3. [DOI] [PubMed] [Google Scholar]
- 25.Baylis C. Renal responses to acute angiotensin II inhibition and administered angiotensin II in the aging, conscious, chronically catheterized rat. Am J Kidney Dis. 1993;22:842–850. doi: 10.1016/s0272-6386(12)70344-x. [DOI] [PubMed] [Google Scholar]
- 26.White BH, Sidhu A. Increased oxidative stress in renal proximal tubules of the spontaneously hypertensive rat: a mechanism for defective dopamine D1A receptor/G-protein coupling. J Hypertens. 1998;16:1659–1665. doi: 10.1097/00004872-199816110-00013. [DOI] [PubMed] [Google Scholar]
- 27.Becker M, Umrani D, Lokhandwala MF, Hussain T. Increased renal angiotensin II AT1 receptor function in obese Zucker rat. Clin Exp Hypertens. 2003;25:35–47. doi: 10.1081/ceh-120017739. [DOI] [PubMed] [Google Scholar]
- 28.Banday AA, Marwaha A, Tallam LS, Lokhandwala MF. Tempol reduces oxidative stress, improves insulin sensitivity, decreases renal dopamine D1 receptor hyperphosphorylation, and restores D1 receptor-G-protein coupling and function in obese Zucker rats. Diabetes. 2005;54:2219–2226. doi: 10.2337/diabetes.54.7.2219. [DOI] [PubMed] [Google Scholar]
- 29.Javkhedkar AA, Lokhandwala MF, Banday AA. Higher renal AT1 receptor affinity exaggerates Ang II induced Na/K-ATPase stimulation in spontaneously hypertensive rats [abstract] FASEB J. 2010;24(suppl):lb707. [Google Scholar]
- 30.Chugh G, Lokhandwala MF, Asghar M. Oxidative stress alters renal D1 and AT1 receptor functions and increases blood pressure in old rats. Am J Physiol Renal Physiol. 2011;300:F133–F138. doi: 10.1152/ajprenal.00465.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marwaha A, Banday AA, Lokhandwala MF. Reduced renal dopamine D1 receptor function in streptozotocin-induced diabetic rats. Am J Physiol Renal Physiol. 2004;286:F451–F457. doi: 10.1152/ajprenal.00227.2003. [DOI] [PubMed] [Google Scholar]
- 32.Fardoun RZ, Asghar M, Lokhandwala M. Role of oxidative stress in defective renal dopamine D1 receptor-G protein coupling and function in old Fischer 344 rats. Am J Physiol Renal Physiol. 2006;291:F945–F951. doi: 10.1152/ajprenal.00111.2006. [DOI] [PubMed] [Google Scholar]
- 33.Hilf G, Gierschik P, Jakobs KH. Muscarinic acetylcholine receptor-stimulated binding of guanosine 5′-O-(3-thiotriphosphate) to guanine-nucleotide-binding proteins in cardiac membranes. Eur J Biochem. 1989;186:725–731. doi: 10.1111/j.1432-1033.1989.tb15266.x. [DOI] [PubMed] [Google Scholar]
- 34.Banday AA, Lokhandwala MF. Loss of biphasic effect on Na/K-ATPase activity by angiotensin II involves defective angiotensin type 1 receptor-nitric oxide signaling. Hypertension. 2008;52:1099–1105. doi: 10.1161/HYPERTENSIONAHA.108.117911. [DOI] [PubMed] [Google Scholar]
- 35.Wilcox CS, Pearlman A. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharmacol Rev. 2008;60:418–469. doi: 10.1124/pr.108.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanada H, Jose PA, Hazen-Martin D, Yu PY, Xu J, Bruns DE, Phipps J, Carey RM, Felder RA. Dopamine-1 receptor coupling defect in renal proximal tubule cells in hypertension. Hypertension. 1999;33:1036–1042. doi: 10.1161/01.hyp.33.4.1036. [DOI] [PubMed] [Google Scholar]
- 37.Kim OJ, Gardner BR, Williams DB, Marinec PS, Cabrera DM, Peters JD, Mak CC, Kim KM, Sibley DR. The role of phosphorylation in D1 dopamine receptor desensitization: evidence for a novel mechanism of arrestin association. J Biol Chem. 2004;279:7999–8010. doi: 10.1074/jbc.M308281200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rankin ML, Marinec PS, Cabrera DM, Wang Z, Jose PA, Sibley DR. The D1 dopamine receptor is constitutively phosphorylated by G protein-coupled receptor kinase 4. Mol Pharmacol. 2006;69:759–769. doi: 10.1124/mol.105.019901. [DOI] [PubMed] [Google Scholar]
- 39.Tiberi M, Nash SR, Bertrand L, Lefkowitz RJ, Caron MG. Differential regulation of dopamine D1A receptor responsiveness by various G protein-coupled receptor kinases. J Biol Chem. 1996;271:3771–3778. doi: 10.1074/jbc.271.7.3771. [DOI] [PubMed] [Google Scholar]
- 40.Felder RA, Sanada H, Xu J, Yu PY, Wang Z, Watanabe H, Asico LD, Wang W, Zheng S, Yamaguchi I, Williams SM, Gainer J, Brown NJ, Hazen-Martin D, Wong LJ, Robillard JE, Carey RM, Eisner GM, Jose PA. G protein-coupled receptor kinase 4 gene variants in human essential hypertension. Proc Natl Acad Sci U S A. 2002;99:3872–3877. doi: 10.1073/pnas.062694599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Premont RT, Macrae AD, Stoffel RH, Chung N, Pitcher JA, Ambrose C, Inglese J, MacDonald ME, Lefkowitz RJ. Characterization of the G protein-coupled receptor kinase GRK4: identification of four splice variants. J Biol Chem. 1996;271:6403–6410. doi: 10.1074/jbc.271.11.6403. [DOI] [PubMed] [Google Scholar]
- 42.Watanabe H, Xu J, Bengra C, Jose PA, Felder RA. Desensitization of human renal D1 dopamine receptors by G protein-coupled receptor kinase 4. Kidney Int. 2002;62:790–798. doi: 10.1046/j.1523-1755.2002.00525.x. [DOI] [PubMed] [Google Scholar]
- 43.Jose PA, Soares-da-Silva P, Eisner GM, Felder RA. Dopamine and G protein-coupled receptor kinase 4 in the kidney: role in blood pressure regulation. Biochim Biophys Acta. 1802:1259–1267. doi: 10.1016/j.bbadis.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanada H, Yatabe J, Midorikawa S, Hashimoto S, Watanabe T, Moore JH, Ritchie MD, Williams SM, Pezzullo JC, Sasaki M, Eisner GM, Jose PA, Felder RA. Single-nucleotide polymorphisms for diagnosis of salt-sensitive hypertension. Clin Chem. 2006;52:352–360. doi: 10.1373/clinchem.2005.059139. [DOI] [PubMed] [Google Scholar]
- 45.Felder RA, Jose PA. Mechanisms of disease: the role of GRK4 in the etiology of essential hypertension and salt sensitivity. Nat Clin Pract Nephrol. 2006;2:637–650. doi: 10.1038/ncpneph0301. [DOI] [PubMed] [Google Scholar]
- 46.Ullian ME, Gelasco AK, Fitzgibbon WR, Beck CN, Morinelli TA. N-acetylcysteine decreases angiotensin II receptor binding in vascular smooth muscle cells. J Am Soc Nephrol. 2005;16:2346–2353. doi: 10.1681/ASN.2004060458. [DOI] [PubMed] [Google Scholar]
- 47.Ullian ME, Beck CN, Walker LP, Fitzgibbon WR, Morinelli TA. Thiol antioxidants regulate angiotensin II AT1 and arginine vasopressin V1 receptor functions differently in vascular smooth muscle cells. Am J Hypertens. 2009;22:221–227. doi: 10.1038/ajh.2008.323. [DOI] [PubMed] [Google Scholar]
- 48.Leclerc PC, Proulx CD, Arguin G, Belanger S, Gobeil F, Jr, Escher E, Leduc R, Guillemette G. Ascorbic acid decreases the binding affinity of the AT1 receptor for angiotensin II. Am J Hypertens. 2008;21:67–71. doi: 10.1038/ajh.2007.1. [DOI] [PubMed] [Google Scholar]
- 49.Padia SH, Howell NL, Siragy HM, Carey RM. Renal angiotensin type 2 receptors mediate natriuresis via angiotensin III in the angiotensin II type 1 receptor-blocked rat. Hypertension. 2006;47:537–544. doi: 10.1161/01.HYP.0000196950.48596.21. [DOI] [PubMed] [Google Scholar]
- 50.Padia SH, Kemp BA, Howell NL, Fournie-Zaluski MC, Roques BP, Carey RM. Conversion of renal angiotensin II to angiotensin III is critical for AT2 receptor-mediated natriuresis in rats. Hypertension. 2008;51:460–465. doi: 10.1161/HYPERTENSIONAHA.107.103242. [DOI] [PubMed] [Google Scholar]
- 51.Padia SH, Kemp BA, Howell NL, Siragy HM, Fournie-Zaluski MC, Roques BP, Carey RM. Intrarenal aminopeptidase N inhibition augments natriuretic responses to angiotensin III in angiotensin type 1 receptor-blocked rats. Hypertension. 2007;49:625–630. doi: 10.1161/01.HYP.0000254833.85106.4d. [DOI] [PubMed] [Google Scholar]
- 52.Padia SH, Kemp BA, Howell NL, Gildea JJ, Keller SR, Carey RM. Intrarenal angiotensin III infusion induces natriuresis and angiotensin type 2 receptor translocation in Wistar-Kyoto but not in spontaneously hypertensive rats. Hypertension. 2009;53:338–343. doi: 10.1161/HYPERTENSIONAHA.108.124198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sanada H, Jones JE, Jose PA. Genetics of salt-sensitive hypertension. Curr Hypertens Rep. 13:55–66. doi: 10.1007/s11906-010-0167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baines RJ, Brunskill NJ. Tubular toxicity of proteinuria. Nat Rev Nephrol. 7:177–180. doi: 10.1038/nrneph.2010.174. [DOI] [PubMed] [Google Scholar]