Abstract
Background
Pegylated interferon (PEG-IFN) treatment for hepatitis C virus (HCV) infection has neuropsychiatric side effects. Data on the impact of HCV treatment on mental health among injecting drug users (IDUs) are limited. We assessed mental health during treatment of recently acquired HCV, within a predominantly IDU population.
Methods
Participants with HCV received PEG-IFN α-2a (180µg/week) for 24 weeks; HCV/HIV received PEG-IFN with ribavirin. Depression was assessed using the Mini-International Neuropsychiatric Interview (MINI). Logistic regression was used to identify factors associated with depression at enrolment and during treatment. Also, the impact of depression prior to and during treatment on SVR was assessed.
Results
Of 163 participants, 111 received treatment (HCV, n=74; HCV/HIV, n=37), with 76% ever reporting IDU. At enrolment, 16% had depression (n=25). In adjusted analysis, depression at enrolment occurred less often in participants full-/part-time employed (AOR 0.23; 95% CI: 0.06, 0.82, P=0.023) and more often in recent IDUs (AOR 3.04; 95% CI: 1.19, 7.72, P=0.019). During treatment, 35% (n=31) developed new-onset depression. In adjusted analysis, poorer social functioning (higher score) was associated with new-onset depression (score ≤9 vs. score ≥17; OR 5.69; 95% CI: 1.61, 20.14, P=0.007). SVR was similar among participants with and without depression at enrolment (60% vs. 61%, P=0.951) and in those with and without new-onset depression (74% vs. 63%, P=0.293).
Conclusions
Although depression at enrolment and during treatment was common among participants with recent HCV, neither impacted SVR. Participants with poor social functioning may be most at risk of developing depression during HCV therapy.
Keywords: injecting drug users, HCV, depression, anxiety, psychiatric
INTRODUCTION
Pegylated interferon (PEG-IFN) and ribavirin is the standard of care for treatment of hepatitis C virus (HCV) infection1–2. PEG-IFN treatment is complicated by neuropsychiatric side effects including suicidal thoughts and development or worsening of depressive symptoms3–4. During PEG-IFN treatment, up to 48% of participants develop depressive symptoms4–8. However, the incidence and severity of IFN-induced depression varies between studies, presumably related to several factors including different socio-demographic characteristics between studies, varying doses and durations of therapy, differences in study designs, variations in the methodological approaches for depression assessment and different classifications of depression9.
Since people with HCV infection have a high prevalence of psychiatric illness, studying neuropsychiatric side effects during HCV treatment is important. The lifetime prevalence of major depressive disorder is higher in individuals with HCV as compared to the general population (22–49% vs. 17%)10–12. Moreover, in the developed world, 50–80% of individuals with HCV infection are injecting drug users (IDUs)13, who also have a high prevalence of co-morbid psychiatric disease14–16.
HCV treatment is often withheld from IDUs and individuals with co-morbid psychiatric disease due to concerns of poor adherence, ongoing drug use, psychosocial instability and exacerbation of pre-existing psychiatric disease mediated by interferon17. However, studies have consistently demonstrated that among IDUs and individuals with psychiatric disease treated with interferon-based therapy, treatment completion and sustained virological response (SVR) are comparable to non-IDUs18 and those without a history of psychiatric disease8. Although a number of studies have assessed depression during HCV treatment4–8, there is still a limited understanding of depression prior to and during HCV treatment among IDUs, particularly in the setting of recently acquired infection.
The Australian Trial in Acute Hepatitis C (ATAHC) was designed specifically to investigate treatment for recent HCV, predominantly in those with IDU-acquired infection. The aims of this study were to evaluate depression prior to and during treatment for HCV infection, identify risk factors associated with depression and assess the impact of depression on response to HCV treatment in the ATAHC study.
METHODS
Study design
ATAHC was a multicenter, prospective cohort study of the natural history and treatment of recent HCV infection, as previously described19. Recruitment of HIV infected and uninfected participants was from June 2004 through November 2007. Recent infection with either acute or early chronic HCV infection with the following eligibility criteria:
First positive anti-HCV antibody within 6 months of enrolment; and either
Acute clinical hepatitis C infection, defined as symptomatic seroconversion illness or alanine aminotransferase (ALT) level greater than 10 times the upper limit of normal (>400 IU/L) with exclusion of other causes of acute hepatitis, at most 12 months before the initial positive anti-HCV antibody; or
Asymptomatic hepatitis C infection with seroconversion, defined by a negative anti-HCV antibody in the two years prior to the initial positive anti-HCV antibody.
All participants with HCV RNA during the screening period (maximum 12 weeks) were assessed for HCV treatment eligibility. Heavy alcohol intake and active drug use were not exclusion criteria. From enrolment, participants were followed for up to 12 weeks to allow for spontaneous HCV clearance and if HCV RNA remained detectable were offered treatment.
All study participants provided written informed consent. The study protocol was approved by St Vincent’s Hospital, Sydney Human Research Ethics Committee (primary study committee) as well as through local ethics committees at all study sites. The study was registered with clinicaltrials.gov registry (NCT00192569).
HCV treatment
Participants who began HCV treatment received PEG-IFN -α2a 180 micrograms weekly for 24 weeks. Due to non-response at week 12 in the initial two participants with HCV/HIV co-infection, the study protocol was amended to provide PEG-IFN and ribavirin combination therapy for 24 weeks in HIV positive individuals. Ribavirin was prescribed at a dose of 1000–1200 mg for those with genotype 1 infection and 800 mg in those with genotype 2/3.
Study assessments
Participants who did not receive HCV treatment were seen at study enrolment and 12 weekly intervals for up to 144 weeks. Among treated participants, additional study visits occurred at enrolment, every two weeks from baseline (beginning of treatment) to week 8 and every four weeks from week 8 until the end of treatment. At each study visit, data on concomitant medications (including psychiatric medications) were collected.
Questionnaires were administered at enrolment and every 12 weeks during the first year (every 24 weeks during second and third years), to obtain information on injecting drug use, social functioning [Opiate Treatment Index (OTI) - Social Functioning Scale]20, psychological parameters [Mini-International Neuropsychiatric Interview (M.I.N.I.)21 and the short version of the Depression Anxiety Stress Scale (DASS-21)]22.
Social Functioning Scale of the Opiate Treatment Index (OTI): addresses employment, residential stability, and inter-personal conflict as well as social support20. This scale has been validated among opiate users in Australia and higher scores are indicative of poorer social functioning (range score: 0–48)20.
Mini-International Neuropsychiatric Interview (MINI): is a validated structured diagnostic interview covering 17 Axis I categories in a short format21. It has good correlation with Structured Clinical Interview for DSM-IV-TR Axis I (SCID-I)23 and the Composite International Diagnostic Interview (CIDI)24. The first section of the MINI consists of nine questions assessing the presence of current major depressive episode (MDE). The second section of the MINI consists of six questions assessing current suicide risk. Suicide risk score is then categorized in three levels: low, moderate and high. MINI was used as the primary instrument to assess depression in the current study, given its correlation with Structured Clinical Interview for DSM-IV-TR Axis I diagnosis of depression.
Depression, Anxiety and Stress Scale (DASS-21): is a 21 item self-administered survey consisting of three scales (seven questions each) assessing the severity of the core symptoms of depression, anxiety and stress in the past week22. The score ranges from 0 to 42, with increasing score indicating increasing severity. Depression, anxiety and stress can also be categorized according to normal, mild, moderate, severe and extremely severe. DASS scores also have high internal consistency. DASS corresponds to mood disorders and was used as a secondary instrument to assess depressive symptoms21, 24.
Study definitions
Depression: current major depressive disorder, as assessed by the MINI.
Depressive symptoms: having moderate (score range 14–20), severe (score range 21–27) or extremely severe (score range 28+) levels measured according to the DASS depression scale.
New-onset depression: development of current major depressive episode during treatment among participants who were not depressed prior to the initiation of therapy.
New-onset suicide risk: development of suicide risk (low, moderate or high) during treatment, among those with no suicide risk prior to the initiation of therapy.
Statistical analyses
Characteristics associated with depression at enrolment and new-onset depression during treatment were assessed. The impact of depression prior to treatment and new-onset depression and suicide risk during treatment on SVR were also evaluated. Characteristics were compared using two-sample t-tests for quantitative variables and Chi-squared test, Fisher’s exact test or McNemar’s test as appropriate, for testing differences in proportions. Logistic regression analyses were performed to identify factors associated with depression at enrolment and new-onset depression in those receiving treatment. Potential factors associated with depression were determined a priori and included sex, age, education, accommodation, employment, methadone/buprenorphine treatment, social functioning, IDU at enrolment (ever, past 6 months and past month), alcohol, and HIV/HCV co-infection. For logistic regression analyses, continuous factors were categorized either using the median (age) or tertiles (social functioning and alcohol), given the absence of a linear effect with the outcome variable. Multivariable logistic regression was performed using a backwards stepwise approach subject to a likelihood ratio test, considering factors that were significant at the 0.20 level in unadjusted analyses. We also assessed the impact of pre-treatment depression, pre-treatment suicide risk, new-onset depression and suicide risk on SVR. Statistically significant differences were assessed at p<0.05; p-values are two-sided. All analyses were performed using the statistical package Stata v10.1 (College Station, TX).
RESULTS
Participant characteristics
Overall, 163 participants were enrolled in the ATAHC study between June 2004 and February 2008. The majority of participants were male (71%), Caucasian (91%), the mean age was 34.3 years (standard deviation (SD) ±9.9) and 31% were HCV/HIV co-infected (Table 1). Overall, 76% (n=124) reported a history of injecting drug use, with 44% (55 of 124) injecting in the past month. A total of 111 participants received treatment and 52 participants were untreated.
Table 1.
Characteristics among all participants enrolled in ATAHC (n= 163)
| Total study population (n=163) |
Treated (n=111) |
Untreated HCV RNA positive at enrolment (n=34) |
Untreated HCV RNA negative at enrolment (n=18) |
|
|---|---|---|---|---|
| Male, n (%) | 116 (71%) | 83 (75%) | 22 (65%) | 11 (61%) |
| Age (yrs), mean ±SD | 34.3 ± 9.9 | 34.5 ± 10.4 | 34.7 ± 9.0 | 32.2 ± 8.4 |
| Tertiary education or greater, n (%) | 66 (40%) | 51 (46%) | 9 (26%) | 6 (33%) |
| Full-time or part-time employment, n (%) | 63 (39%) | 52 (47%) | 9 (26%) | 2 (11%) |
| Methadone or buprenorphine treatment | ||||
| Ever (not current) | 17 (10%) | 12 (11%) | 4 (12%) | 1 (6%) |
| Current | 22 (14%) | 12 (11%) | 6 (18%) | 4 (22%) |
| Social functioning score, median (IQR)* | 13 (8–18) | 11 (6–17) | 15 (9–19) | 18 (13–20) |
| Injecting drug use ever, n (%) | 124 (76%) | 84 (76%) | 28 (82%) | 12 (67%) |
| Injected over the past month, n (%)† | 53 (43%) | 31 (37%) | 15 (54%) | 7 (58%) |
| HIV infection, n (%) | 50 (31%) | 37 (33%) | 11 (32%) | 2 (11%) |
| Hepatitis B virus (HBV) surface antigen, n (%) | 2 (1%) | 1 (1%) | 0 (0%) | 1 (6%) |
| Log10 HCV RNA - screening, median (log IU/L) | 5.6 | 5.8 | 4.0 | 0 |
| HCV genotype | ||||
| Genotype 1 | 75 (46%) | 62 (56%) | 13 (38%) | 0 |
| Genotype 2 | 6 (4%) | 4 (4%) | 2 (6%) | 0 |
| Genotype 3 | 56 (34%) | 40 (36%) | 16 (47%) | 0 |
| Genotype 4 | 1 (1%) | 0 (0%) | 1 (3%) | 0 |
| Missing genotype | 25 (15%) | 5 (4%) | 2 (6%) | 18 (100%) |
IQR (interquartile range),
among participants who reported injecting
Among those enrolled (n=163), 145 were HCV RNA positive at enrolment and thus eligible to receive treatment for HCV infection. The remaining 18 participants had spontaneous clearance of HCV infection and were thus not eligible to receive HCV treatment. In Table 1, the characteristics of participants who were treated (n=111), untreated but HCV RNA positive (n=34), and untreated but HCV RNA negative (ineligible to receive treatment) (n=18) is shown. Among HCV RNA positive participants (n=145), depression at study enrolment was more common among untreated as compared to treated participants (26% vs. 9%, P=0.008). As previously shown17, after adjusting for other behavioural and clinical factors, participants with depression demonstrated a trend to be less likely to receive treatment [adjusted odds ratio (AOR) 0.40; 95% Confidence Interval (95% CI) 0.14, 1.17, P=0.093]. Suicide risk (moderate to high) was more common among untreated compared to treated participants (35% vs. 10%, P< 0.001).
Among the final treated population (n=111), 74 HCV mono-infected participants received PEG-IFN, 35 HCV/HIV co-infected participants received PEG-IFN/ribavirin and 2 HCV/HIV participants received PEG-IFN therapy (these participants were excluded from intention-to-treat analyses, given the protocol modification). As reported previously, among participants who received PEG-IFN treatment for HCV infection (n=109), 82% (89 of 109) received ≥80% of scheduled PEG-IFN doses for ≥80% of the scheduled treatment period25. PEG-IFN dose modification occurred in 5% of patients (n = 5)25.
Depression at study enrolment
Among the 163 participants enrolled in ATAHC, 160 participants had available mental health assessments (MINI and DASS) at study enrolment (Table 2). At study enrolment, 16% (n=25) had depression (current major depressive disorder as assessed by the MINI, Table 2). Moderate to high suicide risk was reported in 18% (n=28). Moderate to extremely severe depressive, anxiety and stress symptoms (assessed by the DASS-21) were reported by 36% (n=57), 40% (n=64) and 24% (n=38), respectively (Table 2). The mean DASS-21 score was 11.1 (SD ± 10.7) for depressive, 8.7 (SD ± 8.0) for anxiety and 13.1 (SD ± 9.8) for stress symptoms. Compared to those with HCV mono-infection, HCV/HIV co-infected participants had a lower proportion with depression (6% vs. 20%, P=0.033) and depressive symptoms (19% vs. 44%, P=0.036). Compared to those without recent drug injecting, recent IDUs (over the past month) had a higher proportion with depression (27% vs. 9%, P=0.002) and depressive symptoms (54% vs. 26% P=0.012).
Table 2.
Mental health characteristics among participants enrolled in ATAHC with available mental health assessments at enrolment*
| Total study population |
Treated | Untreated HCV RNA positive at enrolment |
Untreated HCV RNA positive at enrolment |
|
|---|---|---|---|---|
| Mental health parameters/DASS-21 | n= 158 | n= 108 | n= 34 | n= 16 |
| Depression, median (range) | 8 (0–42) | 6 (0–42) | 13 (0–40) | 14 (0–36) |
| Normal, n (%) | 81 (51%) | 64 (59%) | 11 (32%) | 6 (37%) |
| Mild, n (%) | 20 (13%) | 12 (11%) | 6 (18%) | 2 (12%) |
| Moderate, n (%) | 28 (18%) | 18 (17%) | 9 (26%) | 1 (6%) |
| Severe, n (%) | 12 (8%) | 4 (4%) | 4 (12%) | 4 (25%) |
| Extremely severe, n (%) | 17 (11%) | 10 (9%) | 4 (12%) | 3 (19%) |
| Anxiety, median (range) | 6 (0–40) | 6 (0–40) | 10 (0–26) | 11 (0–40) |
| Normal, n (%) | 81 (51%) | 62 (57%) | 12 (35%) | 7 (44%) |
| Mild, n (%) | 13 (8%) | 9 (8%) | 3 (9%) | 1 (6%) |
| Moderate, n (%) | 34 (22%) | 22 (20%) | 8 (24%) | 4 (25%) |
| Severe, n (%) | 15 (9%) | 9 (8%) | 6 (18%) | 0 (0%) |
| Extremely severe, n (%) | 15 (9%) | 6 (6%) | 5 (15%) | 4 (25%) |
| Stress, median (range) | 12 (0–42) | 10 (0–42) | 14 (0–36) | 15 (0–38) |
| Normal, n (%) | 99 (63%) | 73 (68%) | 18 (53%) | 8 (50%) |
| Mild, n (%) | 21(13%) | 13 (12%) | 6 (17%) | 2 (12%) |
| Moderate, n (%) | 17 (11%) | 13 (12%) | 3 (9%) | 1 (6%) |
| Severe, n (%) | 12 (8%) | 5 (5%) | 4 (12%) | 3 (19%) |
| Extremely severe, n (%) | 9 (6%) | 4 (4%) | 3 (9%) | 2 (12%) |
| Mental health parameters/ MINI | n= 160 | n= 110 | n= 34 | n= 16 |
| Current major depressive episode, n (%) | 25 (16%) | 10 (9%) | 9 (26%) | 6 (37%) |
| Suicide risk (moderate and high), n (%) | 28 (18%) | 11 (10%) | 12 (35%) | 5 (31%) |
| Psychiatric medication- total, n (%)¶ | 49 (30%) | 31 (28%) | 12 (35%) | 6 (33%) |
| Antidepressants- total, n (%)¶ | 37 (23%) | 23 (21%) | 8 (24%) | 6 (33%) |
total n=158 for DASS-21 & total n=160 for MINI,
some participants take more than one type of medication
Factors associated with depression at study enrolment in unadjusted analyses included unstable employment (no full-time/part-time employment), recent injecting drug use (past month), higher social functioning score (poorer social function) and not being infected with HIV (Table 3). In adjusted analysis (Table 3), depression occurred less often among those with full-time/part-time employment (AOR 0.23, 95% CI, 0.06, 0.82, P=0.023) and more often among those with recent injecting drug use (AOR 3.04, 95% CI, 1.19, 7.72, P=0.019).
Table 3.
Characteristics associated with major depressive episode prior to treatment for recent HCV infection (n=160) *
| Characteristic | Depressed, n= 25 |
OR | 95% CI | P | P-Overall | AOR | 95% CI | p |
|---|---|---|---|---|---|---|---|---|
| Age, >34 (vs. ≤34) | 11 (14%) | 0.73 | 0.31–1.72 | 0.472 | – | – | – | – |
| Female sex (vs. male) | 9 (20%) | 1.50 | 0.6–3.7 | 0.385 | – | – | – | – |
| Caucasian ethnicity (vs. other) | 21 (14%) | 0.42 | 0.12–1.46 | 0.173 | – | – | – | – |
| Tertiary education or greater (vs. less than tertiary) | 8 (12%) | 0.66 | 0.27–1.64 | 0.376 | – | – | – | – |
| Full time/part time employment (vs. no employment/other) | 3 (5%) | 0.11 | 0.05–0.61 | 0.007 | – | 0.23 | 0.06–0.82 | 0.023 |
| Rental accommodation (vs. owned) | 5 (13%) | 0.72 | 0.24–2.10 | 0.545 | 0.818 | – | – | – |
| Other types of accommodation (vs. owned) | 3 (14%) | 0.81 | 0.21–3.07 | 0.761 | – | – | – | – |
| Methadone/Buprenorphine therapy- ever (vs. never) | 2 (12%) | 0.70 | 0.15–3.33 | 0.655 | 0.882 | – | – | – |
| Current therapy (vs. never) | 3 (14%) | 0.83 | 0.22–3.09 | 0.782 | – | – | – | – |
| Injecting drug use ever (vs. never) | 22 (18%) | 2.47 | 0.69–8.77 | 0.162 | – | – | – | – |
| Injecting drug use past 6 months (vs. not in past 6 months) | 21 (21%) | 3.95 | 1.29–12.15 | 0.016 | – | – | – | – |
| Injecting drug use past 30 days, yes (vs. not in past month) | 15 (27%) | 3.87 | 1.57–9.58 | 0.003 | – | 3.04 | 1.19–7.72 | 0.019 |
| Social functioning score, 10–16 (vs. 0–9)† | 8 (17%) | 3.37 | 0.84–13.57 | 0.088 | 0.064 | – | – | – |
| ≥17 (vs. 0–9) | 11 (24%) | 5.03 | 1.30–19.37 | 0.019 | – | – | – | – |
| Alcohol- standard drinks/day past month, 3–4 (vs. ≤2)† | 2 (7%) | 0.24 | 0.05–1.15 | 0.074 | – | – | – | – |
| ≥5 (vs. ≤2) | 3 (10%) | 0.36 | 0.09–1.38 | 0.136 | 0.103 | – | – | – |
| Antipsychotic medication (vs. no antipsychotic) | 3 (27%) | 2.16 | 0.53–8.80 | 0.280 | – | – | – | – |
| Antidepressant (SSRI, SNRI,TCA,TeCA) (vs. no antidepressant) | 9 (25%) | 2.25 | 0.90–5.64 | 0.084 | – | – | – | – |
| HCV/HIV co-infection (vs. HCV mono-infection) | 3 (6%) | 0.27 | 0.08–0.96 | 0.040 | – | – | – | – |
three participants had missing MINI survey prior to HCV treatment,
variables divided to tertiles by distribution
serotonin-norepinephrine reuptake inhibitor (SNRI), selective serotonin reuptake inhibitor (SSRI), tricyclic antidepressant (TCA), tetracyclic antidepressant (TeCA)
New-onset depression during treatment for recent HCV infection
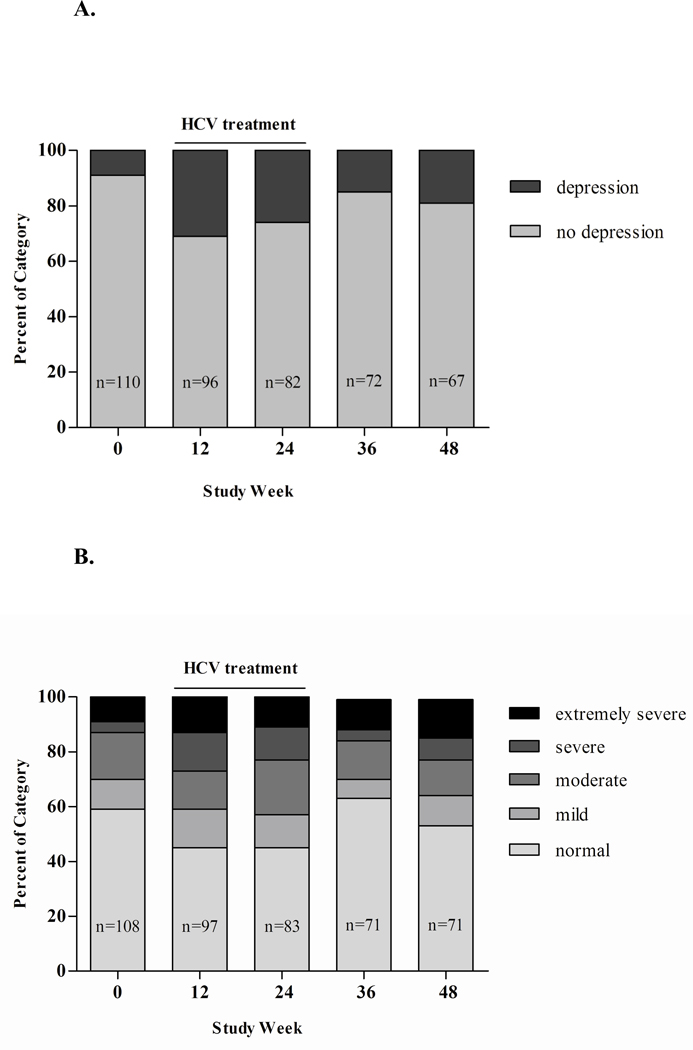
Among those who received HCV treatment (n=111), 88 did not have depression at study enrolment and had available follow-up information following treatment. Among these 88 participants, 35% developed new-onset depression (n=31) during treatment (HCV, 33%; HCV/HIV, 38%, P=0.639) and 35% (11 of 31) of these received antidepressants. Longitudinal changes in depression and depressive symptoms prior to, during and following treatment are shown in Table 4 and Figures 1A and 1B. Participants who developed new-onset depression demonstrated a trend toward higher depressive symptoms prior to treatment compared to those who did not develop new-onset depression (mean score 7.0 vs. 10.2, P=0.093).
Table 4.
Longitudinal changes in MINI and DASS scores in ATAHC among all participants treated for recent HCV infection*
| Scale | Pre-treatment | Week 12 | Week 24 | Week 36 | Week 48 |
|---|---|---|---|---|---|
| MINI | (n= 110) | (n= 96) | (n= 82) | (n= 72) | (n= 67) |
| Major depressive episode, n (%) | 10 (9%) | 30 (31%) | 21 (26%) | 11 (15%) | 13 (19%) |
| Suicide risk, n (%) | 37 (34%) | 33 (34%) | 28 (34%) | 18 (25%) | 21 (32%) |
| Suicide risk level, n (%)¶ | |||||
| Low | 26 (70%) | 20 (61%) | 15 (54%) | 10 (57%) | 12 (60%) |
| Moderate | 7 (19%) | 4 (12%) | 4 (14%) | 2 (11%) | 3 (15%) |
| High | 4 (11%) | 9 (27%) | 9 (32%) | 6 (33%) | 5 (25%) |
| DASS-21 | (n= 108) | (n= 97) | (n= 83) | (n= 71) | (n= 71) |
| Depression | |||||
| Median (IQR)¥ | 6 (1–14) | 12 (4–22) | 10 (2–20) | 8 (2–14) | 8 (2–20) |
| Normal, n (%) | 64 (59%) | 44 (45%) | 37 (45%) | 45 (63%) | 38 (54%) |
| Mild, n (%) | 12 (11%) | 13 (13%) | 10 (12%) | 5 (7%) | 8 (11%) |
| Moderate, n (%) | 18 (17%) | 13 (13%) | 17 (20%) | 10 (14%) | 9 (13%) |
| Severe, n (%) | 4 (4%) | 13 (13%) | 10 (12%) | 3 (4%) | 6 (8%) |
| Extremely severe, n (%) | 10 (9%) | 14 (14%) | 9 (11%) | 8 (11%) | 10 (14%) |
| Anxiety | |||||
| Median (IQR)¥ | 6 (2–11) | 10 (4–18) | 10 (4–16) | 4 (2– 12) | 6 (2–12) |
| Normal, n (%) | 62 (57%) | 41 (42%) | 28 (34%) | 42 (59%) | 37(52%) |
| Mild, n (%) | 9 (8%) | 6 (6%) | 9 (11%) | 4 (6%) | 7 (10%) |
| Moderate, n (%) | 22 (20%) | 18 (19%) | 24 (29%) | 11 (15%) | 14 (20%) |
| Severe, n (%) | 9 (8%) | 12 (12%) | 8 (10%) | 5 (7%) | 3 (4%) |
| Extremely severe, n (%) | 6 (6%) | 19 (21%) | 14 (17%) | 9 (13%) | 10 (14%) |
| Stress | |||||
| Median (IQR)¥ | 10 (4–17) | 16 (6–24) | 14 (6–24) | 12 (4–18) | 10 (4–18) |
| Normal, n (%) | 73 (68%) | 46 (47%) | 45 (54%) | 47 (66%) | 43 (61%) |
| Mild, n (%) | 13 (12%) | 12 (12%) | 8 (10%) | 8 (11%) | 11 (15%) |
| Moderate, n (%) | 13 (12%) | 19 (20%) | 17 (20%) | 9 (13%) | 5 (7%) |
| Severe, n (%) | 5 (5%) | 13 (13%) | 10 (12%) | 5 (7%) | 7 (10%) |
| Extremely severe, n (%) | 4(4%) | 7 (7%) | 3 (4%) | 2 (3%) | 5 (7%) |
number of participants with available MINI and DASS surveys varies at each time point,
among participants with suicide risk,
IQR (interquartile range)
Figure 1.
A. Longitudinal changes of depression among treated participants, assessed by MINI. Depression defined as: current major depressive episode (MDE)
B. Longitudinal changes of depressive symptoms among treated participants, categorized according to DASS severity rating chart: normal (0–9), mild (10–13), moderate (14–20), severe (21–27), and extremely sever (28+)
In unadjusted analysis, factors associated with developing new-onset depression included recent injecting prior to treatment initiation (past six months), alcohol use (>5 standard alcoholic drinks/day over the past month), and higher (poorer) social functioning score (Table 5). In adjusted analysis, poorer social functioning (higher score) was the only factor associated with new-onset depression (score ≤9 vs. score ≥17 OR 5.69, 95% CI, 1.61, 20.14, P=0.007).
Table 5.
Characteristics associated with new onset major depressive episode among participants without depression prior to the initiation of treatment for recent HCV infection (n=88)
| Depressed, n= 31 | OR | 95% CI | P | P-Overall | |
|---|---|---|---|---|---|
| Caucasian ethnicity (vs. other) | 26 (33%) | 0.39 | 0.09–1.58 | 0.189 | – |
| Full time/part time employment (vs. no employment, other) | 13 (29%) | 0.56 | 0.23–1.37 | 0.205 | – |
| HCV/HIV co- infection (vs. HCV mono-infection) | 13 (38%) | 1.24 | 0.51–3.03 | 0.639 | – |
| Injecting drug use ever (vs. never) | 24 (37%) | 1.46 | 0.53–4.02 | 0.467 | – |
| Injecting drug use past 6 months (vs. not in past 6 months) | 21 (45%) | 2.50 | 1.00–6.26 | 0.050 | – |
| Injecting drug use past 30 days (vs. not in past month) | 9 (41%) | 1.45 | 0.53–3.93 | 0.464 | – |
| Social functioning score, 10–16 (vs. ≤9)* | 11 (42%) | 1.59 | 0.49–5.15 | 0.439 | – |
| ≥17 (vs. ≤9) | 10 (53%) | 5.69 | 1.61–20.14 | 0.007 | 0.018 |
| Alcohol-number of drinks/day past month, 3–4 (vs. ≤2)* | 2 (12%) | 0.14 | 0.03–0.76 | 0.022 | – |
| ≥5 (vs. ≤2) | 5 (24%) | 0.31 | 0.09–1.11 | 0.071 | 0.035 |
| Depression by DASS, moderate to extremely severe symptoms (vs. normal, mild symptoms) | 12 (52%) | 2.73 | 1.02–7.29 | 0.046 | – |
| Depression by DASS, mild to extremely severe symptoms (vs. normal symptoms) | 15 (47%) | 2.29 | 0.92–5.73 | 0.075 | – |
| Antidepressant (SSRI, SNRI, TCA, TeCA) (vs. no antidepressant) | 9 (45%) | 1.71 | 0.62–4.73 | 0.301 | – |
variables divided to tertiles by distribution
serotonin-norepinephrine reuptake inhibitor (SNRI), selective serotonin reuptake inhibitor (SSRI), tricyclic antidepressant (TCA), tetracyclic antidepressant (TeCA)
Impact of depression and suicide risk on HCV treatment response
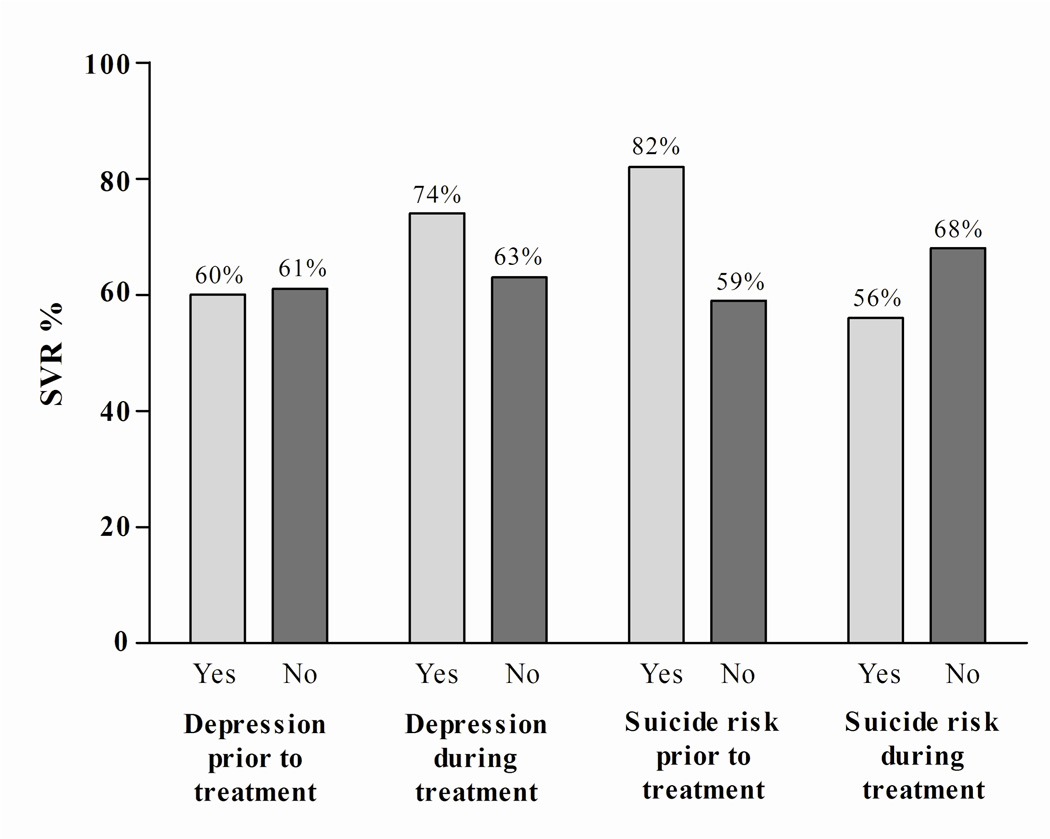
Depression and suicide risk prior to and during HCV treatment did not impact SVR (Figure 2). Of the 110 participants who received treatment, SVR was similar in those with (60%, 6 of 10) and without (61%, 61 of 100) depression at study enrolment (P=0.951). SVR was also similar in those with (82%, 9 of 11) and without (59%, 58 of 99) suicide risk (moderate to high) at study enrolment (P=0.196). SVR was similar in those who did (74%, 23 of 31) and did not (63%, 36 of 57) develop new-onset depression (P=0.293). Lastly, there was no significant difference in SVR among participants with (56%, 9 of 16) and without (68%, 48 of 71) new-onset suicide risk (moderate to high) during treatment (P=0.388).
Figure 2.
Sustained virological response (SVR) among participants with and without depression and suicide risk (moderate to high levels)
Overall, 6% of treated participants (n=7) discontinued therapy early due to psychiatric side effects. At enrolment, none of these seven participants demonstrated depression as measured by MINI and all participants were in the normal/mild range of depressive symptoms as assessed by the DASS. Two of these participants were receiving antidepressants prior to HCV treatment. However, four of the seven developed new-onset depression during therapy.
Use of antidepressant medication prior to and during HCV treatment
Among treated participants, 41% (46 of 111) received antidepressants at any time from study enrolment to the end of HCV treatment: half (n=23) were receiving antidepressants at enrolment (six of whom discontinued antidepressants before HCV treatment initiation) and half (n=23) initiated antidepressants during treatment. Thirty five percent (11 of 31) of participants with new-onset depression initiated antidepressants during HCV treatment, while 16% (9 of 57) of those without enrolment or new-onset depression initiated antidepressants during HCV therapy (3 participants did not have available MINI assessments). Participants receiving antidepressants during HCV treatment were more likely to achieve SVR compared to those not receiving antidepressants (77% vs. 51%, P=0.006).
Post-treatment depression
Among treated participants with available assessments at both enrolment and six months following HCV treatment, there was no significant difference in the proportion with depression (9%, n=6 vs. 14%, n=9; P=0.065) and moderate/high suicide risk (14%, n=9, 12%, n=8; P=1.00) at enrolment compared to six months following treatment.
DISCUSSION
In this study of treatment of recently acquired HCV infection among a predominantly IDU population, depression prior to and during treatment was common, but did not impact response to therapy. Further, depression was an important indicator of treatment deferral, with relatively low rates of depression at study enrolment among those commencing treatment. Social marginalization characteristics were the major predictors of enrolment and new-onset depression. New-onset depression was also reversible, with similar rates of depression and suicide risk six months post-treatment compared to enrolment levels. Favourable HCV treatment outcomes among participants with enrolment and new-onset depression suggest that appropriate clinical and psychiatric management enabled successful delivery of therapy.
At study enrolment, the proportion with depression was three-fold higher among the untreated group with detectable HCV RNA who were eligible for therapy. This is consistent other studies which have demonstrated that depression is associated with HCV treatment deferral26–30. The association of depression with treatment deferral indicates appropriate initial clinical and psychiatric assessment.
In the ATAHC study, depression at study enrolment was associated with sociodemographic characteristics, including recent IDU and lack of employment. There has been limited evaluation of factors associated with depression among HCV-infected IDUs (prior to IFN based treatment). However, poor health related quality of life and personal wellbeing in IDUs with31 and without HCV infection32–33 is well documented in previous cross-sectional studies. Further, among IDUs, depression has been associated with sociodemographic factors such as unemployment and recent public injection14. In ATAHC, social functioning was associated with new-onset depression. Measurement of social functioning prior to treatment may provide a useful tool for predicting who may be at risk of developing psychiatric side effects during HCV treatment and require enhanced psychiatric assistance and monitoring.
Favourable HCV treatment outcomes were observed among participants with depression at enrolment and those with new-onset depression. The observation that psychiatric disease does not impair HCV treatment response is consistent with other studies of IDUs34 and non-IDUs8, 35. A recent study in patients with chronic HCV infection which excluded IDUs and those with severe psychiatric disorders, also did not find any significant association between new-onset depression and SVR rates36. In fact, some studies have demonstrated a higher SVR in those with depression. The suggested mechanism for an observed increase in SVR among those with depression is that depression may act as a pharmacodynamic surrogate for adequate drug levels of PEG-IFN36–38. In the ATAHC study, similar SVR rates among those with and without new-onset depression would appear to indicate appropriate psychiatric monitoring and management. Interestingly, only a third of participants with new-onset depression were commenced on anti-depressants, with high SVR in this group compared to the overall treated population.
This study has a number of limitations. Our definition of depression was based on participant self-report, rather than a medical diagnosis following consultation with a psychiatrist. However, MINI has good correlation with Structured Clinical Interview for DSM-IV-TR Axis I (SCID-I)23 and the Composite International Diagnostic Interview (CIDI)24. The MINI categorizes participants based on the presence or absence of major depressive episode as a dichotomous variable. The result of this is two-fold. First, the proportion with depression at enrolment may be underestimated, given that some patients with mild symptoms of depression may not have been detected using the MINI. Second, those with mild depression at baseline may have had a lower threshold for the development of new-onset depression, thus overestimating the proportion that developed new-onset depression during treatment. Lastly, the results may not be generalizable to other populations with HCV infection and particularly non-IDUs.
In conclusion, this study identified depression as an indicator of HCV treatment deferral at enrolment. Given the association between lower social functioning and depression at enrolment and during treatment, social functioning assessment may be a useful method to identify those at increased risk of depression before and/or during treatment. In addition, it was shown that mental health parameters (depression and/or suicide ideation) at enrolment or during treatment do not impact SVR. Therefore, given appropriate monitoring, patients with depression should be considered for HCV treatment.
Acknowledgments
Acknowledgments and disclosures:
This work was supported by the National Institutes of Health/National Institute of Drug Abuse grant [grant number RO1 DA 15999-01]; National Health and Medical Research Council Practitioner Research Fellowships to [GD and PH and AL]; a National Health and Medical Research Council Career Development Award and a VicHealth Senior Research Fellowship to [MH] and National Health and Medical Research Council Research Fellowship to [JK]. The Kirby institute for infection and immunity in society is funded by the Australian Government Department of Health and Ageing and is affiliated with the Faculty of Medicine, University of New South Wales. Roche Pharmaceuticals supplied financial support for pegylated IFN–alfa-2a/ribavirin.
ATAHC Study Group
Protocol Steering Committee members:
John Kaldor (The Kirby Institute), Gregory Dore (The Kirby Institute), Gail Matthews (The Kirby Institute), Pip Marks (The Kirby Institute), Andrew Lloyd (UNSW), Margaret Hellard (Burnet Institute, VIC), Paul Haber (University of Sydney), Rose Ffrench (Burnet Institute, VIC), Peter White (UNSW), William Rawlinson (UNSW), Carolyn Day (University of Sydney), Ingrid van Beek (Kirketon Road Centre), Geoff McCaughan (Royal Prince Alfred Hospital), Annie Madden (Australian Injecting and Illicit Drug Users League, ACT), Kate Dolan (UNSW), Geoff Farrell (Canberra Hospital, ACT), Nick Crofts (Nossal Institute, VIC), William Sievert (Monash Medical Centre, VIC), David Baker (407 Doctors).
The Kirby institute ATAHC Research Staff:
John Kaldor, Gregory Dore, Gail Matthews, Pip Marks, Barbara Yeung, Jason Grebely, Brian Acraman, Kathy Petoumenos, Janaki Amin, Carolyn Day, Anna Doab, Therese Carroll.
Burnet Institute Research Staff:
Margaret Hellard, Oanh Nguyen, Sally von Bibra.
Immunovirology Laboratory Research Staff:
UNSW Pathology - Andrew Lloyd, Suzy Teutsch, Hui Li, Alieen Oon, Barbara Cameron.
SEALS – William Rawlinson, Brendan Jacka, Yong Pan.
Burnet Institute Laboratory, VIC – Rose Ffrench, Jacqueline Flynn, Kylie Goy.
Clinical Site Principal Investigators:
Gregory Dore, St Vincent’s Hospital, NSW; Margaret Hellard, The Alfred Hospital, Infectious Disease Unit, VIC; David Shaw, Royal Adelaide Hospital, SA; Paul Haber, Royal Prince Alfred Hospital; Joe Sasadeusz, Royal Melbourne Hospital, VIC; Darrell Crawford, Princess Alexandra Hospital, QLD; Ingrid van Beek, Kirketon Road Centre; Nghi Phung, Nepean Hospital; Jacob George, Westmead Hospital; Mark Bloch, Holdsworth House GP Practice; David Baker, 407 Doctors; Brian Hughes, John Hunter Hospital; Lindsay Mollison, Fremantle Hospital; Stuart Roberts, The Alfred Hospital, Gastroenterology Unit, VIC; William Sievert, Monash Medical Centre, VIC; Paul Desmond, St Vincent’s Hospital, VIC.
Reference
- 1.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. New England Journal of Medicine. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 2.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 3.Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology. 2002;36:S237–S244. doi: 10.1053/jhep.2002.36810. [DOI] [PubMed] [Google Scholar]
- 4.Schaefer M, Engelbrecht MA, Gut O, et al. Interferon alpha (IFNalpha) and psychiatric syndromes: a review. [Review] [125 refs] Progress in Neuro Psychopharmacology & Biological Psychiatry. 2002;26:731–746. doi: 10.1016/s0278-5846(01)00324-4. [DOI] [PubMed] [Google Scholar]
- 5.Dieperink E, Ho SB, Thuras P, Willenbring ML. A prospective study of neuropsychiatric symptoms associated with interferon-alpha-2b and ribavirin therapy for patients with chronic hepatitis C. Psychosomatics. 2003;44:104–112. doi: 10.1176/appi.psy.44.2.104. [DOI] [PubMed] [Google Scholar]
- 6.Hauser P, Khosla J, Aurora H, et al. A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Molecular Psychiatry. 2002;7:942–947. doi: 10.1038/sj.mp.4001119. [DOI] [PubMed] [Google Scholar]
- 7.Kraus MR, Schafer A, Faller H, Csef H, Scheurlen M. Psychiatric symptoms in patients with chronic hepatitis C receiving interferon alfa-2b therapy. Journal of Clinical Psychiatry. 2003;64:708–714. doi: 10.4088/jcp.v64n0614. [DOI] [PubMed] [Google Scholar]
- 8.Schaefer M, Hinzpeter A, Mohmand A, et al. Hepatitis C treatment in "difficult-to-treat" psychiatric patients with pegylated interferon-alpha and ribavirin: response and psychiatric side effects. Hepatology. 2007;46:991–998. doi: 10.1002/hep.21791. [DOI] [PubMed] [Google Scholar]
- 9.SchÄfer A, Wittchen H-U, Seufert J, Kraus MR. Methodological approaches in the assessment of interferon-alfa-induced depression in patients with chronic hepatitis C – a critical review. International Journal of Methods in Psychiatric Research. 2007;16:186–201. doi: 10.1002/mpr.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [Erratum appears in Arch Gen Psychiatry. 2005 Jul;62(7):768 Note: Merikangas, Kathleen R [added]] [DOI] [PubMed] [Google Scholar]
- 11.el-Serag HB, Kunik M, Richardson P, Rabeneck L. Psychiatric disorders among veterans with hepatitis C infection. Gastroenterology. 2002;123:476–482. doi: 10.1053/gast.2002.34750. [DOI] [PubMed] [Google Scholar]
- 12.Kraus MR, Schafer A, Csef H, Scheurlen M, Faller H. Emotional state, coping styles, and somatic variables in patients with chronic hepatitis C. Psychosomatics. 2000;41:377–384. doi: 10.1176/appi.psy.41.5.377. [DOI] [PubMed] [Google Scholar]
- 13.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. [Review] [143 refs] The Lancet Infectious Diseases. 2005;5:558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 14.Topp L, Hudson SL, Maher L. Mental health symptoms among street-based psychostimulant injectors in Sydney's Kings Cross. Substance Use & Misuse. 2010;45:1180–1200. doi: 10.3109/10826080903443586. [DOI] [PubMed] [Google Scholar]
- 15.Darke S, Kaye S. Attempted suicide among injecting and noninjecting cocaine users in Sydney, Australia. Journal of Urban Health. 2004;81:505–515. doi: 10.1093/jurban/jth134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darke S, Ross J. Polydrug dependence and psychiatric comorbidity among heroin injectors. Drug & Alcohol Dependence. 1997;48:135–141. doi: 10.1016/s0376-8716(97)00117-8. [DOI] [PubMed] [Google Scholar]
- 17.Grebely J, Petoumenos K, Matthews GV, et al. Factors associated with uptake of treatment for recent hepatitis C virus infection in a predominantly injecting drug user cohort: The ATAHC Study. Drug & Alcohol Dependence. 2010;107:244–249. doi: 10.1016/j.drugalcdep.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hellard M, Sacks-Davis R, Gold J. Hepatitis C treatment for injection drug users: a review of the available evidence. clinical infectious diseases. 2009;49:561–573. doi: 10.1086/600304. [DOI] [PubMed] [Google Scholar]
- 19.Dore GJ, Hellard M, Matthews GV, et al. Effective treatment of injecting drug users with recently acquired hepatitis C virus infection. Gastroenterology. 2010;138:123–135. doi: 10.1053/j.gastro.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darke S, Hall W, Wodak A, Heather N, Ward J. Development and validation of a multi-dimensional instrument for assessing outcome of treatment among opiate users: the Opiate Treatment Index. British Journal of Addiction. 1992;87:733–742. doi: 10.1111/j.1360-0443.1992.tb02719.x. [DOI] [PubMed] [Google Scholar]
- 21.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. [Review] [25 refs] Journal of Clinical Psychiatry. 1998;20:22–33. [PubMed] [Google Scholar]
- 22.Henry JD, Crawford JR. The short-form version of the Depression Anxiety Stress Scales (DASS-21): construct validity and normative data in a large non-clinical sample. British Journal of Clinical Psychology. 2005;44:227–239. doi: 10.1348/014466505X29657. [DOI] [PubMed] [Google Scholar]
- 23.First MB, Spitzer RL, Gibbon M, Williams JBW. Biometrics Research. New York: New York State Psychiatric Institute; 2001. Structured clinical interview for DSM-IV-TR axis I disorders. Research version. Non-patient edition, (SCID-I/NP) [Google Scholar]
- 24.Robins LN, Wing J, Wittchen HU, et al. The Composite International Diagnostic Interview. An epidemiologic Instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Archives of General Psychiatry. 1989;45:1069–1077. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- 25.Grebely J, Matthews GV, Hellard M, et al. Adherence to treatment for recently acquired hepatitis C virus (HCV) infection among injecting drug users. Journal of Hepatology. 2011;55:76–85. doi: 10.1016/j.jhep.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bini EJ, Brau N, Currie S, et al. Prospective multicenter study of eligibility for antiviral therapy among 4,084 U.S. veterans with chronic hepatitis C virus infection. American Journal of Gastroenterology. 2005;100:1772–1779. doi: 10.1111/j.1572-0241.2005.41860.x. [DOI] [PubMed] [Google Scholar]
- 27.Butt AA, Justice AC, Skanderson M, Rigsby MO, Good CB, Kwoh CK. Rate and predictors of treatment prescription for hepatitis C. Gut. 2007;56:385–389. doi: 10.1136/gut.2006.099150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evon DM, Simpson KM, Esserman D, Verma A, Smith S, Fried MW. Barriers to accessing care in patients with chronic hepatitis C: the impact of depression. Alimentary Pharmacology & Therapeutics. 2010;32:1163–1173. doi: 10.1111/j.1365-2036.2010.04460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gidding HF, Law MG, Amin J, Macdonald GA, Sasadeusz JJ, Jones TL, Strasser SI, George J, Dore GJ. Predictors of deferral of treatment for hepatitis C infection in Australian clinics. The medical Journal of Australia. 2011;194:398–402. doi: 10.5694/j.1326-5377.2011.tb03029.x. [DOI] [PubMed] [Google Scholar]
- 30.Rifai MA, Moles JK, Short DD. Hepatitis C treatment eligibility and outcomes among patients with psychiatric illness. Psychiatric Services. 2006;57:570–572. doi: 10.1176/ps.2006.57.4.570. [DOI] [PubMed] [Google Scholar]
- 31.Marcellin F, Preau M, Ravaux I, Dellamonica P, Spire B, Carrieri MP. Self-reported fatigue and depressive symptoms as main indicators of the quality of life (QOL) of patients living with HIV and Hepatitis C: implications for clinical management and future research. HIV Clinical Trials. 2007;8:320–327. doi: 10.1310/hct0805-320. [DOI] [PubMed] [Google Scholar]
- 32.Dalgard O, Egeland A, Skaug K, Vilimas K, Steen T. Health-related quality of life in active injecting drug users with and without chronic hepatitis C virus infection. Hepatology. 2004;39:74–80. doi: 10.1002/hep.20014. [DOI] [PubMed] [Google Scholar]
- 33.Dietze P, Stoove M, Miller P, et al. The self-reported personal wellbeing of a sample of Australian injecting drug users. Addiction. 2010;105:2141–2148. doi: 10.1111/j.1360-0443.2010.03090.x. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt F, Janssen G, Martin G, et al. Factors influencing long-term changes in mental health after interferon-alpha treatment of chronic hepatitis C. Alimentary Pharmacology & Therapeutics. 2009;30:1049–1059. doi: 10.1111/j.1365-2036.2009.04123.x. [DOI] [PubMed] [Google Scholar]
- 35.Schaefer M, Schmidt F, Folwaczny C, et al. Adherence and mental side effects during hepatitis C treatment with interferon alfa and ribavirin in psychiatric risk groups. Hepatology. 2003;37:443–451. doi: 10.1053/jhep.2003.50031. [DOI] [PubMed] [Google Scholar]
- 36.Evon DM, Ramcharran D, Belle SH, et al. Prospective analysis of depression during peginterferon and ribavirin therapy of chronic hepatitis C: results of the Virahep-C study. American Journal of Gastroenterology. 2009;104:2949–2958. doi: 10.1038/ajg.2009.528. [DOI] [PubMed] [Google Scholar]
- 37.Loftis JM, Hauser P. The phenomenology and treatment of interferon-induced depression. [Review] [75 refs] Journal of Affective Disorders. 2004;82:175–190. doi: 10.1016/j.jad.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 38.Maes M, Yirmyia R, Noraberg J, et al. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. [Review] [210 refs] Metabolic Brain Disease. 2009;24:27–53. doi: 10.1007/s11011-008-9118-1. [DOI] [PubMed] [Google Scholar]