Abstract
Cardiovascular disease risk factors (CVDRFs) increase the risk of dementia. The purpose of this study was to examine whether leisure activities (mental, physical, and social activities) modified the effect of CVDRFs on inflammatory markers and cognitive function in middle and old age. A secondary-data analysis study was conducted using data from 405 middle-age participants (40 –59 years) and 342 old-age participants (60 – 84 years) who participated in the Survey of Midlife Development in the United States. CVDRFs were obtained from a combination of self-report medical history and blood-based biomarkers. Three CVDRF groups (≤1, 2, and ≥3 CVDRFs) were identified. More CVDRFs were significantly associated with higher levels of inflammatory markers in both age groups, and associated with lower levels of executive function in the old age group. CVDRFs were not related to the frequency of leisure activities in either age group. After controlling for covariates, higher levels of physical activities were significantly associated with lower levels of inflammatory markers, and higher levels of mental activities were associated with higher levels of cognitive function. In the old age group, physical activities also moderated the effect of CVDRFs on episodic memory, and mental activities moderated the effect of CVDRFs on interleukin-6. Multiple CVDRFs may be associated with poorer cognitive function and higher inflammatory markers, but middle-age and older adults with CVDRFs may not engage in frequent physical and cognitive activities that may be protective. It is important to develop strategies to facilitate engagement in these activities from midlife.
Keywords: physical activities, mental activities, cardiovascular disease risk factors, inflammatory markers, cognition
Introduction
Dementia is the sixth leading cause of mortality in the United States (Alzheimer’s Association, 2010). Alzheimer’s disease (AD) and vascular dementia (VaD) are the two most prevalent types of dementia, together accounting for 80% of all cases of dementia (Alzheimer’s Association, 2010). Recent converging evidence points to several cardiovascular disease risk factors (CVDRFs) associated with development of AD and VaD (Profenno et al., 2011; Yaffe et al., 2009). Over 40 million Americans aged 60 years or older have one or more types of cardiovascular disease (Roger et al., 2011). While it is important to control those known CVDRFs, it is also important to maintain cognitive function, an important indicator of functional independence and high quality of life, when individual are unable to maintain cardiovascular health. However, it is unclear whether older adults with CVDRFs are making efforts to prevent cognitive decline.
High levels of inflammatory markers, especially interleukin-6 (IL-6) and C-reactive protein (CRP), are common in individuals with CVDRFs. Inflammation further increases the risk of later cardiovascular events and cognitive decline (Kuo et al., 2005; Swardfager et al., 2011). An observational prospective study of older adults with metabolic syndrome found that higher levels of inflammatory markers were associated with cognitive decline (Yaffe et al., 2004). In contrast, in a study of middle- and old-age adults with diabetes, CRP was not associated with the severity and progression of white matter lesions (Umemura et al., 2011), suggesting a further clarification of the association between different CVDRFs and inflammatory markers in a wider age range is needed. If inflammation is one of the potential mechanisms linking CVDRFs and cognitive deficits in old age, it is possible that effectively managing inflammation in persons with CVDRFs may indirectly modify cognitive decline.
One way to potentially delay cognitive decline and prevent dementia is to engage in physical, social, and mental activities that may facilitate positive physiological changes in the central nervous system and brain (Stern, 2009). Recent studies have shown that physical activities, including walking, may help preserve cognition in older women with CVDRFs (Vercambre et al., 2011); and increased physical activities are also associated with decreased IL-6 in older adults with impaired glucose tolerance (Yates et al., 2011). Less is known about the protective effect of social or mental activities on cognitive function and inflammatory process in individuals with CVDRFs. There is evidence to suggest that only those activities which challenge established neuronal connections may act to reduce risk for dementia, possibly through increased neural plasticity. For example, in one study, reading and internet use stimulated brain regions important for memory and other higher order cognitive abilities (Small et al., 2009), while passive or less cognitively demanding activities which accommodate with existing cognitive abilities may not have a protective effect. For example, watching TV was found to have a negative association with cognitive performance in old age (Akbaraly et al., 2009).
The purpose of this study was to examine whether leisure activities (mental, physical, and social activities) modified the effect of number of CVDRFs on inflammatory markers (IL-6, and CRP) and cognitive function in middle and old age. While we cannot determine directionality of any putative association with a cross-sectional design, the comparison of two age groups will allow us to affirm such an association exists across adulthood. We attempted to address three specific aims: 1) to compare inflammatory markers, cognitive function, and leisure activities in middle- and old-age individuals with various CVDRFs, 2) to examine whether inflammatory markers would mediate the association between CVDRFs and cognitive function, and 3) to examine whether leisure activities would moderate the effect of number of CVDRFs on inflammatory markers and cognitive function.
Methods
Participants
The dataset used in this secondary-data analysis study was from the Survey of Midlife Development in the United States (MIDUS), an on-going nationally representative longitudinal survey of 7,108 non-institutionalized respondents, including twins (Brim et al., 2004). The baseline data (MIDUS I) collected between 1995 and 1996 focused on socio-demographic and psychosocial assessments. These assessments were repeated between 2004 and 2006 as MIDUS II projects 1 and 2 from 4963 participants (adjusted retention rate for mortality was 75% from MIDUS I to MIDUS II, more details can be found in Radler and Ryff’s study, 2010). Three new projects were added in MIDUS II, they were: assessments of cognitive function (MIDUS II project 3), biomarkers (project 4), and neuroscience (project 5). Data from MIDUS II projects 1, 3, and 4 was used for this analysis.
Project 3 was conducted using a series of cognitive tests administered over telephone. Project 4 included three General Clinical Research Centers (GCRCs) from the West Coast, Midwest, and East Coast. It involved a two-day clinic visit. Upon arrival at the GCRC, participants completed a detailed medical history interview with GCRC clinicians as well as a set of self-administrated questionnaires. Participants were asked to bring all current medications, which were recorded by project staff. Fasting blood samples were obtained the next morning to determine levels of biomarker of interest including IL-6 and CRP. Serum was isolated from all samples, aliquoted, frozen, shipped on dry ice to the appropriate laboratory, and stored for assay. The temperature of the sample was maintained at −80°C during the process. A previous study compared the sample characteristics between participants in Project 4 and those not in Project 4 but in Project 1. The education level was significantly higher in participants involved in Project 4, but other socio-demographic and health characteristics were similar (Morozink et al., 2011).
We included participants from project 4 (n = 1255), but excluded participants without completed biomarker data on CVDRFs (n = 11), without completed data on two domains of cognitive function (n = 37), younger than 40 years of age at MIDUS II (n = 437), and CRP > 10 pg/mL that is often indicative of acute infection (n = 23). The final analysis included 405 middle-age participants (40 – 59 years of age) and 342 old-age participants (60 – 84 years of age) (see Supplemental Figure Flow Chart). Among the final sample, there were 82 pairs of twins. To clarify the potential genetic influence on the relationships examined in this study, a supplemental analysis is reported at the end of the results.
Measures
CVDRFs
Six well-established indicators were considered as CVDRFs in this study according to AHA Guidelines for Primary Prevention of Cardiovascular Disease and Stroke (Pearson et al., 2002) and National Cholesterol Education Program Third Adult Treatment Panel guidelines (Adult Treatment Panel III, 2001). They were abdominal obesity, hypertension, hypercholesterolemia, hypertriglyceridemia, diabetes, and current smoker. The measurements and cutoff scores for each indicator are summarized in the Supplemental Table. Participants’ laboratory-based biomarkers and self-reported medical history were available from Projects 3 and 1, separately, at MIDUS II. A combination of both data was used in this study to avoid any potential bias from only self-reported data and to detect a subset of diagnosed cases that had been well-managed by medications.
Inflammatory Markers
Inflammatory factors were measured by assaying serum samples for IL-6 and CRP levels at MIDUS II project 4. Quantikine® high-sensitivity enzyme linked immunosorbent assay (ELISA) kits were used to measure the serum level of IL-6 (R&D Systems, Minneapolis, MN). The laboratory intra-assay coefficient of variance was 4.09% and the inter-assay coefficient of variance was 13% for IL-6. CRP was measured using the BNII nephelometer from Dade Behring utilizing a particle enhanced immunonepholometric assay (Dade Behring Inc., Deerfield, IL). The laboratory intra-assay coefficient of variance was 2.3 – 4.4% and the inter-assay coefficient of variance was 2.1 – 5.7% for CRP.
Cognitive Function
Cognitive function was measured using the Brief Test of Adult Cognition by Telephone (BTACT) (Tun and Lachman, 2006) at MIDUS II project 3, a neuropsychological battery of six cognitive tests, including measures of episodic verbal memory (Word List Immediate and Delayed), working memory span (Digits Backward), verbal fluency (Category Fluency), inductive reasoning (Number Series), and processing speed (Backward Counting). The telephone-version of BTACT test has been validated in the healthy elderly population (Tun and Lachman, 2006). Participants also completed by phone the Stop and Go Switch Task testing their attention switching and inhibitory control (Tun and Lachman, 2008). Two factor scores, episodic memory and executive function, were derived using exploratory and confirmatory factor analysis of the seven cognitive tests. In the exploratory factor analysis, the two factors were correlated, r(3341) = 0.42, p < 0.001, and accounted for 49% of the total variance in the original study; in the confirmatory factor analysis, the two factor solution provided a good fit (Lachman et al., 2011). More details regarding the factor analysis are provided elsewhere (Lachman et al., 2011). Participant’s individual Z-scores for each factor were used in the data analysis.
Leisure Activities
Leisure activities were separated and measured by three distinct checklists: mental, physical, and social. Participants were asked about frequency of engagement of these activities as part of the MIDUS II project 1. Mental activities included reading, doing word games, playing cards, attending lectures, writing, and using a computer. Each participant indicated the frequency of engaging in these activities using a 6-point ordinal scale ranging from 1 (daily) to 6 (never). Each response was reverse coded with higher mean scores indicating more frequent cognitive activities. This scale was used in previous study to examine the association with education and cognitive function (Lachman et al., 2011).
The checklist of moderate physical activities asked about the frequency of engaging in leisurely sports such as light tennis, slow or light swimming, low impact aerobics, golfing without a power cart, brisk walking, and mowing the lawn with a walking lawnmower during summer and winter time. Participants were asked to respond to each item on a 6-point ordinal scale ranging from 1 (several times a week) to 6 (never), and each response was reverse coded with higher mean scores indicating more frequent physical activities. Internal consistency (Cronbach’s α) of the 6 items was 0.85 in this study.
Social activities were measured with three items inquired about the frequency of attending meetings of union, sports, or other social groups outside the job in a typical month. The total number of times involved in these social activities was calculated. Although the pattern of response for social activities questions was not the same as that for the cognitive or physical activities, questions for the three types of activities were similar in terms of obtaining the frequency of engagement. The level of social activities was significantly related to the level of mental activities (r = 0.29, p < .001), but not physical activities (r = 0.03, p = .63) in this study.
Demographic and Health Information
Demographic information included age, sex, education (12 levels, from “no education” to “doctoral degree”), and race/ethnicity. Depression was measured using the 20-item Center for Epidemiological Studies Depression Inventory (CES-D) (Roberts and Vernon, 1983). Participants were asked whether they had experienced each of the 20 symptoms during the past week using a scale ranging from 0 (rarely or none of the time) to 3 (most or all of the time). The sum scores were calculated with higher scores indicating higher levels of depression. Reliability (internal consistency) was 0.89 at MIDUS II. Histories of cancer and four heart conditions (stroke, heart failure, angina, and heart attack) were collected.
Data Analysis
Analyses were conducted using IBM SPSS 19.0 (Wagner III, 2011). IL-6 and CRP data and frequency of social activities were natural-log transformed before addressing specific aims given their skewness. To examine whether demographic and health characteristics differed by number of CVDRFs in middle- and old-age participants, one-way ANOVA was applied for continuous variables and a Chi-square test was applied for categorical variables. Levene’s test was used to test equality of variances across groups. Bonferroni’s post-hoc test was used for comparisons with equal variances across CVDRFs groups and Games-Howell post-hoc test was used for unequal variances.
To examine whether leisure activities, inflammatory markers, and cognitive function differed by CVDRFs × age group, univariate general linear models were applied taking CVDRFs and age groups as two fixed factors.
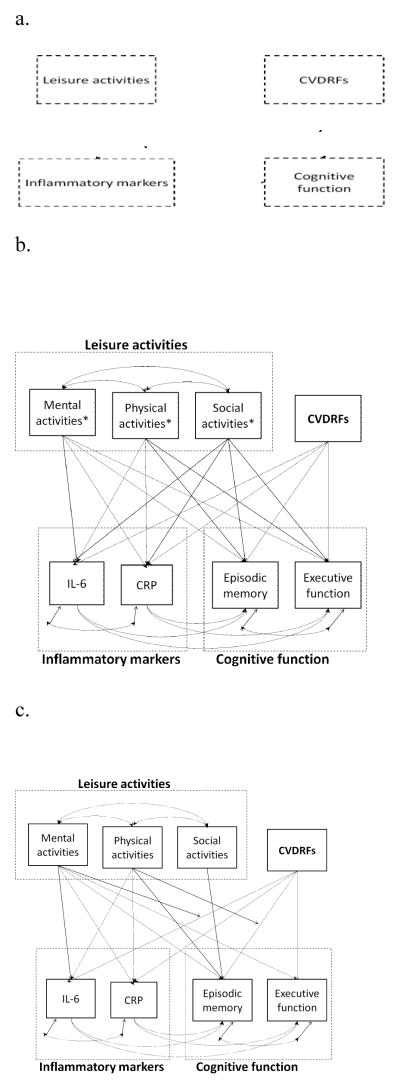
Mplus (Version 5) was used for structural equation modeling (SEM) (Muthén and Muthén, 2007), and the model goodness-of-fit was determined by several fit indices with a minimum criteria for acceptability as CFI > 0.90, RMSEA < 0.08, and SRMR < 0.05 (Browne et al., 1993). Maximum likelihood was used as the estimator. Our conceptual model is shown in Figure 1a. A series of structural models were tested using variables from leisure activities, inflammatory markers, and cognitive function as separate factors as well as grouped as latent variables. Models with all participants, middle-age participants, and old-age participants were tested separately.
Figure 1.
Conceptual and structural models of the relationships between CVDRFs, leisure activities, inflammatory markers, and cognitive function.
a. Conceptual model
b. Overall structural model. Note. Age and sex were covariates of inflammatory markers and cognitive function; education and depression were covariates of cognitive function. * Paths between the interactions of each leisure activities with CVDRFs and each inflammatory factors and cognitive function factors were also included in the model.
c. Final trimmed structural model with best model fit. Note. Age and sex were covariates of inflammatory markers and cognitive function; education and depression were covariates of cognitive function.
Statistical significance on Bonferroni’s test was evaluated using alpha level of 0.018, and the remaining tests were evaluated using an overall alpha level of 0.05.
Results
According to the cut-off scores of measurements for CVDRFs, in middle-age participants, there were 20.5% with 1 CVDRF (n = 83), 29.9% with 2 CVDRFs (n = 121), 25.7% with 3 CVDRFs (n = 104), 17.5% with 4 CVDRFs (n = 71), 5.9% with 5 CVDRFs (n = 24), and 0.5% with 6 CVDRFs (n = 2). In old-age participants, there were 5.0% without any CVDRFs (n = 17), 27.2% with 1 CVDRF (n = 93), 32.7% with 2 CVDRFs (n = 112), 19.9% with 3 CVDRFs (n = 68), 10.5% with 4 CVDRFs (n = 36), and 4.7% with 5 CVDRFs (n = 16). Given the small number of participants without any CVDRFs, participants with 0 or 1 CVDRF were combined into a group, while those with 3 to 6 CVDRFs were combined into a group in the following analysis. Descriptive data for laboratory biomarkers and self-report medical history of CVDRFs, cut-off scores for each measurement, and number of participants with each CVDRF are in the Supplemental Table.
Table 1 displays the demographic and health characteristics of middle- and old-age participants by CVDRFs group.
Table 1.
Descriptive Data for Demographic and Health Characteristics (Total = 747)
| Middle-age group: < 60 years (n = 405)
|
Old-age group: ≥ 60 years (n = 342)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| CVDRFs ≤ 1 (n = 83) | CVDRFs = 2 (n = 121) | CVDRFs ≥ 3 (n = 201) | F or X2 | CVDRFs ≤ 1 (n = 110) | CVDRFs = 2 (n = 112) | CVDRFs ≥ 3 (n = 120) | F or X2 | |
| Age, mean (SD) | 49.47 (5.96) | 50.04 (5.40) | 50.70 (5.93) | 1.45 | 69.68 (6.33) d | 67.56 (6.52) e | 68.33 (6.33) d, e | 3.13* |
| Racial group, n (%) | 0.23 | 3.95 | ||||||
| •White | 77 (92.8%) | 111 (91.7%) | 183 (91.5%) | 107 (97.3%) | 104 (92.9%) | 117 (97.5%) | ||
| •African American | 2 (2.4%) | 3 (2.5%) | 5 (2.5%) | 1 (0.9%) | 4 (3.6%) | 2 (1.7%) | ||
| •Native American | 0 | 1 (0.8%) | 4 (2.0%) | 0 | 2 (1.8%) | 0 | ||
| •Asian | 0 | 1 (0.8%) | 1 (.5%) | 0 | 0 | 0 | ||
| •Others | 4 (4.8%) | 5 (4.1%) | 7 (3.5%) | 2 (1.8%) | 2 (1.8%) | 1 (0.8%) | ||
| Male, n (%) | 26 (31.3%) b | 45 (37.2%) b | 115 (57.2%) c | 21.16*** | 49 (44.5%) | 52 (46.4%) | 66 (55.0%) | 2.90 |
| Education, n (%) | 11.69* | 10.38* | ||||||
| •High School graduate, GED or less | 25 (30.1%) b | 30 (24.8%) b | 42 (20.9%) b | 23 (20.9%) b | 42 (37.5%) c | 38 (31.7%) b, c | ||
| •Some college | 16 (19.3%) b | 30 (24.8%) b, c | 75 (37.3%) c | 32 (29.1%) b | 29 (25.9%) b | 40 (33.3%) b | ||
| •College graduate | 42 (50.6%) b | 61 (50.4%) b | 84 (41.8%) b | 55 (50.0%) b | 41 (36.6%) b, c | 41 (34.2%) c | ||
| Depression (CES-D), mean (SD) | 7.95 (7.57) | 9.42 (8.62) | 9.59 (8.89) | 1.14 | 7.01 (6.50) | 5.64 (6.35) | 7.45 (5.98) | 2.59 |
| Self-reported history of cancer, n (%) | 8 (9.6%) | 7 (5.8%) | 13 (6.5%) | 1.25 | 25 (22.7%) | 23 (20.5%) | 27 (23.0%) | 0.23 |
| Self-reported history of stroke, n (%) | 1 (1.2%) | 0 | 0 | - a | 0 | 1 (0.9%) | 1 (0.8%) | - a |
| Self-reported history of heart failure, n (%) | 0 | 0 | 2 (1.0%) | - a | 0 | 0 | 1 (0.8%) | - a |
| Self-reported history of angina, n (%) | 0 | 1 (0.8%) | 1 (0.5%) | - a | 2 (1.8%) | 0 | 2 (1.7%) | - a |
| Self-reported history of heart attack, n (%) | 3 (3.6%) | 1 (0.8%) | 1 (0.5%) | - a | 3 (2.7%) | 5 (4.5%) | 3 (2.5%) | 2.26 |
Note.
Sample sizes in subgroups were too small to compare the difference.
Each subscript letter denotes a subset of education categories whose column proportions do not differ significantly from each other.
p < .05;
p < .01;
p < .001.
Inflammatory Markers, Cognitive Function, and Leisure Activities by CVDRFs Group in Middle- and Old-Age Participants
Table 2 displays the inflammatory markers, cognitive function, and leisure activities by CVDRFs × age group. Compared to the old-age group, the middle-age group engaged in significantly higher levels of physical activities (t = 3.07, p = .002), had significantly lower levels of IL-6 (t = −3.28, p = .001) and higher levels of episodic memory (t = 7.08, p < .001) and executive function (t = 9.75, p < .001). The two age groups did not differ in the levels of mental or social activities or levels of CRP. Within middle-age participants, the CVDRFs groups significantly differed in inflammatory markers. The groups with ≤ 1 CVDRFs had significantly lower levels of IL-6 and CRP than the other two groups. Within old-age participants, the CVDRFs groups significantly differed in inflammatory markers and cognitive function. The groups with ≤ 1 or 2 CVDRFs had significantly lower levels of IL-6 and CRP and better episodic memory and executive function than the group with ≥ 3 CVDRFs. No difference was found in the engagement in any types of leisure activities by CVDRFs group in either age group.
Table 2.
Descriptive Data for Leisure Activities, Inflammation, and Cognitive Function
| Middle-age group | Old-age group | |||||||
|---|---|---|---|---|---|---|---|---|
| CVDRFs ≤ 1 (n = 83) | CVDRFs = 2 (n = 121) | CVDRFs ≥ 3 (n = 201) | F | CVDRFs ≤ 1 (n = 110) | CVDRFs = 2 (n = 112) | CVDRFs ≥ 3 (n = 120) | F | |
| Mental activities, mean (SD) | 3.34 (0.73) | 3.25 (0.75) | 3.21 (0.82) | 0.88 | 3.23 (0.87) | 3.18 (0.84) | 3.21 (0.90) | 0.10 |
| Physical activities, mean (SD) b | 3.52 (1.35) | 3.61 (1.26) | 3.71 (1.37) | 0.59 | 3.26 (1.36) | 3.47 (1.47) | 3.26 (1.44) | 0.78 |
| Social activities a, mean (SD) | 1.07 (1.01) | 1.06 (0.96) | 1.02 (0.98) | 0.11 | 1.19 (1.02) | 1.29 (1.03) | 1.10 (1.01) | 1.00 |
| IL-6 a, mean (SD) b | 0.52 (0.60) c | 0.71 (0.70) d | 0.80 (0.65) d | 5.24** | 0.78 (0.64) c | 0.75 (0.64) c | 1.08 (0.72) d | 8.78*** |
| CRP a, mean (SD) | −0.04 (1.08) c | 0.44 (1.01) d | 0.60 (0.94) d | 12.43*** | 0.002 (1.00) c | 0.28 (0.92) c | 0.59 (0.92) d | 11.02*** |
| Episodic memory, mean (SD) b | 0.22 (0.74) | 0.28 (0.85) | 0.14 (0.89) | 1.00 | −0.08 (0.91) c | −0.28 (0.95) c, d | −0.39 (0.85) d | 3.30* |
| Executive function, mean (SD) b | 0.41 (0.76) | 0.31 (0.82) | 0.32 (0.83) | 0.39 | −0.19 (0.77) c, d | −0.15 (0.90) c | −0.40 (0.79) d | 3.21* |
Note.
Log-transformed scores.
Scores of the variables were significantly differed by age group.
Each subscript letter denotes a subset of education categories whose column proportions do not differ significantly from each other.
p < .05;
p < .01;
p < .001.
Associations of the Number of CVDRFs, Leisure Activities, Inflammatory Markers, and Cognitive Function
After testing a series of models using observational variables separately or grouped as latent variables, the best model generated was using all observational variables separately. Thus, path analysis was used in the following steps. The overall models were first developed including paths between each two variables using the whole sample and two age groups separately (Figure 1b and Supplemental Table 2). We failed to identify any significant mediating effect of inflammatory markers on the association between CVDRFs and cognitive function (the “Indirect effect” in Supplemental Table 2).
Next, the trimmed models (Figure 1c) were developed excluding some paths that were not statistically significant in any overall models. Model fit was acceptable (see Table 3). There was no significant change of X2 between the overall and trimmed models (ΔX2 : 9.14 – 11.31, Δdf = 10, p > 0.05), but the discrepancy values of RMSEA and SRMR were smaller in the trimmed models than those in the overall models. All covariates (i.e., age, sex, education, and depression) were significantly related to one or more main variables in the trimmed models.
Table 3.
Model Fit and Estimates of Direct and Indirect Effect between CVDRFs, Leisure Activities, Inflammatory Markers, and Cognitive Function in Final Trimmed Models
| Total sample (N = 747) | Middle-age group (n = 405) | Old-age group (n = 342) | |
|---|---|---|---|
| Model fit | X2 = 36.93, df = 16, p = 0.002; CFI = 0.973; RMSEA = 0.042; SRMR =0.020. | X2 = 30.78, df = 16, p = 0.014; CFI = 0.956; RMSEA = 0.048; SRMR =0.025. | X2 = 26.55, df = 16, p = 0.047; CFI = 0.966; RMSEA = 0.044; SRMR = 0.021. |
| Direct effect | |||
| •CVDRFs – IL-6 | 0.146 | 0.136 | 0.166 |
| •CVDRFs – CRP | 0.302 | 0.331 | 0.292 |
| •CVDRFs – EM | −0.022 | 0.060 | −0.091 |
| •CVDRFs – EF | −0.060 | −0.011 | −0.122 |
| •Mental activities – IL-6 | 0.093 | 0.010 | 0.162 |
| •Mental activities – CRP | 0.101 | 0.133 | 0.086 |
| •Mental activities – EM | 0.131 | 0.137 | 0.126 |
| •Mental activities – EF | 0.279 | 0.270 | 0.299 |
| •Physical activities – IL-6 | −0.062 | −0.048 | −0.065 |
| •Physical activities – CRP | −0.117 | −0.122 | −0.109 |
| •Physical activities – EM | −0.073 | 0.116 | −0.263 |
| •Social activities – EM | −0.029 | 0.031 | −0.096 |
| Interaction with CVDRFs | |||
| •Mental activities – IL-6 | −0.043 | 0.005 | −0.089 |
| •Physical activities – EM | 0.042 | −0.036 | 0.125 |
Note. Note. Age and sex were covariates of inflammatory markers and cognitive function; education and depression were covariates of cognitive function. Bold data indicate significant estimates. EM = Episodic memory; EF = Executive function.
After controlling for age, sex, education, and depression, more CVDRFs were significantly related to higher levels of inflammatory markers. Engaging in higher levels of mental activities were significantly related to higher levels of episodic memory and executive function. Engaging in higher levels of physical activities was significantly related to lower levels of inflammatory markers. Results were similar when separating participants into middle- and old-age groups. But in the old-age group, more CVDRFs were significantly related to lower levels of executive function; while in the middle-age group, higher levels of mental activities were significantly related to higher levels of CRP (the “Direct effect” in Table 3). In the old-age group, physical activities significantly moderated the effect of CVDRFs on episodic memory, and mental activities significantly moderated the effect of CVDRFs on IL-6 (the “Interaction with CVDRFs” in Table 3).
Supplemental Analysis: Influence of Twin Status on Cognitive Function and Inflammation Factors
An additional analysis was done to assess whether twin status affected the findings from the regression models reported above. Four regression models were recomputed with the subsample randomly excluding one member from each twin pair (n = 307). Changes in the size of standardized coefficients did not influence the statistical significance of any results from previous tests.
Discussion
Our cross-sectional study included 747 middle-age and older adults participating in a national longitudinal community study, 97.8% of whom had at least one CVDRF based on a combination of laboratory biomarkers and self-reported medical history. Older but not middle-age participants with various CVDRFs significantly differed in levels of executive function. Both age groups with various CVDRFs significantly differed in levels of inflammatory markers, but they were similar in their engagement in three types of leisure activities (mental, physical, and social). We failed to identify the mediating role of inflammatory markers between CVDRFs and cognitive function. However, across middle and late adulthood, engaging in more mental activities were significantly associated with better cognitive function, while engaging in more physical activities were significantly associated with lower levels of inflammation, considering the influence of demographic and health characteristics and number of CVDRFs. Furthermore, in the old age group, physical activities moderated the effect of CVDRFs on episodic memory, and cognitive activities moderated the effects of CVDRFs on IL-6.
Expanding from the findings of previous studies that the presence of individual CVDRFs influences cognitive function and inflammatory markers (Peters et al., 2008; Raffaitin et al., 2011; Wang et al., 2011), our study found that the levels of inflammatory markers differed by the number of CVDRFs in both middle and old age, but the negative association between cognitive function and number of CVDRFs was only found in old age. The major difference occurred between groups with ≥2 CVDRFs and with single CVDRFs in both age groups. The finding is consistent with several studies that co-existing diabetes and hypertension increase the risk of developing dementia by six-fold (Posner et al., 2002), while co-existing diabetes, hypertension, obesity, hypercholesterolemia, and smoking increase the risk of developing AD by 20-fold (Reitz et al., 2011). The underlying mechanism is unclear, but it is likely that these CVDRFs share similar endothelial dysfunction and atherosclerosis, and co-existence CVDRFs may independently and/or interactively enhance inflammatory process, cerebral glucose and energy metabolism and cerebrovascular pathological changes that are related to cognitive decline (Kuczynski et al., 2009; Panza et al., 2011). However, such accumulated effect of CVDRFs on cognitive function would not start manifesting until old age.
Our findings extend our understanding of the relationship between CVDRFs and cognitive function in several ways. First, regardless of the number of CVDRFs, we found no difference in the frequency of engagement in leisure activities in neither middle- nor old-age group. This is surprising as it suggests that middle-age and older adults with CVDRFs may not be fully aware of the benefits of leisure activities on inflammation or cognitive function. Second, when considering the influence of demographic and health characteristics, number of CVDRFs, and other leisure activities, engaging in a higher frequency of mental activities was significantly associated with higher cognitive function, and higher frequency of physical activities was associated with lower levels of inflammatory markers. Further, in the old age group, mental activities moderated the effect of number of CVDRFs on levels of IL-6, and physical activities moderated the effect of CVDRFs on episodic memory. Evidence from meta-analysis of longitudinal cohort studies and randomized controlled trials support that there is a significant protective effect of mental activities on cognition in healthy older adults without cognitive impairment and those with mild cognitive impairment (Martin et al., 2011; Valenzuela and Sachdev, 2009; Valenzuela and Sachdev, 2006). Our findings suggest that mental activities, along with physical exercise, may independently protect cognition or the inflammatory process, in middle- and old-age adults with various CVDRFs. The mechanisms that might link mental and physical activities to inflammatory markers and cognitive function are likely multifactorial. The first hypothesis may be applied in any aging group in addition to those with neurodegenerative or metabolic disorders. Engaging in mental and physical activities provides an enriched environment that enhances neural plasticity in terms of stimulating brain-derived neurotrophic factor (BDNF) and neuronal growth, and results in reserve against cognitive decline (Kramer et al., 2004), while low levels of BDNF were found in patients with diabetes and obesity (Pedersen et al., 2009). The second hypothesis may be specific to people with vascular risk. That is, stimulating activities may also enhance brain vascular health by enhancing endothelial function, while inflammatory markers are an important marker of endothelial function (Fratiglioni et al., 2004). The third hypothesis is based on the stress theory. Leisure activities may help with relaxation, which reduces the secretion of corticosterone as well as alleviate inflammatory process. Chronic hypersecretion of corticosterone and inflammatory processes can cause permanent damage of neurons that lead to cognitive deficits and dementia. Moreover, a previous longitudinal study identified the protective effect of physical exercise (regular walking) on cognitive change over time in older women with at least 3 coronary risk factors (Vercambre et al., 2011). Our data are in line with this observation with respect to the protective effect of exercise against high CVDRFs load.
What should be noticed is that physical exercise can influence various health conditions; thus, individuals with CVDRFs may recognize its importance even though they may not be aware of the possible relationship between physical exercise and cognitive decline. In contrast, mental activities have been theorized to have relatively specific protective effects on cognitive functioning (Stern, 2009). Older adults with a large number of CVDRFs may not recognize the risk of developing cognitive deficits nor the relationship between mental activities and cognitive functioning; thus, they may not engage in mental behaviors. It may be equally important to develop strategies to facilitate their engagement in cognitive activities as well as to deliver cognitive training programs for older adults with CVDRFs.
As one of the strengths of this study, we defined CVDRFs using a combination of laboratory-based biomarkers and self-reported medical history to obtain an accurate profile of CVDRFs. Second, we included measures of peripheral concentrations of inflammatory factors (IL-6 and CRP). Our results showing an interaction between cognitively stimulating activities and CVDRFs in predicting IL-6 adds new evidence of a potential protective effect of cognitively stimulating activities on cognitive function, while previous studies focused on examining mental activities and cognitive tests results (Akbaraly et al., 2009). Third, we comprehensively measured stimulating leisure activities covering physical, social, and cognitive types. Finally, we involved a sample across middle- and late-adulthood. It is interesting that the middle- and old-age groups did not differ in their engagement in mental activities. Although there was no significant association between number of CVDRFs and cognitive function in the middle-age group, using preventative strategies such as mental activities to slow cognitive decline in late life should be initiated as early as midlife.
A major limitation of this study relates to the cross-sectional nature of the design. Although mental and physical activities were found to be associated with inflammatory markers and cognitive function, we were not able to determine their causal relationships. Empirical literature on these relationships has been mixed. For example, one study found childhood cognitive abilities predicted late-life inflammation (Luciano et al., 2009), and another study found participation of leisure activities was related to the level but not the change of cognitive function over time (Bielak et al., 2011). A future prospective design should be able to address the causal relationships between cognitive or physical activities on cognitive function as mediated by underlying inflammatory process in older adults with high CVDRFs load. Second, given the relatively small sample, the associations of individual CVDRFs and self-reported cognitive factors with objective cognitive factors were not explored further. The common co-existence of CVDRFs in old age, however, reduces the likelihood of the influence of individual CVDRFs on cognitive deficits. Third, this study included a relatively young old age group, while the risks of both CVDRFs and cognitive decline substantially increased in the old-old age group. However, because we included a relatively young age group, we were able to study a group that might be considered relatively free of overt dementia.
Middle-age and older adults with multiple CVDRFs may be at risk of impaired inflammatory circulation; further, older adults with multiple CVDRFs may also be at risk of cognitive decline. However, they may not engage in frequent physical and cognitive activities that may be protective against the effect of CVDRFs on cognitive function and inflammatory process. Given the high prevalence of CVDRFs in middle-age and older adults and the high likelihood of cognitive decline in older adults with CVDRFs, our findings help define areas for intervention in these individuals from midlife and lay the groundwork for future prospective studies.
Supplementary Material
Acknowledgments
MIDUS II was supported by from the National Institute on Aging (P01-AG020166) to conduct a longitudinal follow-up of the MIDUS (Midlife in the U.S.) investigation.
Footnotes
CONFLICT OF INTEREST STATEMENT:
None of the authors has any financial or personal relationships with other people or organizations that could inappropriately influence their work.
The authors report no personal or financial disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Feng Lin, Email: vankee_lin@urmc.rochester.edu, School of Nursing, University of Rochester, Helen Wood Hall, 601 Elmwood Avenue, Rochester, NY 14642, Telephone: 585-276-6002, Fax: 585-273-1270.
Elliot Friedman, Institute on Aging, University of Wisconsin-Madison.
Jill Quinn, School of Nursing, University of Rochester.
Ding-Geng (Din) Chen, School of Nursing, University of Rochester, Department of Biostatics and Computational Biology, School of Medicine and Dentistry, University of Rochester.
Mark Mapstone, Department of Neurology, School of Medicine and Dentistry, University of Rochester.
References
- Adult Treatment Panel III. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- Akbaraly TN, Portet F, Fustinoni S, Dartigues JF, Artero S, Rouaud O, Touchon J, Ritchie K, Berr C. Leisure activities and the risk of dementia in the elderly: results from the Three-City Study. Neurology. 2009;73:854–861. doi: 10.1212/WNL.0b013e3181b7849b. [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Association. Alzheimers Dement. 2010/03/20. 2010. Alzheimer’s disease facts and figures; pp. 158–194. [DOI] [PubMed] [Google Scholar]
- Bielak AA, Anstey KJ, Christensen H, Windsor TD. Activity engagement is related to level, but not change in cognitive ability across adulthood. Psychol Aging. doi: 10.1037/a0024667. [DOI] [PubMed] [Google Scholar]
- Brim OG, Ryff CD, Kessler RC. How healthy are we?: A national study of well-being at midlife. University of Chicago Press; 2004. [Google Scholar]
- Browne MW, Cudeck R. Alternative ways of assessing model fit. SAGE FOCUS EDITIONS. 1993;154:136–136. [Google Scholar]
- Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3:343–353. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Bherer L, Colcombe SJ, Dong W, Greenough WT. Environmental influences on cognitive and brain plasticity during aging. J Gerontol A Biol Sci Med Sci. 2004;59:M940–957. doi: 10.1093/gerona/59.9.m940. [DOI] [PubMed] [Google Scholar]
- Kuczynski B, Jagust W, Chui HC, Reed B. An inverse association of cardiovascular risk and frontal lobe glucose metabolism. Neurology. 2009;72:738–743. doi: 10.1212/01.wnl.0000343005.35498.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo HK, Yen CJ, Chang CH, Kuo CK, Chen JH, Sorond F. Relation of C-reactive protein to stroke, cognitive disorders, and depression in the general population: systematic review and meta-analysis. The Lancet Neurology. 2005;4:371–380. doi: 10.1016/S1474-4422(05)70099-5. [DOI] [PubMed] [Google Scholar]
- Lachman ME, Agrigoroaei S, Murphy C, Tun PA. Frequent cognitive activity compensates for education differences in episodic memory. Am J Geriatr Psychiatry. 2011;18:4–10. doi: 10.1097/JGP.0b013e3181ab8b62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano M, Marioni RE, Gow AJ, Starr JM, Deary IJ. Reverse causation in the association between C-reactive protein and fibrinogen levels and cognitive abilities in an aging sample. Psychosom Med. 2009;71:404–409. doi: 10.1097/PSY.0b013e3181a24fb9. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, Clare L, Altgassen AM, Cameron MH, Zehnder F. Cognition-based interventions for healthy older people and people with mild cognitive impairment. Cochrane Database Syst Rev. 2011:CD006220. doi: 10.1002/14651858.CD006220.pub2. [DOI] [PubMed] [Google Scholar]
- Morozink JA, Friedman EM, Coe CL, Ryff CD. Socioeconomic and psychosocial predictors of interleukin-6 in the MIDUS national sample. Health Psychol. 2011;29:626–635. doi: 10.1037/a0021360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. 2007. [Google Scholar]
- Panza F, Frisardi V, Capurso C, Imbimbo BP, Vendemiale G, Santamato A, D’Onofrio G, Seripa D, Sancarlo D, Pilotto A, Solfrizzi V. Metabolic syndrome and cognitive impairment: current epidemiology and possible underlying mechanisms. J Alzheimers Dis. 2011;21:691–724. doi: 10.3233/JAD-2010-091669. [DOI] [PubMed] [Google Scholar]
- Pearson TA, Blair SN, Daniels SR, Eckel RH, Fair JM, Fortmann SP, Franklin BA, Goldstein LB, Greenland P, Grundy SM, Hong Y, Miller NH, Lauer RM, Ockene IS, Sacco RL, Sallis JF, Jr, Smith SC, Jr, Stone NJ, Taubert KA. AHA Guidelines for Primary Prevention of Cardiovascular Disease and Stroke: 2002 Update: Consensus Panel Guide to Comprehensive Risk Reduction for Adult Patients Without Coronary or Other Atherosclerotic Vascular Diseases. American Heart Association Science Advisory and Coordinating Committee. Circulation. 2002;106:388–391. doi: 10.1161/01.cir.0000020190.45892.75. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Pedersen M, Krabbe KS, Bruunsgaard H, Matthews VB, Febbraio MA. Role of exercise-induced brain-derived neurotrophic factor production in the regulation of energy homeostasis in mammals. Exp Physiol. 2009;94:1153–1160. doi: 10.1113/expphysiol.2009.048561. [DOI] [PubMed] [Google Scholar]
- Peters R, Poulter R, Warner J, Beckett N, Burch L, Bulpitt C. Smoking, dementia and cognitive decline in the elderly, a systematic review. BMC Geriatr. 2008;8:36. doi: 10.1186/1471-2318-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner HB, Tang MX, Luchsinger J, Lantigua R, Stern Y, Mayeux R. The relationship of hypertension in the elderly to AD, vascular dementia, and cognitive function. Neurology. 2002;58:1175–1181. doi: 10.1212/wnl.58.8.1175. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008;40:879. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Profenno LA, Porsteinsson AP, Faraone SV. Meta-analysis of Alzheimer’s disease risk with obesity, diabetes, and related disorders. Biol Psychiatry. 2011;67:505–512. doi: 10.1016/j.biopsych.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Radler BT, Ryff CD. Who participates? Accounting for longitudinal retention in the MIDUS national study of health and well-being. J Aging Health. 2010;22:307–331. doi: 10.1177/0898264309358617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaitin C, Feart C, Le Goff M, Amieva H, Helmer C, Akbaraly TN, Tzourio C, Gin H, Barberger-Gateau P. Metabolic syndrome and cognitive decline in French elders: the Three-City Study. Neurology. 2011;76:518–525. doi: 10.1212/WNL.0b013e31820b7656. [DOI] [PubMed] [Google Scholar]
- Reitz C, Tang MX, Schupf N, Manly JJ, Mayeux R, Luchsinger JA. A summary risk score for the prediction of Alzheimer disease in elderly persons. Arch Neurol. 2011;67:835–841. doi: 10.1001/archneurol.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RE, Vernon SW. The Center for Epidemiologic Studies Depression Scale: its use in a community sample. Am J Psychiatry. 1983;140:41–46. doi: 10.1176/ajp.140.1.41. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J American Heart Association Statistics C, Stroke Statistics S. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small GW, Moody TD, Siddarth P, Bookheimer SY. Your brain on Google: patterns of cerebral activation during internet searching. Am J Geriatr Psychiatry. 2009;17:116–126. doi: 10.1097/JGP.0b013e3181953a02. [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swardfager W, Lanctot K, Rothenburg L, Wong A, Cappell J, Herrmann N. A meta-analysis of cytokines in Alzheimer’s disease. Biol Psychiatry. 2011;68:930–941. doi: 10.1016/j.biopsych.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Tun PA, Lachman ME. Telephone assessment of cognitive function in adulthood: the Brief Test of Adult Cognition by Telephone. Age Ageing. 2006;35:629–632. doi: 10.1093/ageing/afl095. [DOI] [PubMed] [Google Scholar]
- Tun PA, Lachman ME. Age differences in reaction time and attention in a national telephone sample of adults: education, sex, and task complexity matter. Dev Psychol. 2008;44:1421–1429. doi: 10.1037/a0012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemura T, Kawamura T, Umegaki H, Mashita S, Kanai A, Sakakibara T, Hotta N, Sobue G. Endothelial and inflammatory markers in relation to progression of ischaemic cerebral small-vessel disease and cognitive impairment: a 6-year longitudinal study in patients with type 2 diabetes mellitus. J Neurol Neurosurg Psychiatry. 2011;82:1186–1194. doi: 10.1136/jnnp.2010.217380. [DOI] [PubMed] [Google Scholar]
- Valenzuela M, Sachdev P. Can cognitive exercise prevent the onset of dementia? Systematic review of randomized clinical trials with longitudinal follow-up. Am J Geriatr Psychiatry. 2009;17:179–187. doi: 10.1097/JGP.0b013e3181953b57. [DOI] [PubMed] [Google Scholar]
- Valenzuela MJ, Sachdev P. Brain reserve and dementia: a systematic review. Psychol Med. 2006;36:441–454. doi: 10.1017/S0033291705006264. [DOI] [PubMed] [Google Scholar]
- Vercambre MN, Grodstein F, Manson JE, Stampfer MJ, Kang JH. Physical Activity and Cognition in Women With Vascular Conditions. Arch Intern Med. 2011 doi: 10.1001/archinternmed.2011.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner WE., III . Using IBM?SPSS?Statistics for Social Statistics and Research Methods. Pine Forge Press; 2011. [Google Scholar]
- Wang S, Jacobs D, Andrews H, Tsai WY, Luo X, Bergmann C, Sano M. Cardiovascular risk and memory in non-demented elderly women. Neurobiol Aging. 2011;31:1250–1253. doi: 10.1016/j.neurobiolaging.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, Shorr RI, Tylavsky FA, Newman AB. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. 2004;292:2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Weston AL, Blackwell T, Krueger KA. The metabolic syndrome and development of cognitive impairment among older women. Arch Neurol. 2009;66:324–328. doi: 10.1001/archneurol.2008.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates T, Davies MJ, Gorely T, Talbot D, Bull F, Sattar N, Khunti K. The effect of increased ambulatory activity on markers of chronic low-grade inflammation: evidence from the PREPARE programme randomized controlled trial. Diabet Med. 2011;27:1256–1263. doi: 10.1111/j.1464-5491.2010.03091.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.