Abstract
The inhibitors of apoptosis (IAP) are important regulators of apoptosis. However, little is known about the capacity of Smac mimetics (IAP inhibitor) to overcome virally-associated-lymphoma’s (VAL) resistance to apoptosis. Here, we explored the pro-apoptotic effect of a novel Smac mimetic, RMT5265.2HCL (RMT) in VAL cells. RMT improved the sensitivity to apoptosis in EBV- and to some extend in HTLV-1- but not in HHV-8-VAL. Furthermore, we identified that RMT promotes caspase 3 and 9 cleavage by inhibiting XIAP and inducing the mitochondrial efflux of Smac and cytochrome C. This investigation further support exploring the use of Smac inhibitors in VAL.
Keywords: XIAP, EBV, HTLV-1, HHV-8, lymphomas, Smac mimetics
1. Introduction
Lymphomas associated with viral infections are common events in immunocompromised hosts and carry a poor prognosis [1–6]. Inhibition of apoptosis is an essential mechanism for the maintenance of VAL homeostasis and resistance to chemotherapy. Commonly, VAL avoids apoptosis by expressing inhibitors of the caspase cascade such as the inhibitors of apoptosis (IAP) [7–9]. However, little is known on the potential therapeutic use of XIAP inhibitors in VAL. Once apoptosis is triggered, mitochondria undergo a series of changes that result in the release of cytochrome C. In the cytoplasm, cytochrome C forms a complex with procaspase 9, ATP and APAF-1 (apoptosome) to promote the activation of caspase 9. Once activated, caspase 9 triggers the activation of the “executioner” caspases 3 and 7. Tumors can modify the activation of caspases in part by expressing the inhibitors of apoptosis (IAP) [10]. IAP inhibit key initiator and executioner caspases (3, 7, 8 and 9) [10, 11]. In particular, X-chromosome-linked IAP (XIAP) is recognized as a ubiquitously expressed protein with a potent anti-apoptotic role. Its baculoviral IAP repeat (BIR) 3 domain inhibits the monomeric inactive form of caspase-9 and its BIR 2 domain binds to the catalytic sites of the activated forms of the common “executioner” caspases 3 and 7 [12–14].
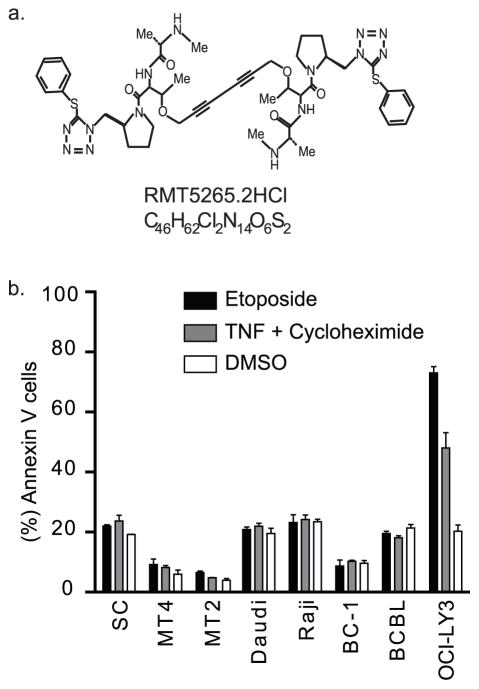
Cells sensitive to the mitochondrial apoptotic pathway overcome XIAP regulatory function by releasing from the mitochondria the second mitochondrial activator of caspases (Smac). In the cytoplasm, Smac relieves the XIAP-associated inhibition of the caspases by binding XIAP’s BIR domains and by promoting their auto-ubiquitination and rapid degradation [15, 16]. The central role in cell death of XIAP and its clinical relevance has led to the development of novel agents that overcome IAP’s anti-apoptotic function [17–19]. Previous reports have successfully demonstrated that synthetic peptide analogs of Smac restore sensitivity to apoptosis in different tumor models [20]. Recently, a new bivalent Smac mimetic, RMT5265.2HCL (RMT, Figure 1), has been designed [18]. RMT mimics the C-terminus of Smac and inhibits XIAP and cIAP1/2, through the BIR 3 domain, more potently than other Smac mimetics. This compound penetrates cell membranes and binds XIAP with an affinity equal to that of Smac itself. Furthermore, RMT has been shown to sensitize ovarian and breast cancer cells more effectively than other Smac mimetics to activators of the receptor apoptotic pathway such as the tumor necrosis factor (TNF) and TNF-related apoptosis-inducing ligand [18]. However, the mechanism of action of Smac mimetics in tumor models with defects in the receptor apoptotic pathway, such as those associated with viral infections, remains unknown [21–23]. In this study, we address this question in vitro using three virally associated lymphoma (VAL) models characterized for having defects in the activation of the TNF receptor, such as human T-lymphotropic virus 1 (HTLV-1)-associated adult T-cell leukemia/lymphoma (ATL), Epstein-Barr virus (EBV)-associated Burkitt’s lymphoma, and human herpes virus 8 (HHV-8)-associated primary effusion lymphomas [24–29].
Figure 1.
VAL are resistant to inducers of the apoptotic pathways. (A) Chemical structures of the RMT. (B) HTLV-1 Tax transgenic cell line: SC, HTLV-1-immortalized human lymphoid cells: MT2 and MT4, EBV(+) lymphoma cells: Daudi and Raji and HHV-8-associated lymphoma cells: BCBL and BC1 or control cell lines (OCI-LY3) were treated for 24 hours with etoposide or TNFα with cycloheximide (see Methods section for details). Apoptosis was determine by flow cytometry and measured by the proportion of annexin V positive cells. Data is presented as the mean ± standard deviation of independent experiments.
2. Material and Methods
2.1. Cell Lines
EBV(+) Burkitt’s lymphoma cell lines (Daudi and Raji), HTLV-1(+) adult T-cell leukemia/lymphoma cell lines (MT2 and MT4), and HHV-8(+) primary effusion lymphoma cell lines (BCBL and BC1) were maintained in RPMI medium. SC, a large granular lymphocytic cell line derived from a HTLV-1 Tax transgenic mouse model [30], and OCI-LY3 (non-virally associated diffuse large cell lymphoma cell line, kindly provided by I. Lossos, University of Miami) were maintained in Iscove’s medium.
Culture media were supplemented with 10% fetal bovine serum, 1% L-glutamine, 1 mM sodium pyruvate, and 50 μg/ml penicillin-streptomycin. OCI-LY3 medium was supplemented with fresh human plasma (Innovative Research). Culture medium for the SC cell line was additionally supplemented with 250–500 units/ml of human interleukin-2 (IL-2).
2.2. Immunoblotting and Mitochondrial Extraction
Cell lysates were prepared using a mammalian cell lysis buffer (50 mM Tris-Cl, pH 8/ 5 mM EDTA/ 100 mM NaCl/ 0.5% Triton X-100 plus protease and phosphatase inhibitors). Twenty micrograms of lysate was separated by 10% SDS/PAGE and transferred to polyvinylidene difluoride membranes, blocked with 0.01% Tween 20 and 5% dry milk in TBS (TBST), and probed overnight with primary antibody. After washing with TBST, horseradish peroxidase-labeled secondary antibody was added (1:1000 dilution, goat anti-rabbit IgG and goat anti-mouse IgG; Pierce). Immunodetection was performed with enhanced chemiluminescence reagents (Supersignal West Femto Sensitivity Substrate, Pierce).
Immunoblots were performed using antibodies directed against the following antigens: cleaved caspase- 3 (9761), pro- and cleaved caspase- 9 (9508), Bcl-2 (2876), Bcl-xL (2762) and XIAP (2042) from Cell Signaling Technology and procaspase-8 (sc-7890), cIAP1/2 (sc-12410), cIAP1 (sc-7943), Fas-associated death domain-like IL-1β-converting enzyme inhibitory protein (c-FLIPS/L, sc-5276) and GAPDH (sc-25788) from Santa Cruz Biotechnology. Antibodies were used at a dilution of 1:1000. Densitometric measurements for band intensities were performed using ImageJ software.
The localization of Smac and cytochrome C was determined with Smac and cytochrome C polyclonal antibodies from Santa Cruz Biotechnology (sc-12683, sc-7159, respectively). The cytoplasmic fraction and mitochondrial fraction were obtained following the mitochondrial fractionation kit protocol (Active Motif).
2.3. Apoptosis and Proliferation Studies
For apoptosis studies, 106 cells were treated with etoposide (180 nM, Sigma), or 10 ng/ml TNFα (Sigma) and cycloheximide (CHX, 10 μg/ml, Sigma), or titration doses (25 and 50 nM) of RMT5265.2HCl (RMT, kindly provided by PG Harran, University of Texas SouthWestern Medical Center at Dallas) or dimethyl sulfoxide (DMSO, as control). Twenty-four hours later, 105 cells were stained with FITC-conjugated antibody against annexin V (Molecular Probes). Apoptotic cells were measured with a FACScan flow cytometer (Becton Dickinson), quantitating annexin V(+) cells. Statistics were performed by analysis of variance (ANOVA) using Prism statistical software.
Proliferation was measured by Bromodeoxyuridine (Brdu) incorporation following FITC BrdU Flow Kit protocol (BD Pharmingen). Brdu positive cells were measured at baseline and after 24 hour treatment with RMT with a FACScan flow cytometer (Becton Dickinson).
2.4. Plasmids
Small hairpin RNA (shRNA) against XIAP and Luciferase (as control) were expressed under the control of the U6 human promoter and were generated using PLKopuro.1 vector (provided by S. Stewart, Washington University). Complementary shRNA oligos were annealed and cloned into a vector digested with AgeI and EcoRII, and confirmed by sequencing analysis. The sense shRNA oligonucleotide probes were as follows: murine and human XIAP: GATAGGAATTTCCCAAAT and murine and human Bcl-xL: GGAGATGCAGGTATTGGTGAG. Control plasmid expressing shRNA against Luciferase was provided by S. Stewart [31]. Recombinant lentiviruses were generated in 293T cells. Infection of SC, MT2, Daudi and BCBL cell lines was performed for 48 hours and then cells were placed in selection media using 0.8–10 μg/mL puromycin.
3. Results
3.1. Virally-Associated Lymphomas are Resistant to Inducers of the Extrinsic and Intrinsic (mitochondrial) Apoptotic Pathways
The resistance of VAL to TNFα has been associated with down regulation of TNFR1 (HTLV-1 and EBV[+] tumors) or the expression of viral-FLIP (HHV-8 [+] tumors, [25–29]). To confirm VAL resistance to inducers of the extrinsic apoptotic pathway, we measured the percent of Annexin V positive cells after treatment with TNFα (in combination with CHX). As shown in Figure 1, VALs were resistant to the apoptotic effect of TNFα compared to the non-VAL cell line OCI-LY3. Similarly, VAL cells were more resistant to inducers of the intrinsic (etoposide) apoptotic pathway than OCI-LY3 cells (p<0.001).
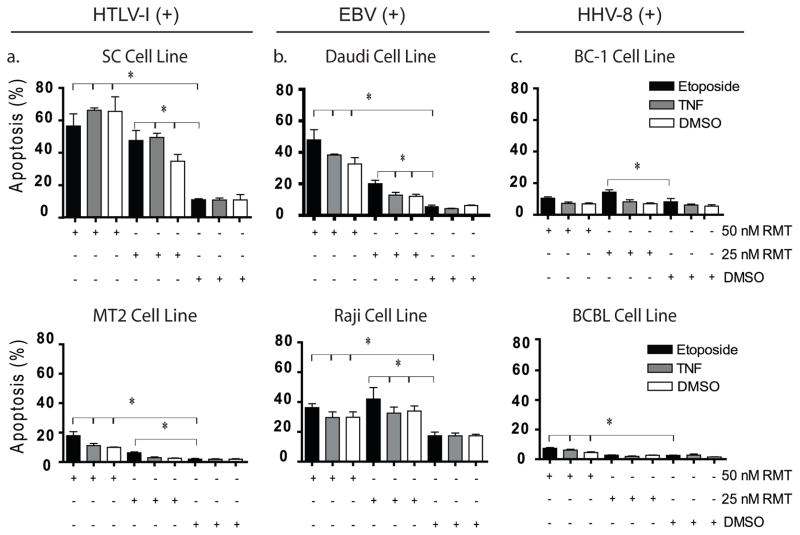
3.2. The Smac Mimetic RMT Induces Apoptosis and Enhances the Apoptotic Activity of Etoposide in EBV- and HTLV-1-VAL cells
Based on the resistance of VAL cell lines to the apoptotic effect of TNF and Etoposide, we investigated whether RMT was able to restore the apoptotic sensitivity of VAL cells. To this end, we treated different VAL cell lines with RMT or RMT in combination with etoposide. The sensitivity of VAL cells to RMT treatment demonstrated significant degree of variability between cell lines; however, a trend towards a higher sensitivity in HTLV-1 and EBV(+) VAL cells emerged. RMT, at 25 nM, led to a minimal but statistically significant increase in apoptosis in HTLV-1 and EBV(+) VAL cells compared to HHV-8 VAL cells (BCBL and BC-1, Figure 2). However, at a higher dose (50nM), HTLV-1 Tax(+) cells (SC and MT2) and EBV(+)- VAL cell lines (Daudi and Raji, p<0.05) demonstrated higher levels of apoptosis than HHV-8 VAL cells cell lines. Similar to MT2 and Daudi, the non-VAL cell OCI-LY3 cells were highly sensitive to the apoptotic effect of RMT at 25 and 50 nM (Supplemental Figure 1A).
Figure 2.
Treatment with RMT restores apoptosis and increases the effect of etoposide in EBV(+)- and HTLV-1(+)-associated lymphoma cells. The induction of apoptosis by RMT ± etoposide was examined in (A) HTLV-1 Tax transgenic cell line SC, HTLV-1 immortalized human lymphoid cells MT2, (B) EBV(+) VAL: Daudi and Raji and (C) HHV-8 VAL cells: BCBL and BC1. Apoptosis was measured by FACS analysis using annexin V after 24 hours of treatment with RMT (25 nM or 50 nM) ± etoposide (150 nM). (* p<0.001). . Data is presented as the mean ± standard deviation of independent experiments.
The addition of etoposide in HTLV-1 Tax(+) and EBV(+) VAL cell lines minimally increased the apoptotic effect of RMT at high concentration (50nM, P=0.2), however at 25nM an increase of ~1.5- to 2-fold over DMSO or RMT alone was observed (p<0.02, Figure 2A–B). Furthermore, similar to the RMT treatment alone, HHV-8 (+) VAL cells (BCBL and BC-1) demonstrated a great degree of resistance RMT in combination with etoposide.
To determine whether VAL sensitivity to RMT relates to differences in cell proliferation, we compared the baseline proliferation rate of RMT sensitive (Daudi and MT2) and RMT resistant VAL cells using a Brdu incorporation assay. RMT sensitive cells demonstrated higher Brdu incorporation than RMT resistant cells (60% vs. 40%, p<0.001). In addition, when the proliferation rate was measured after RMT treatment, VAL cells displayed no changes in their baseline Brdu incorporation levels (Supplemental Figure 1B).
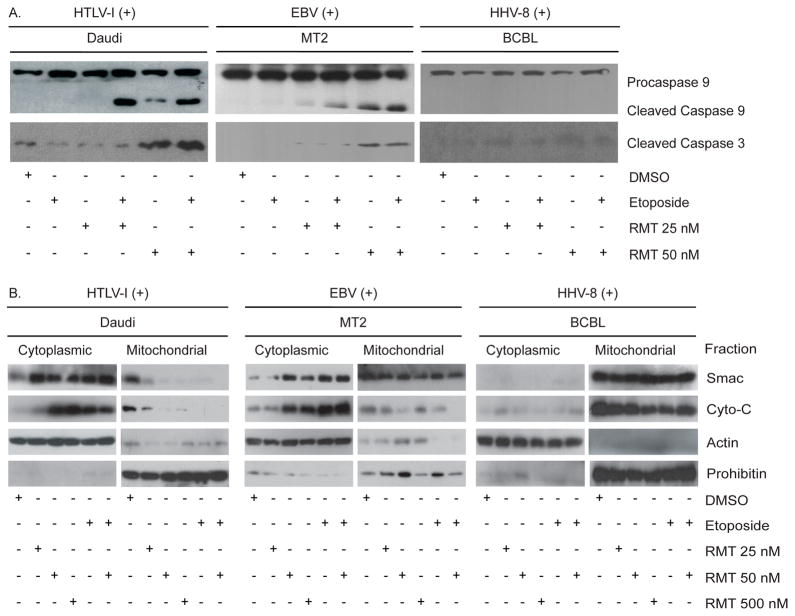
3.3. RMT Promotes Caspase 3 and 9 Cleavage by Inducing Mitochondrial Efflux of Smac and Cytochrome C
To further understand the effects of RMT on the caspase cascade, we evaluated the protein levels of caspase 3, 7 and 9 and the release of cytochrome C from the mitochondria. To this end, we first measured the protein levels of cleaved caspase 3, 7 and 9 in two RMT sensitive cells (Daudi and MT2) and an RMT-resistant cell line (BCBL) after treatment with RMT or RMT in combination with etoposide. At lower concentrations of RMT (25 nM) the levels of cleaved caspase 9 increased minimally while no increase was seen in cleaved caspase 3. When RMT (25 nM) was combined with etoposide, we observed an increase in the levels of cleaved caspase 9 without increasing cleaved caspase 3 levels (Figure 3A). On the other hand, RMT at 50 nM or in combination with etoposide increased the levels of cleaved caspase-9 and caspase-3 in MT2 and Daudi cells compared with DMSO treated cells (Figure 3A and Supplemental table 1 for densitometry measurement of the band intensities). In contrast to these results, both concentrations of RMT failed to promote caspase 3 and 9 cleavage in the BCBL cell line. When procaspase 7 levels were measured, RMT sensitive cells display higher levels than BCBL cells. However, RMT treatment had little effect on procaspase 7 levels, suggesting that RMT’s effect is caspase 3 dependent (Supplemental Figure 1C). Overall, these results suggest the presence of defects in the activation of the mitochondrial caspase cascade in BCBL cells.
Figure 3.
RMT increases cytochrome C and Smac mitochondrial efflux and promotes caspase 3 and 9 activation. (A) The effect on activation of caspase 3 and 9 was examined after 24 hours of RMT ± etoposide treatment in Daudi, MT2 and BCBL cells. (B) Mitochondrial and cytoplasmic cytochrome C and Smac western blot of Daudi and BCBL cells treated with RMT for 24 hours. Each fraction was blotted for Smac, cytochrome C (cyto-C), prohibitin (mitochondrial loading control) and actin (cytoplasmic loading control) as described in the Methods section.
Based on our observation that caspase 9 is cleaved during RMT treatment in RMT-sensitive cells, we reasoned that either RMT is functioning upstream of caspase 9 activation or that VALs have a constitutively low level of apoptosome formation that must be controlled by XIAP. To discriminate between these two possibilities, we asked whether RMT induces mitochondrial cytochrome C release. To answer this question, we compared the mitochondrial function of RMT-sensitive (Daudi and MT2) and resistant cells (BCBL) by measuring the mitochondrial and cytoplasmic levels of Smac and cytochrome C after RMT or etoposide treatment. Figure 3B show that 24-hour treatment with RMT (25, 50 or 500 nM) and etoposide increased the cytoplasmic levels of Smac and cytochrome C in RMT-sensitive but not in RMT-resistant cells (BCBL). Furthermore, the correlation between the cytoplasmic levels of cytochrome C, but not of Smac, with the increasing concentrations of RMT in sensitive cells suggests a dose-dependent effect. Overall, these results suggest that the mitochondrial efflux of Smac and cytochrome C plays an important role in RMT’s apoptotic effect.
To substantiate the role that activation of the mitochondrial apoptotic pathway has on RMT-induced apoptosis in RMT-sensitive cells, we treated Daudi cells with a caspase 8 inhibitor (Z-IETD-FMK) during RMT treatment. Our data demonstrate that despite caspase 8 inhibition, RMT was able to induce caspase 9 and caspase 3 cleavage (Supplemental Figure 1D).
3.4. XIAP inhibition is important for RMT to promote apoptosis in RMT-sensitive cell lines
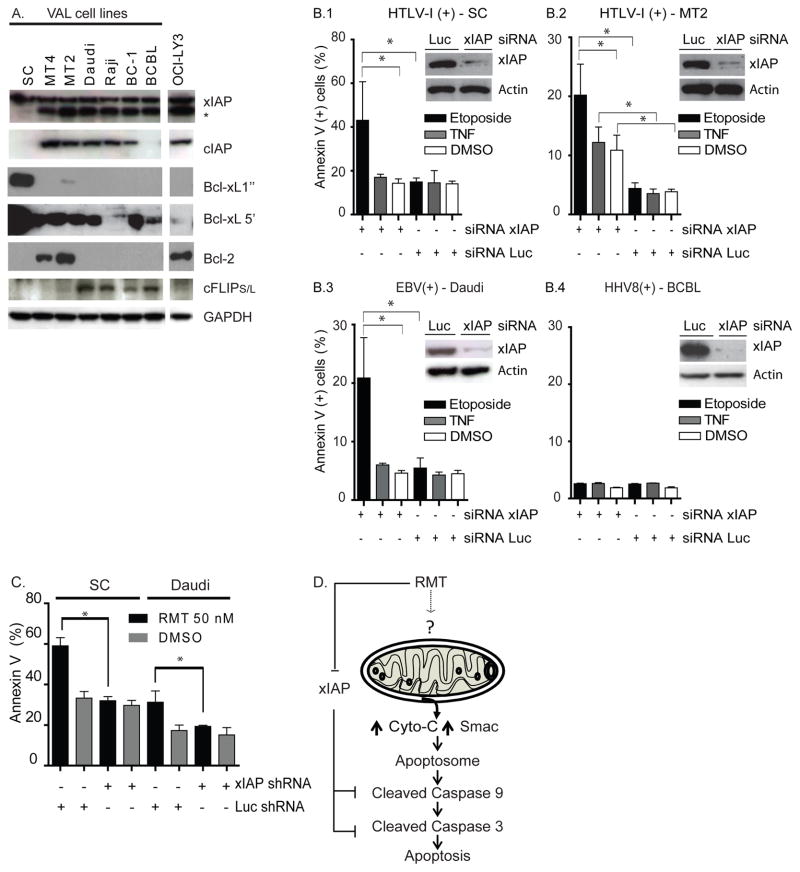
To evaluate whether XIAP levels explain the difference in RMT response, we examined the protein levels of several regulators of the mitochondrial apoptotic pathway. Figure 4A shows that XIAP was ubiquitously expressed in all lymphoma cell lines. In addition, HTLV-1-associated lymphoma cells (MT2 and MT4) overexpressed BCL-2 while BCBL, MT2 and SC overexpressed Bcl-xL.
Figure 4.
xIAP inhibition is important for RMT to promote apoptosis in RMT-sensitive cells. (A) Levels of FLIP, cIAP1 and cIAP2, xIAP, Bcl-2, Bcl-xL and GAPDH are shown for VAL cells and control (OCI-LY3). Two exposure times are provided for Bcl-xL Western blots because of the abundance of this protein in SC cell lines. (B) xIAP shRNA improves the apoptotic effect of etoposide in EBV(+)- and HTLV1(+)- VAL cell lines. VAL cells SC (B.1), MT2 (B.2), Daudi (B.3) and BCBL (B.4) were infected with lentivirus expressing xIAP shRNA (murine or human sequence). xIAP levels of knock down in xIAP-shRNA expressing cells compared to Luciferase (Luc)-shRNA are shown. The effects on apoptosis shown in VAL-shRNA expressing cells were measured by flow cytometry after 24 hours of treatment with etoposide or TNFα (* p<0.001). Non-specific bands of lower molecular weight (*) were also identified in immunoblots with antibodies to xIAP. (C) xIAP is essential for RMT- induced apoptosis in HTLV-1 (+) and EBV (+) associated lymphomas. RMT sensitive cells (SC and Daudi) expressing xIAP or Luciferase-siRNA were treated with RMT (50 nM) or DMSO for 24 hours. In all these experiments apoptosis was measured by flow cytometry and was defined by positivity of Annexin V. Figure represent the mean ± SD of three independent experiments. (D) Diagrammatic representation of the proposed molecular mechanistic model of action of RMT in RMT-sensitive cells. (?) Undetermined mechanism.
Based on the lack of correlation between XIAP protein levels and RMT response, we evaluated the importance of XIAP as an anti-apoptotic mechanism in our in vitro models. To achieve this goal, we measured the level of apoptosis in VAL cells treated with etoposide after expression of a shRNA directed to XIAP. XIAP-shRNA reduced the endogenous levels of XIAP by 70–90% without causing apoptosis in SC, MT2, Daudi or BCBL cell lines (XIAP protein levels did not decrease in cells treated with etoposide, Supplemental Figure 1E). However, when these XIAP-shRNA expressing cells were treated with etoposide, we observed an increase in the extent of apoptosis by 2–2.5-fold in the three RMT-sensitive cell lines (SC, MT2 and Daudi) as compared to cell lines expressing a control shRNA (Luciferase, p<0.001, Figure 4B.1–.4). In contrast, XIAP knock down failed to increase the apoptotic effect of etoposide in BCBL cells. The observed apoptotic effect of XIAP-shRNA expression combined with etoposide treatment and the lack of apoptosis induced by XIAP-shRNA expression suggest that inhibition of XIAP alone cannot fully explain the activity of RMT.
To evaluate the role of XIAP inhibition in RMT’s apoptotic effect, we tested the apoptotic levels in RMT-sensitive cells (SC or Daudi) when XIAP-shRNA was expressed. The levels of annexin V after 24 hours of RMT treatment (50nM) in RMT-sensitive cells expressing the control shRNA (Luciferase-shRNA) decrease by 2-fold when XIAP-shRNA was expressed (figure 4C). Taken together, these results demonstrate that inhibition of XIAP is necessary but not sufficient for RMT-induced apoptosis.
3.4. BCBL’s resistance to RMT is not associated to BCL-xL overexpression
The resistance of BCBL cells to RMT’s apoptotic effector the combination of XIAP-shRNA and etoposide leaves open the possibility that another survival mechanism may be responsible for RMT’s resistance. Based on the protein baseline expression levels of BCL-xL, XIAP and c-IAP in BCBL cells, we compared their change after treating BCBL and Daudi cells with RMT (50 nM). Treatment with RMT induced the expression of BCL-xL in BCBL cells while it reduced moderately the expression of BCL-xL, XIAP and c-IAP in Daudi cells (Supplemental Figure 1F). To evaluate whether the overexpression of BCL-xL is responsible for BCBL’s RMT resistance, we generate a BCL-xL-shRNA BCBL expressing cell line and tested RMT’s apoptotic effect. As shown in Supplemental Figure 1G, RMT treatment (50nM) was ineffective on inducing apoptosis in BCL-xL- and luciferase-shRNA BCBL expressing cells. These results suggest that other potential mechanism inhibition of the mitochondrial apoptotic pathway may play a role in BCBL’s RMT apoptotic resistance.
4. Discussion
In this study we provide evidence that the bivalent Smac mimetic RMT can induce apoptosis in EBV(+)-VAL cells, and to some extend in HTLV1(+)-VAL cells, through activation of the intrinsic apoptotic pathway. We demonstrated that RMT’s apoptotic effect results from its capacity to induce the mitochondrial efflux of Smac and cytochrome C and inhibit XIAP (Figure 4D).
We demonstrated in two independent lines of investigation that XIAP, a target of RMT, plays an important role in EBV(+)- and HTLV-1(+)-lymphoma cell’s survival. First, we found that knocking down XIAP facilitated etoposide-induced apoptosis in HTLV-1(+) and EBV(+) lymphoma cell lines. Second, although RMT has been shown to bind the BIR 3 domains of XIAP and cIAP1/2 [18, 20, 32, 33], our results are consistent with others showing that the Smac mimetic inhibitory effect on XIAP is important for inducing apoptosis in VAL models [20, 34]. XIAP’s important role in VAL cell survival, particularly in EBV(+)- lymphoma cells, are in line with the cell survival and expansion observed when wild type XIAP is overexpressed in EBV-transformed B cells obtained from XIAP-deficient patients and the central role of XIAP’s survival efffect in Syk signaling observed in cell lines derived from EBV(+) post-transplant lymphoproliferative disorders [35] [7]. Overall, these findings highlight the importance of XIAP and the lack of redundancy among IAP proteins in EBV- and potentially in HTLV-1(+) lymphomas. In addition to inhibiting XIAP, we provide evidence that the mitochondria play an important role in RMT induction of apoptosis. The evidence that RMT increased mitochondrial efflux of Smac and cytochrome C only in sensitive cells and that shRNA-XIAP expression alone did not induce apoptosis support this model.
In addition, this model is consistent with the lack of apoptosis in BCBL cells produced by the combination of RMT and BCL-xL-shRNA, where HHV-8 provides an inhibitory effect on the mitochondrial activating potential [36]. However, a more detailed examination into how RMT promotes cytochrome C release is warranted. Overall, the dual effect of RMT (the mitochondrial efflux of cytochrome C and the inhibition of regulators of the caspases activation such as XIAP) supports the concept that the mitochondrial apoptotic pathway is a two-signal system and provides an explanation for the discordant results observed between the limited effects in cell survival seen in the murine models deleted in XIAP or Smac genes and the Smac mimetics [37, 38].
Several investigators have suggested that other Smac mimetics require activation of caspase 8 to induce apoptosis. It is believed that activation of caspase 8 occurs via an autocrine production of TNF. In contrast, RMT promotes the activation of caspase 8 by inducing cIAP proteasomal degradation and not by inducing TNF production [18]. Although, it was previously unclear whether RMT requires the induction of cytochrome C efflux to promote apoptosis [39–42], our study has addressed this question and established in our model that the mitochondrial cytochrome C and Smac release are an important step during the induction of apoptosis by RMT.
RMT constitutes a promising therapeutic or chemosensitizing agent to target EBV(+) and potentially HTLV-1(+) VAL. Our work improved the understanding of the mechanistic model for RMT-induced apoptosis. We showed that RMT’s apoptotic effect involves activation of the mitochondria, the subsequent mitochondrial efflux of Smac and cytochrome C and the inhibition of XIAP (Figure 4D). However, further studies are needed to evaluate in detail the mechanisms through which RMT or other Smac mimetics activate the mitochondria.
Supplementary Material
Acknowledgments
We thank Ms. Anthea Hammond for editing assistance and Dr. Morgan Mclemore for his critical review and helpful comments.
Funding Source
L.H.B received funding support from the NIH (CA127910 and CA129968) and a GCCDSW, L.R. was supported by the National Institute of Health (NIH, CA94056, CA10073 and CA63417) and L.B-M received funding from the Crissey Hematology and Medical Oncology Research Fund.
Footnotes
Contribution
S.R and J.C contributed equally in this study. S.R, J.C designed research, performed research, analyzed data and wrote paper. A.L., Y.H, X.G. performed research and analyzed data. L.H.B, H.F., L.R., H.J.K. contributed with vital reagents and analytical tools, and analyzed data. L. B-M designed research, performed research, contributed vital new reagents and analytical tools, analyzed data, and wrote the paper
Conflict of Interest Statements
Conflict of interest: H.F is a compensated consultant for Sigma-Aldrich. The other authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruth FJ. Viruses and lymphoma/leukaemia. The Journal of Pathology. 2006;208:176–86. [Google Scholar]
- 3.Tanosaki R, Tobinai K. Adult T-cell leukemia–lymphoma: current treatment strategies and novel immunological approaches. Expert Review of Hematology. 2010;3:743–53. doi: 10.1586/ehm.10.73. [DOI] [PubMed] [Google Scholar]
- 4.Jacobson CA, LaCasce AS. Lympoma: risk and response after solid organ transplant. Oncology (Williston Park) 2010;24:936–44. [PubMed] [Google Scholar]
- 5.Nourse JP, Jones K, Gandhi MK. Epstein–Barr Virus-Related Post-Transplant Lymphoproliferative Disorders: Pathogenetic Insights for Targeted Therapy. American Journal of Transplantation. 2011;11:888–95. doi: 10.1111/j.1600-6143.2011.03499.x. [DOI] [PubMed] [Google Scholar]
- 6.Sunil M, Reid E, Lechowicz M. Update on HHV-8-Associated Malignancies. Current Infectious Disease Reports. 2010;12:147–54. doi: 10.1007/s11908-010-0092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hatton O, Phillips LK, Vaysberg M, Hurwich J, Krams SM, Martinez OM. Syk activation of PI3K/Akt prevent HtrA2-dependent Loss of XIAP to promote survival of Epstein-Barr virus (EBV)+ B cell lymphomas. Journal of Biological Chemistry. 2011 doi: 10.1074/jbc.M111.255125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor JM, Nicot C. HTLV-1 and apoptosis: role in cellular transformation and recent advances in therapeutic approaches. Apoptosis : an international journal on programmed cell death. 2008;13:733–47. doi: 10.1007/s10495-008-0208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma X, Kalakonda S, Srinivasula SM, Reddy SP, Platanias LC, Kalvakolanu DV. GRIM-19 associates with the serine protease HtrA2 for promoting cell death. Oncogene. 2007;26:4842–9. doi: 10.1038/sj.onc.1210287. [DOI] [PubMed] [Google Scholar]
- 10.LaCasse EC, Mahoney DJ, Cheung HH, Plenchette S, Baird S, Korneluk RG. IAP-targeted therapies for cancer. Oncogene. 27:6252–75. doi: 10.1038/onc.2008.302. [DOI] [PubMed] [Google Scholar]
- 11.Roy N, Deveraux QL, Takahashi R, Salvesen GS, Reed JC. The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. EMBO J. 1997;16:6914–25. doi: 10.1093/emboj/16.23.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silke J, Hawkins CJ, Ekert PG, Chew J, Day CL, Pakusch M, et al. The anti-apoptotic activity of XIAP is retained upon mutation of both the caspase 3- and caspase 9-interacting sites. J Cell Biol. 2002;157:115–24. doi: 10.1083/jcb.200108085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gough NR. XIAP as a Positive Feedback Regulator of Caspase Activation. Sci STKE. 2006;2006:tw351. [Google Scholar]
- 14.Tenev T, Zachariou A, Wilson R, Ditzel M, Meier P. IAPs are functionally non-equivalent and regulate effector caspases through distinct mechanisms. Nat Cell Biol. 2005;7:70–7. doi: 10.1038/ncb1204. [DOI] [PubMed] [Google Scholar]
- 15.Du C, Fang M, Li Y, Li L, Wang X. Smac, a Mitochondrial Protein that Promotes Cytochrome c-Dependent Caspase Activation by Eliminating IAP Inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 16.Vaux DL, Silke J. IAPs, RINGs and ubiquitylation. Nat Rev Mol Cell Biol. 2005;6:287–97. doi: 10.1038/nrm1621. [DOI] [PubMed] [Google Scholar]
- 17.Wu H, Tschopp J, Lin S-C. Smac Mimetics and TNF[alpha]: A Dangerous Liaison? Cell. 2007;131:655–8. doi: 10.1016/j.cell.2007.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li L, Thomas RM, Suzuki H, De Brabander JK, Wang X, Harran PG. A Small Molecule Smac Mimic Potentiates TRAIL- and TNF{alpha}-Mediated Cell Death. Science. 2004;305:1471–4. doi: 10.1126/science.1098231. [DOI] [PubMed] [Google Scholar]
- 19.Gaither A, Porter D, Yao Y, Borawski J, Yang G, Donovan J, et al. A Smac Mimetic Rescue Screen Reveals Roles for Inhibitor of Apoptosis Proteins in Tumor Necrosis Factor-{alpha} Signaling. Cancer Res. 2007;67:11493–8. doi: 10.1158/0008-5472.CAN-07-5173. [DOI] [PubMed] [Google Scholar]
- 20.Cillessen SAGM, Reed JC, Welsh K, Pinilla C, Houghten R, Hooijberg E, et al. Small-molecule XIAP antagonist restores caspase-9 mediated apoptosis in XIAP-positive diffuse large B-cell lymphoma cells. Blood. 2008;111:369–75. doi: 10.1182/blood-2007-04-085480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nalan Akyurek YR, Rassidakis Georgios Z, Schlette Ellen J, Jeffrey Medeiros L. Expression of inhibitor of apoptosis proteins in B-cell non-Hodgkin and Hodgkin lymphomas. Cancer. 2006;107:1844–51. doi: 10.1002/cncr.22219. [DOI] [PubMed] [Google Scholar]
- 22.Nishimura K, Ueda K, Guwanan E, Sakakibara S, Do E, Osaki E, et al. A posttranscriptional regulator of Kaposi's sarcoma-associated herpesvirus interacts with RNA-binding protein PCBP1 and controls gene expression through the IRES. Virology. 2004;325:364–78. doi: 10.1016/j.virol.2004.04.041. [DOI] [PubMed] [Google Scholar]
- 23.Sanda T, Asamitsu K, Ogura H, Iida S, Utsunomiya A, Ueda R, et al. Induction of cell death in adult T-cell leukemia cells by a novel I[kappa]B kinase inhibitor. Leukemia. 2006;20:590–8. doi: 10.1038/sj.leu.2404129. [DOI] [PubMed] [Google Scholar]
- 24.Guasparri I, Keller SA, Cesarman E. KSHV vFLIP Is Essential for the Survival of Infected Lymphoma Cells. J Exp Med. 2004;199:993–1003. doi: 10.1084/jem.20031467. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Guasparri I, Bubman D, Cesarman E. EBV LMP2A affects LMP1-mediated NF-{kappa}B signaling and survival of lymphoma cells by regulating TRAF2 expression. Blood. 2008;111:3813–20. doi: 10.1182/blood-2007-03-080309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Y-C, Hsu T-Y, Lin R-H, Su I-J, Chen J-Y, Yang C-S. Short Communication: Resistance to Tumor Necrosis Factor-α-Induced Apoptosis in Human T-Lymphotropic Virus Type I-Infected T Cell Lines. AIDS Research and Human Retroviruses. 2002;18:207–12. doi: 10.1089/08892220252781266. [DOI] [PubMed] [Google Scholar]
- 27.Chuang H-C, Lay J-D, Chuang S-E, Hsieh W-C, Chang Y, Su I-J. Epstein-Barr Virus (EBV) Latent Membrane Protein-1 Down-Regulates Tumor Necrosis Factor-{alpha} (TNF-{alpha}) Receptor-1 and Confers Resistance to TNF-{alpha}-Induced Apoptosis in T Cells: Implication for the Progression to T-Cell Lymphoma in EBV-Associated Hemophagocytic Syndrome. Am J Pathol. 2007;170:1607–17. doi: 10.2353/ajpath.2007.061026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chuang H-C, Lay J-D, Hsieh W-C, Wang H-C, Chang Y, Chuang S-E, et al. Epstein-Barr virus LMP1 inhibits the expression of SAP gene and upregulates Th1 cytokines in the pathogenesis of hemophagocytic syndrome. Blood. 2005;106:3090–6. doi: 10.1182/blood-2005-04-1406. [DOI] [PubMed] [Google Scholar]
- 29.Sun Q, Matta H, Chaudhary PM. The human herpes virus 8-encoded viral FLICE inhibitory protein protects against growth factor withdrawal-induced apoptosis via NF-kappa B activation. Blood. 2003;101:1956–61. doi: 10.1182/blood-2002-07-2072. [DOI] [PubMed] [Google Scholar]
- 30.Grossman WJ, Ratner L. Cytokine expression and tumorigenicity of large granular lymphocytic leukemia cells from mice transgenic for the tax gene of human T-cell leukemia virus type I. Blood. 1997;90:783–94. [PubMed] [Google Scholar]
- 31.Stewart SA, Dykxhoorn DM, Palliser D, Mizuno H, Yu EY, An DS, et al. Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA. 2003;9:493–501. doi: 10.1261/rna.2192803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carter BZ, Gronda M, Wang Z, Welsh K, Pinilla C, Andreeff M, et al. Small-molecule XIAP inhibitors derepress downstream effector caspases and induce apoptosis of acute myeloid leukemia cells. Blood. 2005;105:4043–50. doi: 10.1182/blood-2004-08-3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu G, Chai J, Suber TL, Wu J-W, Du C, Wang X, et al. Structural basis of IAP recognition by Smac/DIABLO. Nature. 2000;408:1008–12. doi: 10.1038/35050012. [DOI] [PubMed] [Google Scholar]
- 34.Sun H, Nikolovska-Coleska Z, Yang C, Qian D, Lu J, Qiu S, et al. Design of Small-Molecule Peptidic and Non-Peptidic Smac Mimetics. Acc Chem Res. 2008;41:264–1277. doi: 10.1021/ar8000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rigaud S, Fondaneche M-C, Lambert N, Pasquier B, Mateo V, Soulas P, et al. XIAP deficiency in humans causes an X-linked lymphoproliferative syndrome. Nature. 2006;444:110–4. doi: 10.1038/nature05257. [DOI] [PubMed] [Google Scholar]
- 36.Feng P, Park J, Lee B-S, Lee S-H, Bram RJ, Jung JU. Kaposi’s Sarcoma-Associated Herpesvirus Mitochondrial K7 Protein Targets a Cellular Calcium-Modulating Cyclophilin Ligand To Modulate Intracellular Calcium Concentration and Inhibit Apoptosis. Journal of Virology. 2002;76:11491–504. doi: 10.1128/JVI.76.22.11491-11504.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arnt CR, Kaufmann SH. The saintly side of Smac//DIABLO: giving anticancer drug-induced apoptosis a boost. Cell Death Differ. 10:1118–20. doi: 10.1038/sj.cdd.4401294. [DOI] [PubMed] [Google Scholar]
- 38.Fulda S, Wick W, Weller M, Debatin K-M. Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nat Med. 2002;8:808–15. doi: 10.1038/nm735. [DOI] [PubMed] [Google Scholar]
- 39.Cillessen SAGM, Hess CJ, Hooijberg E, Castricum KCM, Kortman P, Denkers F, et al. Inhibition of the Intrinsic Apoptosis Pathway Downstream of Caspase-9 Activation Causes Chemotherapy Resistance in Diffuse Large B-Cell Lymphoma. Clin Cancer Res. 2007;13:7012–21. doi: 10.1158/1078-0432.CCR-06-2891. [DOI] [PubMed] [Google Scholar]
- 40.Vince JE, Wong WW-L, Khan N, Feltham R, Chau D, Ahmed AU, et al. IAP Antagonists Target cIAP1 to Induce TNF[alpha]-Dependent Apoptosis. Cell. 2007;131:682–93. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 41.Adrain C, Creagh EM, Martin S. Apoptosis-associated release of Smac/DIABLO from mitochondria requires active caspases and is blocked by Bcl-2. The EMBO Journal. 2001;23:6627–36. doi: 10.1093/emboj/20.23.6627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cuvillier O, Levade T. Sphingosine 1-phosphate antagonizes apoptosis of human leukemia cells by inhibiting release of cytochrome c and Smac/DIABLO from mitochondria. Blood. 2001;98:2828–36. doi: 10.1182/blood.v98.9.2828. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.