Abstract
T cell receptor (TCR) signaling plays a critical role in regulatory T cell (Treg) development. However, the mechanism for tissue specific induction of Tregs in the periphery remains unclear. We observed that surfactant protein A (SP-A) deficient mice have impaired expression of Foxp3 and fewer CD25+Foxp3+ Treg cells after ex vivo stimulation and after stimulation with lipopolysaccharide (LPS) in vivo. The addition of exogenous SP-A completely reversed this phenotype. Although SP-A is known to inhibit T cell proliferation under certain activation conditions, both IL-2 levels as well as active TGFβ levels increase on extended culture with exogenous SP-A, providing a key mechanism for the maintenance and induction of Tregs. In addition, kinetic suppression assays demonstrate that SP-A enhances the frequency of functional Foxp3+Tregs in responder T cell populations in a TGFβ-dependent manner. In mice treated with LPS in vivo, Tregs increased ~160% in WT mice compared to only a 50% increase in LPS treated SP-A−/− mice eight days after exposure. Together, these findings support the hypothesis that SP-A affects T cell immune function by the induction of regulatory T cells during activation.
Introduction
The lung is a remarkably exposed tissue and as such, occupies a unique environmental niche. It must allow for sufficient immune responses to combat infection while limiting that same response to prevent chronic inflammation. This balance is partially mediated by surfactant proteins. The lung collectin, surfactant protein A (SP-A) is one of the predominant immune proteins with in the airspaces. It has well-established roles as a modulator of innate immune cell function and as an opsonin. SP-A also regulates the production of inflammatory cytokines and reactive species and is thought to mediate a balance between upregulating inflammatory mediators to protect the host, while preventing an exaggerated inflammatory response that could damage the delicate lung epithelium and impair gas exchange (reviewed in (1), (2)).
This balance between protective immunity and suppression of prolonged inflammation requires that CD4+ T cells initially get appropriately activated and then differentiate into effector/memory or regulatory subsets (3). The expression of Foxp3 defines the regulatory T cell (Treg) lineage, and is required for Treg suppressive function in both thymus and periphery (4–6). Loss of function mutations in Foxp3 result in systemic autoimmunity caused by immune dysregulation, polyadenopathy, enteropathy and X-linked syndrome (IPEX) in humans, and have been linked to the Scurfy phenotype in mice (7–9). The best characterized CD4+Foxp3+ T cells are the distinct lineage of naturally occurring Tregs present in the thymus. In the periphery, circulating natural Treg cells (nTregs) normally comprise up to 5% of all T cells. Under certain circumstances, conventional T cells can be induced to express Foxp3 and exhibit regulatory activity both in vitro and in vivo (iTregs). Evidence for the generation of this induced population of Foxp3+ suppressor Treg cells outside the thymus was derived from the observation that chronic and low dose Ag-presentation leads to the generation of these cells (10–12). Foxp3+ Tregs are also notable for their poor proliferative response in vitro, for their ability to suppress other T cells, and for their lack of effector cytokine production. A recent study by D’Alessio et al(13), reported that regulatory T cells are important in mediating recovery from acute lung injury via a mechanism involving transforming growth factor beta (TGFβ), a pleiotropic cytokine, with multiple effects on both immune and non-immune cell types in the lung. A large body of literature implicates TGFβ in the maintenance, induction and function of Tregs (14, 15). Experiments with TGFβ1−/− mice demonstrated that TGFβ was not required for the generation of nTregs in the thymus, but was necessary for Treg maintenance in the periphery (16). T cells can also be induced ex vivo to become functional Foxp3+ iTregs with in vivo and in vitro suppressive capability upon TCR mediated stimulation in the presence of exogenous TGFβ (17, 18). In two animal models of autoimmunity, adoptive transfer of Tregs could prevent autoimmunity only in the presence of T cells having an intact intracellular TGFβ signaling pathway (19, 20). Thus, the inductive molecules or signals required for Foxp3 expression and generation of iTregs remain an active area of research.
Previous work by our lab and collaborators suggested that association between SP-A and latent TGFβ1 provides a possible novel mechanism to regulate TGFβ1-mediated inflammation and fibrosis in the lung. In the present study, we hypothesized that SP-A interacts with TGFβ and T cells to enhance the frequency of Foxp3+ Tregs in responder T cell populations. We observed that T cells harvested from SP-A−/− mice have impaired expression of Foxp3 and fewer CD25+Foxp3+ Treg phenotype cells after extended ex vivo culture, compared to T cells purified from wild type (WT) mice. The addition of exogenous SP-A completely restored and even enhanced the level of Foxp3 expression in T cells. In addition, kinetic suppression assays demonstrate that SP-A enhances the frequency of functional Foxp3+Tregs in responder T cell populations in a TGFβ dependent manner. To induce Tregs in the lung in vivo, we utilized a modification of the extended LPS model first described by d’Alessio et al (13). While the proportion of Tregs were nearly identical in untreated SP-A−/− and WT, eight days after LPS exposure Tregs increased to a much greater extent in WT mice compared to LPS treated SP-A−/− mice. Together, these findings suggest that SP-A exerts long-term effects on T cell immune function by the induction of regulatory T cells late during activation in a TGFβ dependent manner.
Materials and Methods
Mice
SP-A−/− mice were generated as previously described (21) and back-crossed to C57BL/6 background for 12 generations. WT mice were obtained from littermates in heterogenous breedings or from Charles River Laboratories (CRL, Wilmington MA). Mice aged 8–12 weeks were used for all experiments, which were performed independently with both male and female mice. All mice were housed in a barrier facility, and all procedures were performed according to local and National Institutes of Health guidelines and were approved by the Duke University Institutional Animal Care and Use Committee.
SP-A preparation
SP-A was purified from the lung lavage fluid of patients with alveolar proteinosis as described previously (22). Briefly, the lavage fluid was initially treated with butanol to extract the SP-A. The resulting pellet was then sequentially solubilized in the detergent octylglucoside and 5 mMTris, pH 7.4. Extracted SP-A was then passed over a polymyxin B-agarose column to reduce endotoxin contamination. SP-A preparations had final endotoxin concentrations of <0.01pg/mg SP-A as determined by the Limulus amoebocyte lysate assay according to manufacturers’ instructions (QCL-1000, Lonza (BioWhittaker), MD). While no active TGFβ was assayed in the SP-A preparations utilized for this study, we did find varying amounts of inactive TGFβ ranging from ~1.6–2.8 pg/μg of purified SP-A using both a bioassay as well as an ELISA as described below.
Media and antibodies
RPMI 1640 with 5% heat inactivated FBS, 25 mM HEPES, 5 μM 2-mercaptoethanol, penicillin-streptomycin (100 U/ml), and 2 mM L-glutamine (all from Life Technologies/Gibco) was utilized as the primary culture medium (complete RPMI). For all suppression assays and some primary culture, we utilized the glutamine supplemented serum free OpTmizer medium (Gibco). All antibodies used for activation or flow cytometry were obtained from BioLegend, eBioScience or BD Pharmingen. Low-endotoxin azide free anti-CD3 (clone 145-2C11) and anti-CD28 (clone 38.51) were used for ex vivo activation assays. Anti-TGFβ1 or anti-TGFβ1,2,3 was obtained from R&D Systems (clone 9016 and 1D11 respectively). LAP (R&D Systems), and fetuin (α-fetoprotein) from Sigma were also utilized in blocking studies.
In vivo studies
Intratracheal LPS (Sigma, E. coli O55:B5) administration was performed by an oropharyngeal (o.p.) aspiration method. Briefly, mice were anesthetized by isoflurane, and suspended by their upper incisors with wire on a ~70° inclined frame. The tongue was gently extended and 40 μl of LPS solution (1 mg/ml) in saline or USP saline (Sigma) was pipetted into the mouth. Brief occlusion of the nose forced the animal to inhale through the mouth, thereby aspirating the solution into the respiratory tract in one or two breaths. Animals were subsequently removed from the support, placed with access to a warming pad, and observed closely until fully recovered from anesthesia. In some experiments (Figure 5A), a high dose of CFSE (125 μM in 40 μl volume instead of the 0.5 μM dose used for labeling cells in vitro) (Molecular Probes #C34554) was instilled along with saline or LPS to label cells in vivo. Excess, unbound dye is typically cleared in 12–15 h, and previous studies have determined that CFSE is non-toxic at these concentrations (23). After the indicated time points, spleen, lungs and bronchio-alveolar lavage fluid (total lung volume lavage) were obtained for downstream analyses. Cytocentrifugations from BALF were performed onto VWR Superfrost slides on a Shandon Cytospin according to manufacturer instructions, followed by H&E staining and blinded differential cell counts.
T cell isolation
Splenocytes were gently teased out from the tissue onto uncoated polystyrene plates. Lungs were diced and digested with collagenase A (Roche) and DNAse I (Worthington) for 30 minutes, and the reaction was stopped with excess EDTA and FBS. Cells from either source were filtered through a 40-μm nylon strainer to obtain a single cell suspension. The cell suspension was then subjected to density gradient centrifugation using Ficoll-Hypaque 1083 (Sigma). RBC lysing solution (BioLegend) was utilized to remove any residual RBCs. Purified T cells were then obtained by negative selection using an appropriate MACS antibody cocktail and paramagnetic microbeads (MiltenyiBiotec (some experiments in Figure 3) or Dynal Invitrogen) to deplete contaminating cells. For some experiments, CD4+ T cells or CD4+CD25− T cells were negatively selected using a second purification with paramagnetic microbeads. T cell purities averaged 95–98% for pan-T cells, and 99% for highly purified subpopulations.
Flow cytometry
Flow cytometry was performed using a BD LSRII (BD Biosciences, CA) at the Duke University Human Vaccine Institute Flow Cytometry Core, which is supported by the National Institutes of Health Award AI-51445. Multiple panels of multi-color surface staining on T cells were set up using anti-CD3 (145-2C11), CD4 (GK1.5 or RM4-5), CD25 (PC61), CD44 (IM7), CD62L (MEL-14), CD45 (30-F11) and FR4 (TH6) to distinguish specific cell populations. For intracellular staining using Foxp3 antibodies (three separate clones: FJK-16s, MF-14, 150D/E4), cells were washed well after surface staining, fixed in 10% neutral buffered formalin, and permeabilized with 0.3% saponin in HBSS+0.5% FBS. All flow cytometric analysis was performed using FloJo v8 or v7.6J software (Treestar, OR). As indicated, data were normalized to proportions of Tregs from spleens from individual mice (in vivo experiments) or mean initial proportion of Tregs (ex vivo experiments) to facilitate comparison across multiple mice and experiments.
Ex vivo activation and suppression assays
Purified, freshly isolated T cells (~100,000 in 150 μl) were incubated under different activation conditions in 96-well round-bottom plates in complete RPMI. Each well received 0.8 μCi of [3H]thymidine (6.7 Ci/mmol; MP Bio) and cells were incubated for another 15 h. Incorporated radioactivity (as an indicator of proliferation) was measured by liquid scintillation using CytoScint ES (MP Bio) on a TriCarb 2100TR (Packard Instruments). Mixed lymphocyte reactions were performed over a 5 day period, with C3BFeJ and C57BL/6J mice as described in Figure 2. Foxp3 expression was assessed by flow cytometry using H2-Kk or H2-Db to differentiate responder cells from stimulator cells. The protocol for suppression assays was modified from Venken et al. to directly address the proportion of Tregs with suppressive capabilities in the initial treatment group, as depicted in Figure 4 for CFSE labeled target cells (24). To track cell divisions, freshly isolated T cells were labeled with 0.4 μM CFSE (Molecular Probes #C34554) or 0.5 μM CPD670 (eBioscience). Excess label was quenched and activation assays were performed. At the indicated time points, cells were harvested, stained as described and data were acquired on a BD LSRII flow cytometer.
ELISA and cytokine bioassays
ELISAs were performed using R&D Systems (TGFβ), eBioScienceSingleplex (TGFβ), Millipore MilliPlex (IL-10), BioLegend MAX Deluxe (IL-2, IL-6, IL-10) or eBioScience Ready-Set-Go (IL-2, TNFα) kits according to manufacturer instructions. To determine very low levels of active TGFβ, in some ELISAs, the ELAST signal amplification system (Perkin-Elmer) was employed according to manufacturer instructions at 1:1000 final concentration to lower the dynamic range of TGFβ detection to as low as 2 pg/ml. The amount of TGFβ/IL-2 in culture supernatants was determined using a 4-parameter fit equation derived from values obtained from known amounts of standard cytokine after background subtraction (BMG Labtech/Optima or BMS FlowCytomixsoftware). A CCL64 mink lung epithelial cell line with a PAI1 luciferase reporter system was also utilized to determine biological activity of TGFβ in culture supernatants (diluted to contain <0.4% FBS during assay) using either purified human platelet derived or recombinant TGFβ (ProSpec, (Israel), Peprotech, R&D Systems) as a reference standard.
Statistics
Data are expressed as mean ± SEM unless otherwise indicated. Statistical significance was tested with an unpaired Student’s t test or ANOVA using Prism 4b or 5 (GraphPad Software Inc, CA) or the Advanced Analysis Toolpak in Excel 2010 (Microsoft Corp., WA). Statistically significant differences were determined by p<0.05.
Results
SP-A enhances Foxp3 expression on T cells after LPS stimulation in vivo
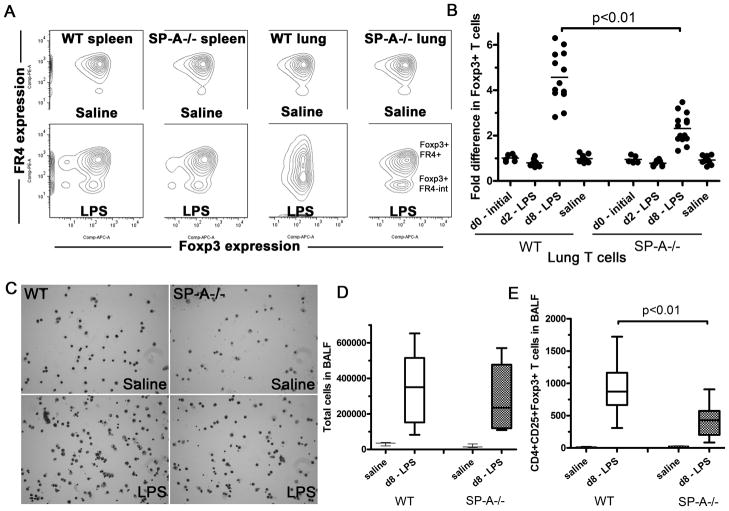
In order to determine whether SP-A plays a direct role in the generation of suppressor T cells, we utilized WT and SP-A−/− mice subjected to a modified version of the extended LPS stimulation model described by d’Alessio et al. T cells purified from spleens or lungs of WT and SP-A−/− mice were probed for CD3, CD4, CD25, FR4 and Foxp3 (Fig. 1A). As seen in Figure 1B, no differences were observed in basal levels of Foxp3 expression in lung derived T cells between WT and SP-A−/− mice, either initially or after 8 days (d8) of o.p. saline exposure (1.2–3.3% of total T cells). In contrast to previously reported data with E. coli LPS serotype O111:B4 (13), we observed only a small, non-significant reduction in Foxp3+ cells 1.5 or 2 days after o.p. LPS exposure (serotype O55:B5). However, 8 days post-exposure, we observed a 4.3-fold increase in Foxp3+ T cells from WT mice over initial levels in the lung. SP-A−/− mice subject to the same treatment only showed a 2.4 fold increase in Foxp3+ T cells, suggesting that SPA present in the lungs was affecting the increase of Foxp3+ T cells in the lungs. The o.p. LPS model did not significantly change Foxp3+ Treg levels in the spleen (1.4–2.9% of total T cells). Two thirds (~67–72%) of all CD4+CD25+ cells expressed Foxp3 at d8. The fraction of cells expressing FR4, a marker for functional, native Tregs was more variable, and inversely correlated with stimulation. While Foxp3+ Tregs in all unstimulated conditions expressed high levels of FR4, a new population of intermediate FR4 expressing Foxp3+ cells was observed in the LPS treated mice. This population was most prominent in the lungs of WT mice treated with LPS. The total numbers of cells in BALF increased in both WT and SP-A−/− mice remained much higher than baseline in both WT and SP-A−/− mice, even 8 days post-LPS exposure (Fig. 1C and D). Absolute CD4+CD25+Foxp3+ T cell numbers in bronchoalveolar lavage fluid (BALF) increased from as little as 2 to 34 cells per mouse to 309–1722 cells in WT mice upon LPS exposure. This increase was over twice that seen in SP-A−/− mice. Previous studies have shown that the levels of key inflammatory mediators such as TNFα, RANTES and IL-6 are greatly reduced by day 4 (d4) post LPS exposure, and essentially undetectable by d10 (13). We were unable to detect any TNFα in either WT or SP-A−/− mice treated with LPS at d8. IL-6 (30–58 pg/ml BALF) or IL-10 (18–25 pg/ml in homogenates) levels also did not significantly differ between LPS treated WT and SP-A−/− mice at d8 (data not shown).
Figure 1. SP-A enhances the formation of Foxp3+Tregs in the lung on extended LPS stimulation in vivo.
LPS O55:B5 was instilled oro-pharyngeallyonce, for a duration of 0, 2 or 8 days, and levels of Foxp3+, FR4-hi and FR4-intermediate cells in lungs or spleen of these mice were detected by flow cytometry(A). The fold induction in Foxp3+ T cells in individual mice (data points) are obtained after normalization to the unchanged proportions of Foxp3+ T cells in spleens of corresponding mice across5 independent experiments (B). C and D, Cytospins and differential T cell counts of BALF obtained from total lung volume lavage of saline or LPS treated mice after 8 days of exposure. Absolute numbers of CD4+CD25+Foxp3+ Tcells present in BALF collected from total lung volume lavage were also determined by flow cytometric analysis (E). The box-and-whiskers plots (D and E) show both the range of cell numbers from highest to lowest, as well as lower quartile, median and upper quartile.
Exogenous SP-A specifically enhances Foxp3 expression after ex vivo activation of primary T cells
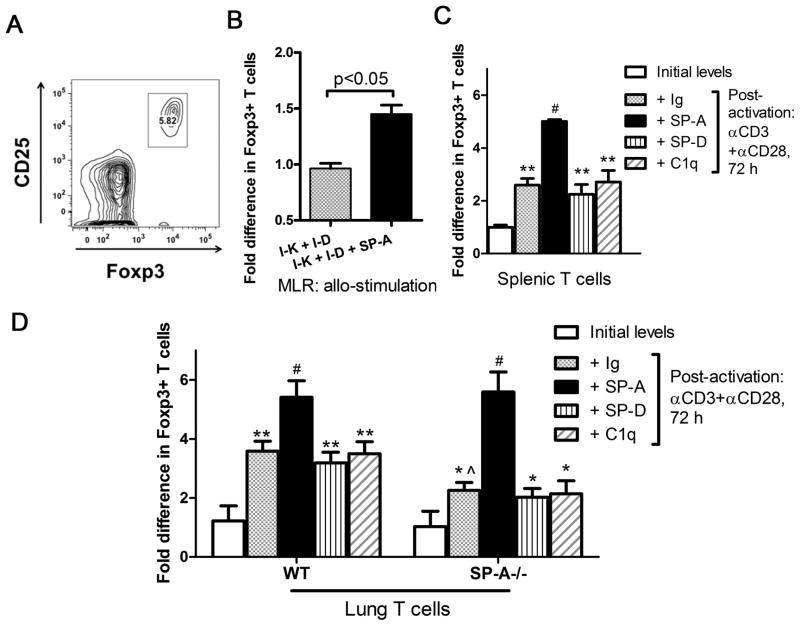
To further confirm this observation and determine if SP-A had a direct effect on the generation of Foxp3+ T cells de novo, we activated purified T cells ex vivo with an antibody-free mixed lymphocyte reaction (MLR) The allogeneic stimulation in the MLR did not show significant differences in baseline levels of Tregs compared to T cells alone from either C57BL/6 or C3BFeJ mice. However, in the presence of exogenous SP-A, we observed a ~47% increase in numbers of Foxp3+ Tregs (Fig. 2B). We also confirmed the SP-A mediated increase in Treg in a controlled activation model, stimulating the TCR using anti-CD3 and anti-CD28 ±SP-A or control proteins. An increase in Foxp3 expression on activation has been previously reported for spleen derived T cells (25, 26)(Fig. 2C). Exogenous SP-A (20 μg/ml) resulted in even greater enhancement of Foxp3+ cells, to over 5-fold greater than baseline.
Figure 2. Exogenous SP-A stimulation enhances the generation of Foxp3+ cells.
Purified T cells were isolated from C3BFeJ mice (2×10^6 cells/ml, allo T cells), stimulated with irradiated splenocytes from C57Bl/6J mice (5×10^6 cells/ml) and cultured for 4 days in the absence or presence of 20 μg/ml of exogenous human SP-A in one representative of three independent experiments with at least 3 mice per group (B). Tregs were defined as CD3+CD4+CD25+Foxp3+ cells and were characterized by flow cytometry (A). Purified T cells from spleen or lungs of WT or SP-A−/− mice were activated with anti-CD3 (1.5 μg/ml) and anti-CD28 (0.5 μg/ml) for ~3 days in the presence of SP-A(20 μg/ml), SP-D (5 μg/ml), C1q (20μg/ml) or control Ig(20 μg/ml)(C and D). The Foxp3+ population was assayed by flow cytometry, represented as initial frequency (white bars), after activation (gray bars), or after activation in the presence of SP-A (black bars) or SP-D or C1q (stipled bars). All data is normalized to initial levels of Foxp3+ T cells in the spleen. * p<0.05, ** p<0.01 compared to initial levels of Foxp3+ cells; ^ p<0.05 compared to post-activation levels in WT mice; #p<0.03 for post-activation SP-A mediated increase over post-activation + Ig conditions, in both WT and SP-A−/− mice, across four independent experiments.
SP-A expression has not been reported in the spleen, making it unlikely that splenic T cells are actively engaged with SP-A in situ. In order to evaluate the effects of SP-A on T cells in the lung, we performed the extended activation assays with lung derived T cells. On activation with anti-CD3 and low amounts of anti-CD28 in extended culture, Foxp3 protein expression increased ~190% in lung derived WT T cells (Fig. 2D), normalized to baseline levels in WT spleens. SP-A deficiency results in a decrease in induction of Foxp3+ cells amongst the responder T cell population in lungs. Exogenous SP-A treatment during ex vivo activation not only ameliorates the decrease, but causes up to a 2-fold increase in the frequency of Foxp3+ T cells. Neither the structurally similar protein C1q, purified Ig, nor the other lung collectin, SP-D, enhanced Foxp3 expression(Figs. 2C and D). Although the extent of Foxp3+ cell induction in this study was lower in splenic T cells compared to lung T cells, we observed that ~82–84% of both CD4+CD25+ spleen and lung derived T cells expressed Foxp3 after 3 days of activation. Thus, TCR stimulation with costimulation in the presence of exogenously added SP-A is sufficient to specifically induce Foxp3+ expression ex vivo.
SP-A enhances active TGFβ production in T cells with concomitant suppression of proliferation
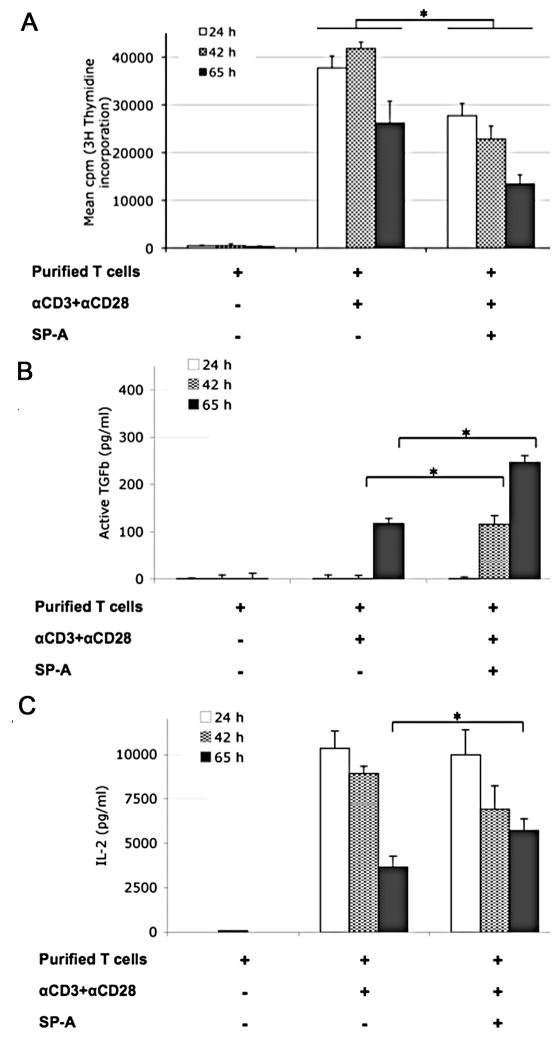
To determine the extent of proliferation at different time points, we measured the incorporation of [3H]-thymidine by actively dividing WT lung-derived T cells, where increased proliferation directly corresponds to increased incorporation of radioactivity. As seen in Figure 3A, anti-CD3 and anti-CD28 greatly enhances proliferation over control, with a peak at time points ranging from 39 to 57 h (with [3H]-thymidine present during the last 15 h) post-addition, and a decline with extended culture (65 h [3H]-thymidine pulse point in an 80 h culture). The addition of exogenous SP-A suppresses T cell proliferation at all time points tested during initial activation. The proportion of hypodiploid cells (discerned by propidium iodide staining and flow cytometric analysis) in the ex vivo activation assays using purified T cells showed a steady increase, peaking at ~13% of the population at 72 h, from a starting value of ~2% immediately after isolation and purification (data not shown). We did not observe any enhancement or significant differences in cell death between T cells activated in the presence or absence of SP-A.
Figure 3. SP-A enhances IL-2 and bio-active TGFβ production in extended culture, even as T cell proliferation is suppressed in a kinetic manner during initial activation.
Purified mouse T cells were left unstimulated or stimulated for the indicated times with anti-CD3 and anti-CD28 in the absence or presence of SP-A (20 μg/ml). (A) Mean cpm of [3H]-thymidine incorporation over 15 h at the indicated time points, by proliferating cells from replicate wells in a representative experiment is depicted. (B) TGFβ levels from culture supernatants were determined by a functional bioassay using CCL64MLE cells as described in Methods.(C) IL-2 levels in culture supernatants at the indicated time points were measured by ELISA. * p<0.05 for fold differences across corresponding time points in three independent experiments by the Mann-Whitney test.
To determine the mechanism responsible for this suppression, we measured TGFβ in culture supernatants. Even as proliferation was reduced by 65 h post-activation, increased levels of bio-active TGFβ were detected in culture supernatants by the luciferase reporter in CCL64MLE cells (Fig. 3B). Active TGFβ levels increased as early as 42 h in the presence of SP-A, with ~2.1-fold higher levels by 65 h post-activation.
IL-2 levels correlate with increased cell proliferation. IL-2 levels peaked at 24 h with anti-CD3 and anti-CD28 in the absence of SP-A, and generally decreased on extended activation. When we added SP-A, we observed ~1.5 fold higher IL-2 levels at 65 h even as proliferation was suppressed. Both IL-2 and TGFβ are critical for Treg induction and maintenance. The lowered proliferation in the presence of SP-A, coupled with relatively high levels of IL-2 and TGFβ, and enhanced induction of Foxp3 (Fig. 2C and D) suggested the presence of a suppressor Treg population.
SP-A enhances the frequency of functional Tregs in responder T cell populations
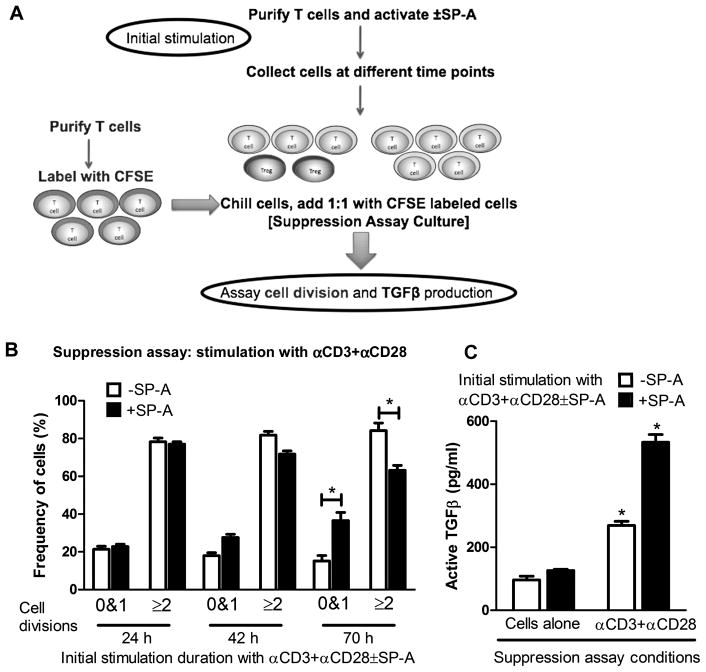
While the previous experiments suggested that the presence of SP-A could induce Foxp3 expression in T cells activated with different stimuli in and ex vivo, we sought to determine the kinetics of induction of this functional suppressor population. As depicted in Figure 4A and 4B, T cells were activated for different periods of time in the absence or presence of exogenous SP-A to allow for the generation of possible suppressor populations. Freshly isolated splenic T cells were labeled with CFSE and used as the target cells in the suppression assay, in which all cells were stimulated with anti-CD3 and anti-CD28. When target cells were cultured with lung T cells that had been subjected to 24 h of initial activation in the absence or presence of SP-A, ~80% of target cells underwent ≥2 divisions in the course of the suppression assay. After 42 h of initial activation in the presence of SP-A, there is a ~55% increase in target cells that are largely undivided (0 and 1 divisions). This increase in suppressor capability in cells initially activated in the presence of SP-A became more pronounced when target cells were co-incubated with T cells previously activated with SP-A for 70 h (~140% increase in target cells with 0 and 1 divisions was observed, in conjunction with a reduction in target cells with >2 divisions). At the same time, we did not observe an increase in the hypodiploid dead and dying population by propidium iodide staining (data not shown).
Figure 4. SP-A enhances the frequency of Foxp3+Tregs in responder T cell populations.
Purified T cells were activated with anti-CD3 and anti-CD28 in the absence or presence of SP-A as shown in the flowchart (A). Cells were harvested, washed and chilled at 24 h, 42 h and 68–70 h post initial activation time points. These cells were used 1:1 in a suppression assay with freshly isolated T cells pulsed with CFSE. In the suppression assay (B), cells were left unactivated as a control or activated with anti-CD3+anti-CD28 for a total of ~70 h, and percentages of divided cells were determined by flow cytometry. Results obtained from cells activated for 24 h, 42 h and 65 h are depicted as the proportion of cells that have either remained undivided or have undergone 2 or more divisions. (C) shows the TGFβ levels from culture supernatants obtained from the cultured cells in the suppression assay, as determined by a functional bioassay using CCL64MLE cells. This data is representative of three independent experiments.*p<0.05.
To determine if this suppressive effect could be mediated by TGFβ, we measured TGFβ production in supernatants from 70 hour suppression assay cultures. When target cells were cultured without any stimulation with cells that had been previously activated for 70 hours with anti-CD3 and anti-CD28 in the absence and presence of SP-A, we observed 96±21 pg/mL and 120±17 pg/mL respectively, of active TGFβ (‘cells alone’ condition in Fig. 4C). However, upon the addition of anti-CD3 and anti-CD28 to suppression assay cultures containing naive target cells and cells previously activated in the presence of SP-A for 70 hours we observed ~98% more active TGFβ than in in the absence of SP-A and ~323% more than the cells alone condition.
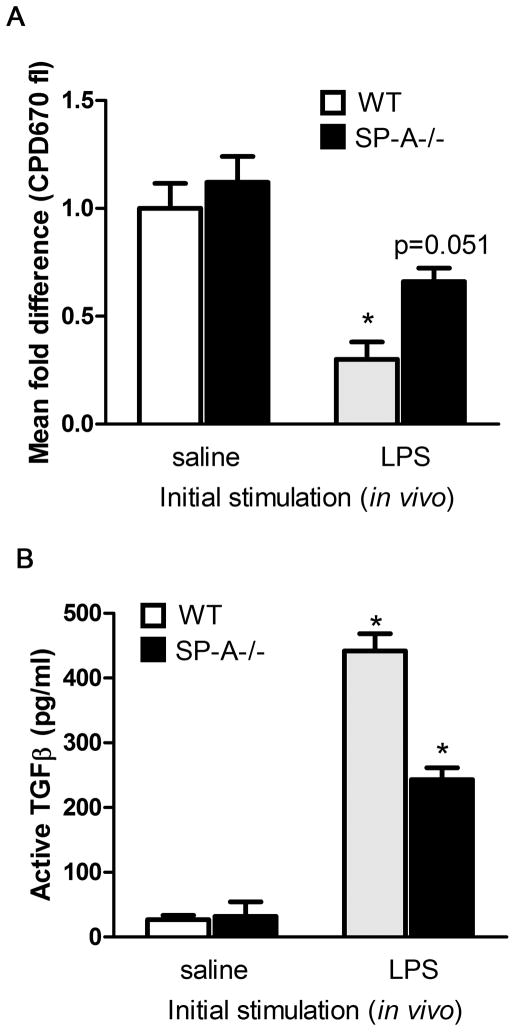
To demonstrate this increase in the generation of suppressor populations in the presence of SP-Ain vivo, we returned to the extended LPS stimulation model. Following extended LPS stimulation in vivo, lung T cells were harvested and used in suppressor assays. We tracked target cell proliferation using CPD670, which is functionally similar to CFSE, but excited with a red laser at 633 nm (Fig. 5A). Target T cells (CPD670-labeled) co-incubated with T cells from WT LPS treated mice showed ~2.2 fold greater suppression of proliferation, than with T cells isolated from SP-A−/− LPS treated mice. We observed enhanced active TGFβ production in lung tissue after extended LPS activation (Fig. 5B). Thus, functional Tregs capable of suppressing other T cells and producing high levels of active TGFβ develop with either extended in vivo stimulation with LPS or ex vivo stimulation with anti-CD3+anti-CD28, and greater proportions of this induced population are observed in the presence of SP-A.
Figure 5. SP-A enhances functional Foxp3+Tregs on extended LPS stimulation in vivo.
LPS was instilled oro-pharyngeally for 8 days, and T cells were harvested and purified from lungs. These cells were used 1:1 in a suppression assay with freshly isolated T cells pulsed with CPD670, and the fold difference in proliferating cells (≥2 divisions) in relation to labeled, activated target cells was calculated (A). A lower level of fluorescence is indicative of greater extent of proliferation, and lower degree of suppression. One lobe of the lung from saline or LPS treated mice was used to prepare lysates for determining amounts of total and active TGFβ levels by ELISA (B). *p<0.05 compared to respective saline treatment condition.
TGFβ blockade reduces induction of Foxp3 and restores T cell proliferation in an ex vivo suppression assay
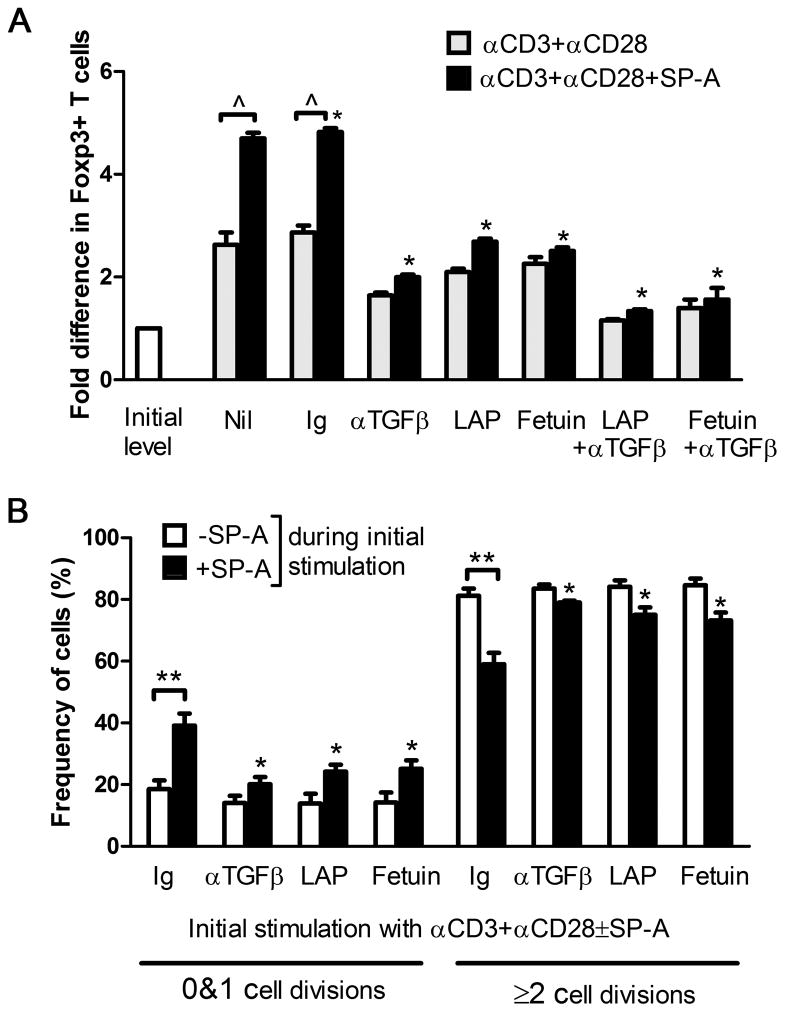
In order to determine whether the induction of functional Tregs by SP-A was a TGFβ dependent phenomenon, we measured the levels of Foxp3 and suppressive function in the presence of Latency Associated Protein (LAP), which can bind and functionally incapacitate active TGFβ, fetuin(α-fetoprotein), which binds TGFβRII to block TGFβ binding, and in the presence of TGFβ blocking antibody. As seen in Figure 6A, WT T cells activated with anti-CD3+anti-CD28 along with control Ig had ~3-fold induction of Foxp3 over initial levels. The level of induction increased to ~5-fold in cells also treated with exogenous SP-A. However, when subject to TGFβ blockade, the level of induction dropped to 2- or ~2.6-fold with anti-TGFβ and LAP respectively. When both anti-TGFβ and LAP, or anti-TGFβ+fetuin were added at the beginning of culture, very little induction of Foxp3 was observed, even in the presence of exogenous SP-A.
Figure 6. TGFβ blockade abrogates SP-A induced Foxp3+Tregs and restores T cell proliferation in an ex vivo suppression assay.
Foxp3 levels were detected by flow cytometry after 70 h of culture in the absence (gray) or presence (black) of SP-A, with control Ig or TGFβ blocking agents as indicated (A). Data is depicted as fold difference in Foxp3+ cells compared to mean initial levels from pooled untreated mice. *p<0.05 with respect to the activated, control Ig+SP-A group across treatment conditions, ^ p<0.01 in −/+SP-A treatment. Suppression assays were performed with anti-CD3+anti-CD28 stimulation using cells subject to initial stimulation in the absence or presence of SP-A (B). The proportion of cells that have either remained undivided or have undergone 2 or more divisions in the suppression assay following initial stimulation under indicated treatment conditions is depicted. *p<0.05 with respect to control Ig+SP-A across treatment conditions, ** p<0.02 in −/+SP-A treatment.
To establish that LAP and anti-TGFβ treatment also reduced the suppressor phenotype of these cells we used cells initially activated with anti CD3 and CD28 with or without SP-A in the presence or absence of LAP, fetuin and anti-TGFβ (Fig. 6B). LAP (0.5 μg/ml) and anti-TGFβ (1.5 μg/ml) showed no increased cytotoxity over control Ig at these saturating concentrations in terms of Foxp3 expression, while the concentration of fetuin was reduced to 0.5 mg/ml to avoid enhanced cytotoxicity. Treatment with anti-TGFβ during the course of activation in the presence of exogenous SP-A resulted in a reduction of functional Tregs generated during extended initial activation. Specifically, both numbers of undivided (0 and 1 divisions) and dividing (≥2 divisions) target cells were similar to that observed with initial stimulation with anti-CD3 and anti-CD28 (~20% and ~80% respectively, versus ~38% and ~60%). This greater proportion of actively dividing cells would potentially allow for clonal expansion that would be half an order of magnitude higher over 10 division cycles. Thus, both Foxp3 induction and generation of a functional suppressor population in the presence of SP-A occur by a TGFβ – dependent mechanism.
Discussion
Several studies over the last few years suggest that T cells in the lung become functionally impaired under certain conditions, (e.g. during the resolution phase of acute lung injury, or following antigen exposure). In this report, we have identified SP-A as a key inducer of a CD4+CD25+Foxp3+ suppressor Treg population, via a TGFβ dependent mechanism.
The transcription factor Foxp3 is probably the most fundamental and best defined signature marker for Tregs in mice. It has been shown to play an essential role in generation of suppressive function. Even a reduction of Foxp3 expression resulted in impaired suppressive function and an aggressive autoimmune syndrome (27). Foxp3 has also been associated with graft-versus-host disease, multiple sclerosis and inflammatory bowel disease, as well as with key T cell signaling pathways, cytokine production and expression of surface receptors like CTLA4 (reviewed in (28)). In WT mice, we detected increased levels of Foxp3 expression in T cells isolated from lungs, but not in T cells from spleen, 8d after high dose o.p. LPS instillation into the airways (Fig. 1). This increase in Foxp3+ T cells could conceivably occur via recruitment, induction, proliferation or any combination of these. To address these possibilities, we administered o.p. CFSE labeling during LPS exposure followed by suppression assay culture with CPD670 labeled target cells and flow cytometric analysis. Low cell proliferation dye levels at d8 suggest active proliferation of Foxp3+ T cells in the lung of WT mice. However, the possibility cannot be excluded that, at this late time point, cells that have not been labeled or have lost the CFSE label cannot be distinguished from cells that have actively proliferated (data not shown). At the same time, SP-A−/− mice had fewer Foxp3+ T cells than WT mice following in vivo or extended ex vivo stimulation (Figs. 1 and 2D), which was restored and enhanced by the addition of exogenous SP-A (Fig. 2).
Previous work from our lab suggests that SP-A may play an important role in enhancing T cell proliferation in response to weak TCR signals (29). Resting Tregs from non-inflamed tissues exhibit little suppressor activity, and must be stimulated to acquire such function. Recent ‘Cutting Edge’ reports by Thompson et al(30), Moran et al(31)and Gottschalk/Corse et al(25, 32) suggest that the quality and quantity of the TCR signal is a key determinant of whether a responding T cell is induced to express Foxp3. We did not observe any significant differences in baseline levels of Foxp3+ T cells in lungs or spleen of WT and SP-A−/− mice, suggesting that SP-A only works in conjunction with T cell activation to enhance Foxp3 expression. Levels of cytokines such as IL-6, which is known to downregulate Foxp3 expression, also did not differ between these mice. However, IL-2 levels are enhanced in ex vivo cultures with exogenous SP-A (Fig. 3C). This is important in the light of recent studies that demonstrate that IL-2 helps maintain Foxp3 expression in TGFβ induced Foxp3+ T cells (33, 34). In these studies, neutralization of IL-2 or disruption of IL-2 signaling by Stat5 knockdown diminished the level of Foxp3 expression and decreased suppressor function of iTregin vivo. Activation induced Tregs, arising in the presence of IL-2 and TGFβ, (whose production/availability is affected by SP-A), appear to be a key component of the increase in Tregs. However, some IL-2 and TGFβ is produced in SP-A−/− mice following activation (~190 and 240 pg/ml, respectively, in d8 lung homogenates). These cytokines are possibly produced by both T cells and macrophages, albeit with differing kinetics owing to the lack of SP-A mediated inhibition. This might account for some of the increase in Tregs in SP-A−/− mice (e.g. in Figs. 1B, 2D and 5). Migration of native Tregs into the lungs, as well as retention of induced Tregs is also a possibility in the in vivo experiments. SP-A appears to help enhance this process and maintain Tregs in the lung, resulting in higher Treg proportions in treated WT lungs. Finally, we determined that there was no significant difference in extent of cell death between T cells from WT and SP-A−/− mice or in the absence and presence of exogenous SP-A, supporting the existence of a suppressor population. Thus, the relatively high levels of both IL-2 and TGFβ present in the lungs in the presence of SP-A may contribute to the enhanced expression of Foxp3, and survival as well as suppressor activity of the iTregs.
Functional suppressor activity by Foxp3+ T cells was observed only when they were induced in the presence of bioactive TGFβ (Fig. 4B and 6B). TGFβ is an extremely pleiotropic cytokine. Post developmental knockdown of TGFβ was shown to cause multifocal inflammatory cell infiltration accompanied by multiorgan failure nearly a decade before Foxp3+ Tregs were identified (35). However, excessive levels of TGFβ have also been linked to autoimmune disorders, susceptibility to opportunistic infections and increased alveolar permeability. As suggested by d’Alessio et al (13), stringent regulation of TGFβ at sites of inflammation might be a key factor in resolution of LPS mediated diffuse lung injury.
TGFβ present in SP-A preparations has been a longstanding concern in studies utilizing SP-A. Using 50 μg/ml of recombinant SP-A produced in CHO cells, which contained over 1000 pg/ml of TGFβ, a level much higher than observed in SP-A purified from other sources, Kunzmann et al (36)demonstrated TGFβRII-dependent SMAD2 phosphorylation in human CD4+ T cells. SP-A preparations used in this study were tested for TGFβ with both a functional bioassay and a highly sensitive low-range ELISA amplification. Assay and did not contain detectable levels of active TGFβ. However, we did find varying amounts of inactive TGFβ ranging from ~32–56pg/ml of purified SP-A used in culture. TGFβ was found to be associated with SP-A irrespective of the purification method, suggesting that these proteins may directly bind each other. Preliminary data from our lab and Kunzmann et al suggest that SP-A might act as a sink or storage reservoir for TGFβ, and enable its steady release under inflammatory conditions. This role of SP-A may even provide the signals to both induce Tregs in conjunction with T cell activation and maintain Tregs in the local physiologic setting of the lung. Indeed, while TGFβ has been somewhat controversially reported to be necessary for Treg suppressive function, recent studies suggest that it may be indispensable for Foxp3 expression and Treg induction as well as influence Treg suppressive activity (20, 37–39). In addition, we did not observe differences in levels of IL-10, another key cytokine that has the potential to mediate Treg suppressive effects in antigen-specific responses. Basal levels of IL-10 in BALF were undetectable. IL-10 was not detected at high or significantly different levels in either culture supernatants, or lung homogenates. IL-10 levels peaked at 32±12 and 40±9 pg/ml following 70 h activation in the absence or presence of exogenous SP-A. However, IL-10 levels in middle right lung homogenates 2 days post-LPS instillation increased, ranging from ~40–50 pg/ml in both both WT and SP-A−/− mice, but these levels dropped down to ~18–25 pg/ml by day 8 post-LPS instillation.
SP-A tends to promiscuously bind many glycosylated proteins including antibody, as well as LPS. Therefore, we also studied the induction of Foxp3 by SP-A in an antibody-free MLR culture system. Also, in order to ensure that the induction of Foxp3 was specific, we used the other immune lung collectin, SP-D, and the structurally similar protein, C1q, as additional controls. In addition to TGFβ, SP-A may also directly interact with cell surface proteins on T cells. This aspect of research remains rather murky, owing to the ability of SP-A to bind many different glycolsylated proteins ranging from the recently identified unconventional myosin 18A (SP-R210) to CD91/calreticulin, immunoglobulins, and even the LPS receptor TLR4(40–43). We are presently engaged in elucidating the biochemical effects of SP-A interaction with key T cell surface activation markers including the CD3 complex, CD28, LFA1, and ICAM.
The pulmonary alveolar epithelium is one of the most environmentally exposed tissues in the body. This study highlights a role for SP-A as an important control mechanism to prevent overzealous immune responses and chronic inflammation in the lung by the TGFβ dependent induction of regulatory T cells during activation.
Acknowledgments
This work was supported by grants from the National Institutes of Health RO1HL-030923, R01HL-068072, P50HL-084917. S.M. is a Pulmonary Fibrosis Foundation nominee. Special thanks to Patti McDermott at the DHVI Flow Cytometry Core, Dr. Julie Ledford for teaching S.M. oro-pharyngeal instillation techniques, and Kathy Evans for exogenous SP-A purification.
Abbreviations
- BALF
Bronchio-alveolar lavage fluid
- LAP
Latency associated Peptide
- MLR
Mixed Lymphocyte Reaction
- o.p
oro-pharyngeal
- SP-A
Surfactant Protein-A
- SP-D
Surfactant Protein-D
- TGFβ
Transforming Growth Factor β
- Treg
Regulatory T cell
References
- 1.Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol. 2005;5:58–68. doi: 10.1038/nri1528. [DOI] [PubMed] [Google Scholar]
- 2.Pastva AM, Mukherjee S, Giamberardino C, Hsia B, Lo B, Sempowski GD, Wright JR. Lung effector memory and activated CD4+ T cells display enhanced proliferation in surfactant protein A-deficient mice during allergen-mediated inflammation. J Immunol. 2011;186:2842–2849. doi: 10.4049/jimmunol.0904190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akbar AN, Vukmanovic-Stejic M, Taams LS, Macallan DC. The dynamic co-evolution of memory and regulatory CD4+ T cells in the periphery. Nat Rev Immunol. 2007;7:231–237. doi: 10.1038/nri2037. [DOI] [PubMed] [Google Scholar]
- 4.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor Foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 5.Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat Immunol. 2007;8:457–462. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- 6.Cobbold SP, Castejon R, Adams E, Zelenika D, Graca L, Humm S, Waldmann H. Induction of FoxP3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J Immunol. 2004;172:6003–6010. doi: 10.4049/jimmunol.172.10.6003. [DOI] [PubMed] [Google Scholar]
- 7.Kasprowicz DJ, Smallwood PS, Tyznik AJ, Ziegler SF. Scurfin (FoxP3) controls T-dependent immune responses in vivo through regulation of CD4+ T cell effector function. J Immunol. 2003;171:1216–1223. doi: 10.4049/jimmunol.171.3.1216. [DOI] [PubMed] [Google Scholar]
- 8.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genetics. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 9.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genetics. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 10.Apostolou I, von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. J Exp Med. 2004;199:1401–1408. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knoechel B, Lohr J, Kahn E, Bluestone JA, Abbas AK. Sequential development of interleukin 2-dependent effector and regulatory T cells in response to endogenous systemic antigen. J Exp Med. 2005;202:1375–1386. doi: 10.1084/jem.20050855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 13.D’Alessio FR, Tsushima K, Aggarwal NR, West EE, Willett MH, Britos MF, Pipeling MR, Brower RG, Tuder RM, McDyer JF, et al. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest. 2009;119:2898–2913. doi: 10.1172/JCI36498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorelik L, Flavell RA. Transforming growth factor-beta in T-cell biology. Nat Rev Immunol. 2002;2:46–53. doi: 10.1038/nri704. [DOI] [PubMed] [Google Scholar]
- 15.Rubtsov YP, Rudensky AY. TGFbeta signalling in control of T-cell-mediated self-reactivity. Nat Rev Immunol. 2007;7:443–453. doi: 10.1038/nri2095. [DOI] [PubMed] [Google Scholar]
- 16.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172:5149–5153. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 19.Marie JC, Liggitt D, Rudensky AY. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity. 2006;25:441–454. doi: 10.1016/j.immuni.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25:455–471. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Korfhagen TR, Bruno MD, Ross GF, Huelsman KM, Ikegami M, Jobe AH, Wert SE, Stripp BR, Morris RE, Glasser SW, et al. Altered surfactant function and structure in SP-A gene targeted mice. Proc Natl Acad Sci U S A. 1996;93:9594–9599. doi: 10.1073/pnas.93.18.9594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McIntosh JC, Swyers AH, Fisher JH, Wright JR. Surfactant proteins A and D increase in response to intratracheal lipopolysaccharide. Am J Respir Cell Mol Biol. 1996;15:509–519. doi: 10.1165/ajrcmb.15.4.8879185. [DOI] [PubMed] [Google Scholar]
- 23.Graziano M, St-Pierre Y, Beauchemin C, Desrosiers M, Potworowski EF. The fate of thymocytes labeled in vivo with CFSE. Exp Cell Res. 1998;240:75–85. doi: 10.1006/excr.1997.3900. [DOI] [PubMed] [Google Scholar]
- 24.Venken K, Thewissen M, Hellings N, Somers V, Hensen K, Rummens JL, Stinissen P. A CFSE based assay for measuring CD4+CD25+ regulatory T cell mediated suppression of auto-antigen specific and polyclonal T cell responses. J Immunol Meth. 2007;322:1–11. doi: 10.1016/j.jim.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 25.Gottschalk RA, Corse E, Allison JP. TCR ligand density and affinity determine peripheral induction of Foxp3 in vivo. J Exp Med. 2010;207:1701–1711. doi: 10.1084/jem.20091999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Josefowicz SZ, Wilson CB, Rudensky AY. Cutting edge: TCR stimulation is sufficient for induction of Foxp3 expression in the absence of DNA methyltransferase 1. J Immunol. 2009;182:6648–6652. doi: 10.4049/jimmunol.0803320. [DOI] [PubMed] [Google Scholar]
- 27.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 28.Ohkura N, Sakaguchi S. Regulatory T cells: roles of T cell receptor for their development and function. Semin Immunopathol. 2010;32:95–106. doi: 10.1007/s00281-010-0200-5. [DOI] [PubMed] [Google Scholar]
- 29.Mukherjee S, Giamberardino C, Thomas J, Evans K, Goto H, Ledford JG, Hsia B, Pastva AM, Wright JR. Surfactant protein a integrates activation signal strength to differentially modulate T cell proliferation. J Immunol. 2012;188:957–967. doi: 10.4049/jimmunol.1100461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson LJ, Valladao AC, Ziegler SF. Cutting edge: De novo induction of functional Foxp3+ regulatory CD4 T cells in response to tissue-restricted self antigen. J Immunol. 2011;186:4551–4555. doi: 10.4049/jimmunol.1003573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corse E, Gottschalk RA, Allison JP. Strength of TCR-Peptide/MHC Interactions and In Vivo T Cell Responses. J Immunol. 2011;186:5039–5045. doi: 10.4049/jimmunol.1003650. [DOI] [PubMed] [Google Scholar]
- 33.Chen Q, Kim YC, Laurence A, Punkosdy GA, Shevach EM. IL-2 Controls the Stability of Foxp3 Expression in TGF-{beta}-Induced Foxp3+ T Cells In Vivo. J Immunol. 2011;186:6329–6337. doi: 10.4049/jimmunol.1100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zorn E, Nelson EA, Mohseni M, Porcheray F, Kim H, Litsa D, Bellucci R, Raderschall E, Canning C, Soiffer RJ, et al. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108:1571–1579. doi: 10.1182/blood-2006-02-004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kunzmann S, Wright JR, Steinhilber W, Kramer BW, Blaser K, Speer CP, Schmidt-Weber C. TGF-beta1 in SP-A preparations influence immune suppressive properties of SP-A on human CD4+ T lymphocytes. Am J Physiol Lung Cell Mol Physiol. 2006;291:L747–756. doi: 10.1152/ajplung.00401.2005. [DOI] [PubMed] [Google Scholar]
- 37.Shevach EM, Tran DQ, Davidson TS, Andersson J. The critical contribution of TGF-beta to the induction of Foxp3 expression and regulatory T cell function. Eur J Immunol. 2008;38:915–917. doi: 10.1002/eji.200738111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fahlen L, Read S, Gorelik L, Hurst SD, Coffman RL, Flavell RA, Powrie F. T cells that cannot respond to TGF-beta escape control by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2005;201:737–746. doi: 10.1084/jem.20040685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piccirillo CA, Letterio JJ, Thornton AM, McHugh RS, Mamura M, Mizuhara H, Shevach EM. CD4(+)CD25(+) regulatory T cells can mediate suppressor function in the absence of transforming growth factor beta1 production and responsiveness. J Exp Med. 2002;196:237–246. doi: 10.1084/jem.20020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang CH, Szeliga J, Jordan J, Faske S, Sever-Chroneos Z, Dorsett B, Christian RE, Settlage RE, Shabanowitz J, Hunt DF, et al. Identification of the surfactant protein A receptor 210 as the unconventional myosin 18A. J Biol Chem. 2005;280:34447–34457. doi: 10.1074/jbc.M505229200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gardai SJ, Xiao YQ, Dickinson M, Nick JA, Voelker DR, Greene KE, Henson PM. By binding SIRPalpha or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell. 2003;115:13–23. doi: 10.1016/s0092-8674(03)00758-x. [DOI] [PubMed] [Google Scholar]
- 42.Oosting RS, Wright JR. Characterization of the surfactant protein A receptor: cell and ligand specificity. Am J Physiol. 1994;267:L165–172. doi: 10.1152/ajplung.1994.267.2.L165. [DOI] [PubMed] [Google Scholar]
- 43.Yamada C, Sano H, Shimizu T, Mitsuzawa H, Nishitani C, Himi T, Kuroki Y. Surfactant protein A directly interacts with TLR4 and MD-2 and regulates inflammatory cellular response. Importance of supratrimeric oligomerization. J Biol Chem. 2006;281:21771–21780. doi: 10.1074/jbc.M513041200. [DOI] [PubMed] [Google Scholar]