Abstract
Flow cytometry specializes in high content measurements of cells and particles in suspension. Having long excelled in analytical throughput of single cells and particles, only recently with the advent of HyperCyt sampling technology has flow cytometry’s multi-experiment throughput begun to approach the point of practicality for efficiently analyzing hundreds-of-thousands of samples, the realm of high throughput screening (HTS). To extend performance and automation compatibility we built a HyperCyt-linked Cluster Cytometer platform, a network of flow cytometers for analyzing samples displayed in high-density, 1536-well plate format. To assess performance we used cell and microsphere based HTS assays that had been well characterized in previous studies. Experiments addressed important technical issues: challenges of small wells (assay volumes 10 μL or less, reagent mixing, cell and particle suspension), detecting and correcting for differences in performance of individual flow cytometers, and the ability to reanalyze a plate in the event of problems encountered during the primary analysis. Boosting sample throughput an additional four-fold, this platform is uniquely positioned to synergize with expanding suspension array and cell barcoding technologies in which as many as 100 experiments are performed in a single well or sample. As high-performance flow cytometers shrink in cost and size, cluster cytometry promises to become a practical, productive approach for HTS and other large scale investigations of biological complexity.
Keywords: Flow cytometry, suspension array, high content analysis, high throughput screening
Introduction
Microscope imaging-based high content screening (HCS) platforms revolutionized the drug discovery enterprise when introduced in the late 1990s, bridging the gap between throughput and information richness of experiments performed with adherent cells(1). Imaging-based HCS technology has improved and expanded over subsequent years to become considered a mainstream screening technology in the pharmaceutical industry(2). Flow cytometry is a complementary technology that specializes in high content measurements of suspension cells such as leukocytes and hematopoetic stem cells. It measures multiple optical signals associated with cells or particles as they pass through a laser based detection system, one at a time. Flow cytometry has long excelled in throughput and information density for analysis of individual cells, now routinely capable of quantifying sixteen or more features per cell at tens-of-thousands of cells per second. However, only recently with the introduction of HyperCyt sampling technology has it begun to achieve levels of multi-experiment throughput compatible with its application in a high throughput screening (HTS) environment(3–5).
HyperCyt uses a peristaltic pump in combination with an autosampler to boost throughput of experimental samples 10- to 20-fold (3). The autosampler moves a sample uptake probe from well to well of a multi-well microplate while the continuously running pump causes uptake of a 1–2 μl sample from each well and an air bubble to separate each sample from the other. Samples from all wells of a microplate are delivered to the flow cytometer in a single round of analysis and stored in a single data file. Specialized software distinguishes and extracts data for individual samples by virtue of temporal gaps in the flow of cells resulting from passage of the sample-separating air bubbles through the laser beam.
The platform has been extensively validated for producing high content, quantitative measurements while processing 384-well plates in 11 minutes or less (6–20). It has been successfully used in small molecule screening assays to produce more than 10 million experimental measurements of small molecule interactions with biological targets, all published on the publically accessible PubChem website (www.ncbi.nlm.nih.gov/pcassay). The conventional HyperCyt platform, of which there are currently more than 100 in laboratories worldwide, is linked to a single flow cytometer and typically processes ~15,000 wells/day. To achieve throughput compatible with screening of libraries containing several hundred thousand compounds or more it has been necessary to use up to four separate platforms running in parallel. This is an expensive and labor intensive approach that requires significant institutional space and complex logistics of operation that are not readily amenable to full automation. To improve performance efficiency and automation compatibility we built and tested a Cluster Cytometer HyperCyt platform that is capable of analyzing samples displayed in high-density, 1536-well plate format.
Materials and Methods
Cells and reagents
Myeloid U937 cells transfected with the human formylpeptide receptor (FPR) were cultured in RPMI-1640 medium (Mediatech 15-041-CV) supplemented with 10% heat-inactivated fetal bovine serum (GIBCO 14160), 2mM L-glutamine-10U/ml penicillin-10 μg/ml streptomycin (Omega Scientific PG-30), 10 mM HEPES (Sigma H-0887), and 4 μg/ml Ciproflaxin (Mediatech MT61-277-RF). Cultures were grown at 37° C in a 5% CO2 atmosphere, and passaged every three days. U937 cells were used that expressed a mutant FPR with glycine and alanine substituted for serine and threonine residues in the C-terminal tail (DeltaST) to prevent receptor internalization (21). Peptide dilution buffer (PDB) consisted of 110 mM NaCl (Sigma S-9625), 30 mM HEPES, 10 mM KCl (Sigma P-3911), 1mM MgCl2 (Sigma M-8266), 10 mM glucose (Sigma G-8270), and 0.1% bovine serum albumin (Sigma B-2518). Protease buffer consisted of 50 mM HEPES, 100 mM NaCl, 1 mg/ml bovine serum albumin, 0.025% Tween-20 (Sigma P-7949), pH 7.4. N-formyl-methionine-leucine-phenylalanine-phenylalanine peptide (fMLFF) was obtained from Sigma (F-3506). Fluorescein-labeled tryptophan-lysine-tyrosine-methionine-valine-D-methionine (WPEP-FITC) was obtained from New England Peptide (custom synthesis) and was previously characterized (22). Multiplexed sets of streptavidin-coated microspheres were obtained from Spherotech (Blue array particle kit, 5.1 μm, SVPAK500-5067-X-4). Quantum FITC MESF low level and high level fluorescein calibration standard microspheres were obtained from Bangs Laboratories (824B and 825B, respectively). Recombinant B. anthracis lethal factor (LF) and C. botulinum neurotoxin light chain A (BoNTALC) were obtained from List Laboratories (169A and 610A, respectively). All test compounds were stored in 100% DMSO (Fisher Scientific D136-1) and diluted 1:100 in each assay so that wells containing test compounds also contain 1% DMSO. Control wells are added with 1% DMSO (vehicle control) to control for potential extraneous effects of DMSO on the assay. A key aspect of assay development is demonstration that the assay output is unaffected by DMSO in amounts expected to be present in test compound containing wells.
Hardware and software
Accuri C6 flow cytometers equipped with CFlow Plus and CFlow Plus Automation software were obtained from BDAccuri, Inc. (Ann Arbor, MI). All had the standard configuration of detectors common to most if not all other C6s. PMTs and other detectors were operated at fixed, manufacturer-specified voltages and gains that were not subject to user modification. The GX274 autosampler and 4-channel peristaltic pump were obtained from Gilson Instruments, Inc. (Middleton, WI). HyperCyt Autosampler control software (version 2.0, IntelliCyt Corp., Albuquerque, NM) was modified to control the GX274 autosampler and Accuri C6 flow cytometers via RS232 serial port and Ethernet TCP/IP network communication, respectively. HyperView software (version 2.5.1, IntelliCyt) was modified to enable linked analysis of the 4 FCS data files generated by processing of each 1536 well plate and automated standardization of fluorescence data from curves generated with fluorescein calibration standard microspheres. A Biomek FX robot (Beckman Coulter, Indianapolis, IN) equipped with a 1536 pintool set containing 100 nL pins (V&P Scientific VP 550A) was used to transfer small molecule test compounds to 1536-well bioassay plates. A MicroFlo Select dispenser equipped with a 1 μL dispense cassette (BioTek Instruments, Winooski, VT) was used to dispense cells, microspheres and reagents into wells. Assays were performed in 1536-well, HiBase polystyrene plates (MPG-782101, Greiner Bio-One, Monroe, NC).
FPR ligand binding inhibition assay
As previously described (23,24), the FPR assay measures the ability of test compounds to compete with a high-affinity fluorescent ligand, WPEP-FITC, for binding to cell membrane FPR. The assay response was quantified on the basis of median green fluorescence intensity (MFI) determinations of cell-bound WPEP-FITC made for individual wells. The assay response range was defined by replicate control wells containing unlabeled fMLFF blocking peptide in PDB (positive controls, with minimum MFI expected from complete inhibition of WPEP-FITC binding) or PDB with 1% DMSO alone (negative controls, with maximum MFI expected from no inhibition of WPEP-FITC binding). For assay performance, additions to wells were in sequence as follows: 1st, 375 nM fMLFF or PDB alone (4 μl/well); 2nd, cells (107/ml, 3 μl/well); 3rd, (after 30 min, 4°C incubation) 17 nM WPEP-FITC (3 μl/well). After an additional 45 min, 4°C incubation, plates were immediately analyzed by flow cytometry with the HyperCyt® platform. All incubations were performed with plates rotating continuously from inverted to upright position to maintain cells in suspension. Test compound inhibition of WPEP-FITC binding to FPR was calculated as 100 x (MFIMAX – MFITEST)/(MFIMAX – MFIMIN), in which MFIMAX and MFIMIN represent the averages for MFI determinations in negative and positive control wells, respectively, and MFITEST represents the MFI measured in wells containing test compounds and WPEP-FITC in combination. Details of gating strategy and an assay schematic are provided in Supplementary Fig. 3.
Protease inhibition assay
Protease inhibition assays were performed as previously described(25), but with modifications. Biotinylated GFP protease substrates for LF, BoNTALC and a protease-resistant substrate (pinpointGFP) were prepared and loaded on streptavidin microspheres as previously described (14,25,26). Additions to wells were in sequence as follows: 1st, 4 μL protease buffer; 2nd, 2 μl of a mixture of 1.5 μM LF and 5 nM BoNTALC in protease buffer; 3rd, 100 nL of test compounds (1 mM in DMSO); and 4th, 4 μL containing 2×105/ml of each set of substrate-bearing microspheres. Plates were sealed and incubated at 24°C overnight (16–18 h), rotating continuously from inverted to upright position until analyzed in the HyperCyt platform the following day. The assay response was quantified on the basis of MFI determinations of microsphere-bound protease substrate-GFP made for individual wells. The assay response range was defined by replicate control wells containing no proteases (positive controls, with maximum MFI reflecting the expected response from complete protease inhibition) or the protease mixture alone (negative controls, with minimum MFI reflecting complete absence of protease inhibition). Test compound inhibition of substrate cleavage by protease resulted in an increase in MFI relative to negative controls and was calculated as 100 x (MFITEST – MFIMIN)/(MFIMAX – MFIMIN), in which MFIMIN and MFIMAX represent the averages for MFI determinations in negative and positive control wells, respectively, and MFITEST represents the MFI measured in wells containing test compounds and protease mixtures in combination. Details of gating strategy and an assay schematic are provided in Supplementary Fig. 4.
GRK2 assay
The GRK2 assay measures the ability of test compounds to displace a fluorescently labeled RNA aptamer that binds with nanomolar affinity to the GRK2 protein. GRK2 protein was biotinylated using biotin N-hydroxysuccinimide ester (Sigma) and conjugated to streptavidin-coated beads (Spherotech) at 4°C overnight in GRK2 assay buffer (20 mM HEPES pH 7.0, 10 mM NaCl, 5 mM MgCl2, 0.1% Lubrol, 2 mM DTT, 1 mM CHAPS). The RNA aptamer was fluorescently labeled at a single site on the 3′ end with 6-FAM (6-carboxyfluorescein), to produce aptamer-3′-FAM (1 FAM group per RNA aptamer, synthesized and labeled by IDT (www.IDTDNA.com)), which was diluted to 6.6 nM in GRK2 assay buffer. A total of volume of 10 μL was added to wells in the following sequence: 1st, 4 μL GRK2 assay buffer; 2nd, 100 nL of 1mM test compounds; 3rd, 3 μL GRK2 conjugated beads, incubated at room temperature for 15 minutes; and 4th, 3 μL 6.6 nM aptamer-3′-FAM. Plates were incubated at room temperature for 1 hr, rotating continuously from inverted to upright position. Plates were then analyzed by flow cytometry with the HyperCyt platform. The assay response was quantified on the basis of aptamer-3′-FAM MFI determinations made for individual wells. The assay response range was defined by replicate control wells containing 1% DMSO vehicle control (negative controls, with maximum MFI expected from no inhibition of aptamer-3′-FAM binding) or 50X unlabeled RNA aptamer in assay buffer (positive controls, with minimum MFI expected from complete inhibition of aptamer-3′-FAM binding). Test compound inhibition of aptamer-3′-FAM binding to GRK2 was calculated as 100 x (MFIMAX – MFITEST)/(MFIMAX – MFIMIN), in which MFIMAX and MFIMIN represent the averages for MFI determinations in negative and positive control wells, respectively, and MFITEST represents the MFI measured in wells containing test compounds and aptamer-3′-FAM in combination.
Assay quality assessment
In all assays, the mean and standard deviation (SD) of MFI measurements from replicate control wells were used to calculate the Z′ score, a dimensionless measure of screening assay quality that reflects both assay signal dynamic range and data variation associated with the signal measurements (27). As illustrated in assay descriptions above, some assays were designed to detect test compound effects that cause an increase in MFI while others were designed to detect MFI decreases. Positive control wells represented the maximum expected MFI in the former case, the minimum in the latter, and vice-versa for negative control wells. For purposes of calculating Z′ the important consideration was only that control wells with minimum and maximum MFI be distinguished as follows:
in which MeanMAX and SDMAX represent the mean and SD of MFI values from control wells with maximum MFI, and MeanMIN and SDMIN are the same for control wells with minimum MFI. Possible values of Z′ range from negative infinity to 1.0. An assay is deemed excellent for screening purposes if Z′ is in the range between 0.5 and 1.0. A Z′ value of 0 is indicative of a screening assay capable of providing only a “yes/no” type of output.
An alternative statistic is the Z-factor, which is calculated in a similar fashion as Z′ except that 1) the terms in the equation pertaining to the negative control wells are substituted with mean and SD MFI values for all wells in the plate excluding the positive control wells, and 2) the absolute value of the difference term in the denominator is used. The Z-factor is subject to influence from a variety of additional factors that don’t affect Z′ such as compound concentration and number of active compounds on the plate. Because the value of Z′ is subject to fewer nuances of interpretation than the Z-factor, we exclusively used Z′ for purposes of evaluating instrument performance in the present studies.
Flow cytometry
All analyses were performed with Accuri C6 flow cytometers (see above). Singlet populations of cells and microspheres were selectively gated for analysis on the basis of forward light scatter (linear scale) and side light scatter (log scale) from a 488 nm laser. Green fluorescence emission detected at 533/30 nm was excited by a 488 nm laser, recorded in the FL1-H channel and displayed in log scale. Red fluorescence emission detected at 675/25 was excited by a 640 nm laser, recorded in the FL4-H channel and displayed in log scale. Representative list mode and GatingML files for any of the figures are available upon request from the corresponding author.
Results
Cluster cytometry
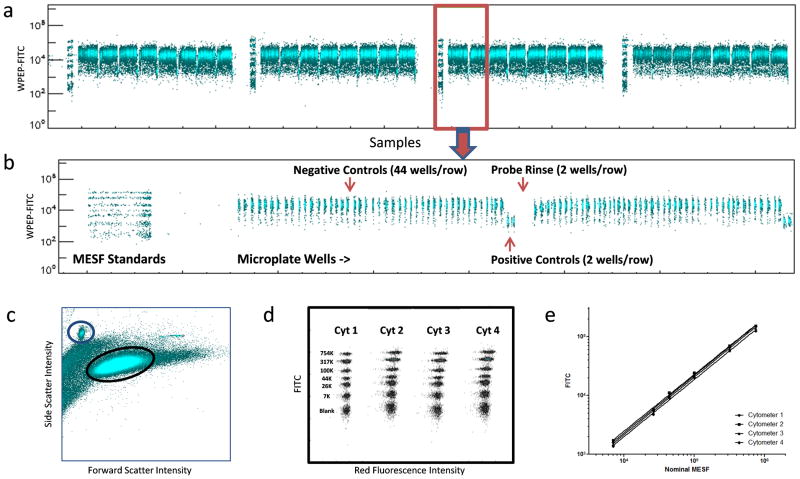
The platform consisted of a high-precision positioning autosampler, a cluster of four small-footprint, moderate-cost Accuri C6 flow cytometers, and a 4-channel peristaltic pump that delivered samples from four sample uptake probes to each of the flow cytometers (Fig. 1A and Supplementary Fig. 2). The cytometers were remotely controlled over an Ethernet network by a netbook computer that coordinated autosampler and cytometer operation, file naming via barcode reader, and data streaming to a centralized storage location where data from the four cytometers were linked and analyzed (Fig. 1A). To enable high-throughput analysis of samples displayed in 1536-well plate format, the four sample uptake probes were positioned at 8-well intervals across the 32-row dimension of the plate (Fig. 1B). The autosampler moved the 4-probe head across the 48-column plate dimension row by row so that each probe sampled a 384-well segment of the plate (Fig. 1C). In initial pilot tests a multiplexed preparation of microspheres consisting of five sets with distinctive red fluorescence intensity profiles (Fig. 1D) was dispensed into a 1536-well plate so that each well contained 2,000 microspheres from each set in a 10 μL volume. Approximately 2 μL was aspirated per well from each plate segment in parallel at a rate that allowed the entire plate to be processed in less than 11 min. In a representative experiment, each cytometer consistently resolved the samples from individual wells as well as the five discrete fluorescence profiles of microspheres in each sample (Fig. 1E). The number of microspheres detected from each of the five fluorescence intensity sets averaged from 299 ± 34 to 328 ± 31 per well over the entire plate and ranged from a minimum of 108 to a maximum of 429 from each set per well.
Fig 1.
Cluster Cytometer performance
Performance of the HyperCyt platform has been extensively validated for both cell and microsphere based HTS assays in 96- and 384-well format. However, the move to 1536 well plates and use of multiple flow cytometers in parallel accentuated several new technical issues: the need to restrict assay volumes to 10 μL or less to accommodate smaller wells, limited options for ensuring adequate mixing of reagents and suspension of cells and particles in such small wells, the potential need to detect and correct for differences in the performance of individual flow cytometers, and the ability to reanalyze a plate in the event of problems encountered with one or more of the cytometers (e.g., clogging) during the primary analysis. To address these issues we used cell and microsphere based HTS assays that had been well characterized in previous studies.
Cell suspension, mixing and cross-cytometer fluorescence response calibration
The formylpeptide receptor (FPR) ligand binding inhibition assay, successfully used to identify high-affinity, small molecule FPR antagonists (24,28), tests the ability of compounds to displace a fluorescent peptide ligand, WPEP-FITC, from FPRs expressed in membranes of intact cells (23,29) (Supplementary Fig. 3D). The original assay volume of 15 μL was reduced to 10 μL by increasing cell and reagent concentrations. Three sequential rounds of droplet deposition with a micro-dispenser were used to fill wells first with control solutions with or without receptor blocking peptide ligand (4 μL), then cells (3 μL) and finally, WPEP-FITC fluorescent ligand. All mixing in wells was exclusively accomplished by rotating the plates end-over-end from inverted to upright position at ~4 RPM during each incubation step as previously described (30). Plates were set up with 44 wells of each row as negative controls (cells plus WPEP-FITC to produce brightly fluorescent cells) and 2 as positive controls (cells and WPEP-FITC plus excess non-fluorescent, blocking peptide ligand to produce dimly fluorescent cells). The four sampling probes were programmed to aspirate a suspension of fluorescein calibration standard microspheres for 10 s prior to sampling of the 1536-well plate. Thus, the analysis results for each cytometer/plate segment had a calibration standards fluorescence profile as an internal control of cytometer fluorescence response performance (Figs. 2A and B). The calibration standard microspheres had a distinctive light scattering profile (Fig. 2C) by which they could be distinguished from the cells and gated for separate analysis (Supplementary Fig. 3A–C). Comparison of the resulting fluorescence calibration curve profiles for the four flow cytometers indicated a high degree of similarity (Fig. 2D and E).
Fig 2.
In a representative plate, a greater than 10-fold response difference between positive and negative controls was observed for each of the four cytometer/plate segments (Table 1). Z′ scores (27) calculated separately for each cytometer/plate segment on the basis of WPEP-FITC green fluorescence intensity ranged from 0.579 to 0.819. When data from the four segments were pooled, as if the entire plate had been analyzed by a single flow cytometer, the Z′ score was 0.704. Transformation to a calibrated scale such as molecules of equivalent soluble fluorescein (MESF) is an accepted means of normalizing data to compensate for differences between relative fluorescence response values produced by different flow cytometers. Using regression coefficients from line fits illustrated in Fig. 2D Calibrated MESF values were calculated for control well data. Unexpectedly, such a transformation resulted in a slightly lower Z′ score for pooled data (0.605) in association with increased coefficients of variation (CVs) for positive and negative controls (Table 1).
Table 1.
Evaluation of the FPR fluorescent ligand binding assay in 1536-well format.
| WPEP-FITC (MFI) | Calibrated MESF | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cyto-meter | Controla | Mean | SD | CV | Z′ | Mean | SD | CV | Z′ |
| 1 | Pos | 2080 | 96 | 4.6 | 0.579 | 10390 | 492 | 4.7 | 0.572 |
| Neg | 22378 | 2750 | 12.3 | 120176 | 15183 | 12.6 | |||
| 2 | Pos | 2140 | 64 | 3.0 | 0.819 | 8544 | 265 | 3.1 | 0.815 |
| Neg | 23067 | 1199 | 5.2 | 100825 | 5428 | 5.4 | |||
| 3 | Pos | 2032 | 44 | 2.2 | 0.819 | 9350 | 207 | 2.2 | 0.816 |
| Neg | 23522 | 1254 | 5.3 | 114534 | 6240 | 5.4 | |||
| 4 | Pos | 1895 | 100 | 5.3 | 0.704 | 8108 | 437 | 5.4 | 0.699 |
| Neg | 23130 | 1998 | 8.6 | 105542 | 9324 | 8.8 | |||
| All | Pos | 2036 | 119 | 5.9 | 0.704 | 9098 | 947 | 10.4 | 0.605 |
| Neg | 23024 | 1950 | 8.5 | 110293 | 12389 | 11.2 | |||
Plate segments analyzed by each cytometer contained 352 negative control wells and 16 positive control wells
To further address this issue, we plotted Calibrated MESF values for both calibration microspheres and cells as a function of nominal MESF for the 4 cytometers (Supplementary Fig. 5A). Calibrated MESF profiles and fitted regression lines for the calibration microspheres were virtually identical for all cytometers, an indication that the linear fit model brought all cytometers into good quantitative agreement. By contrast, the Calibrated MESF values for cells from control wells were less tightly grouped about the overlapping best fit lines and showed relatively small but statistically significant inter-cytometer differences within each control group. These measurements were made on the basis of the height of the fluorescence pulse produced by cell or microsphere passage through the laser beam (FL1-H). Such measurements can be sensitive to differences in laser beam configuration between cytometers (e.g., laser beam diameter), particularly when making quantitative comparisons between particles of different diameters as was the case for the microspheres and cells (~5 vs. ~9 μm, respectively). Therefore, we also investigated the use of fluorescence pulse area measurements (FL1-A) that are considered less sensitive to this potential source on inter-cytometer variation (Supplementary Fig. 5B). While negative control values appeared to group more tightly in FL1-A plots as compared to FL1-H plots, positive control values did not exhibit a similar trend (Supplementary Fig. 5B). It seems likely that plate position effects may have accounted for some of the observed variation, perhaps reflecting assay artifacts due to edge effects or reagent dispensing variation across the 4 quadrants of the 1536-well plate.
Taken together, these results suggested that the innate variation between the four flow cytometers was relatively small and subject to sources of variation that could not be improved by MESF transformation of fluorescence data. Clearly documented was the importance of including assay control wells in all plate quadrants and using fluorescence data from them as the primary means for normalizing results individually for each cytometer. The relative uniformity and signal range of assay response values over the entire plate indicated that adequate mixing had been accomplished without a need for physical interventions such as pipetting or vortexing of assay suspensions. This was confirmed by Z′ scores of 0.780, 0.781 and 0.830 that were obtained when three additional plates were analyzed separately in a similar fashion (pooled WPEP-FITC control well MFI data from the four segments of each plate, data not shown).
Analysis repeatability
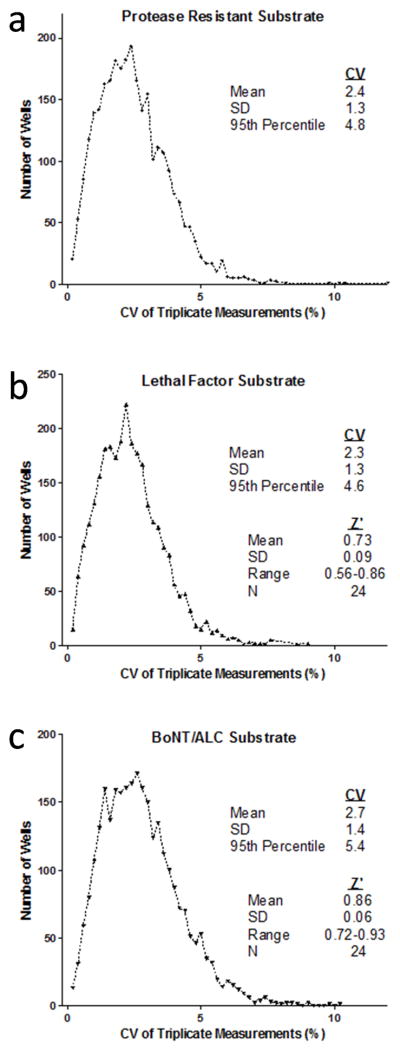
We have previously described microsphere based HTS assays to detect inhibitors of proteases, anthrax lethal factor (LF) and Botulinum neurotoxin A light chain (BoNT/ALC)(14,25,26). The assays employ recombinant fusion proteins consisting of a protease substrate sequence (peptide with cleavage site) fused at one end with a biotinylated attachment sequence and at the other with green fluorescent protein (GFP). The substrate fusion proteins are attached to color-coded streptavidin microspheres via the biotin moiety and protease activity is detected as a loss of microsphere GFP fluorescence intensity resulting from cleavage of bound substrate (Supplementary Fig. 4A). To adapt the protease assay to 1536-well plates, we reduced assay volumes from the original 20–25 μL to 10 μl by concentrating assay components as above. To two plates we separately dispensed mixtures of proteases (LF and BoNT/ALC) and color-coded microspheres bearing GFP fusion proteins (LF substrate, BoNT/ALC substrate and a protein resistant to both proteases). We used a 1536 pintool set to add 1,993 test compounds from the Molecular Libraries Small Molecule Repository (MLSMR) Validation Set (100 nL/well). The plates were incubated overnight on the plate rotator to allow protease reactions to go to completion and processed the following day. Detailed results of the compound screen, which also evaluated an additional protease and microsphere-bound substrate in each well, will be reported separately (PubChem Summary AIDs 566467, 566469 and 566470, updated at completion of each screening stage); however, we took the opportunity to assess the repeatability of results by processing each plate three times in succession.
From 1492 wells on each plate were determined the mean, standard deviation (SD) and corresponding coefficient of variation (CV) for the three sequential measurements of median green fluorescence intensity of each microsphere set in the well. In histograms plotting the distribution of CVs for each set, the 95th percentiles ranged from 4.6% for LF substrate to 5.4% for BoNT/ALC substrate (Fig. 3). Thus, 95% or more of the replicate measurements for each bead set had CVs of less than 6%. For substrates susceptible to protease cleavage, Z′ scores derived from positive and negative control wells (8 of each per plate segment) averaged 0.73 (LF) and 0.86 (BoNT/ALC) over the 24 separate plate segment determinations (Fig. 3B and C, 8 segments of 2 plates evaluated 3 times each). These results indicated that the same plate can be analyzed up to three times with the expectation of consistently good data quality and reproducibility.
Fig 3.
Validation against 384-well plate data
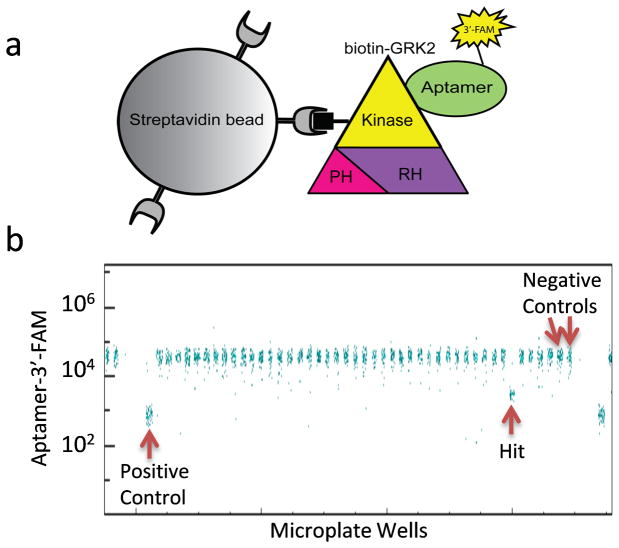
Under the auspices of the NIH-sponsored Molecular Libraries Screening Centers Network and Probe Production Centers Network (MLSCN and MLPCN, respectively), the single-cytometer HyperCyt system has been applied to screening of small molecules from the MLSMR in more than 18 campaigns (>40 separate biological targets) involving a diversity of cell- and microsphere-based assays. To date, our flow cytometry screening has been exclusively performed in 384-well format. To validate performance of the quad-cytometer platform we selected a microsphere based assay that had recently been screened in 384-well format. The assay was designed to detect inhibitors selectively targeting G protein-coupled receptor kinase 2 (GRK2), an enzyme implicated in cardiac failure (31). In the HTS assay, a green fluorescent RNA aptamer is used in a displacement assay to identify small molecules that bind to regions of the GRK2 kinase domain critical for activity (Fig. 4A). Biotin-GRK2 is bound to streptavidin microspheres so that the microspheres become brightly fluorescent in the presence of the aptamer. Hit detection is based upon a decrease in microsphere fluorescence intensity that results when a test compound disrupts aptamer binding to GRK2 (Fig.4B, and http://pubchem.ncbi.nlm.nih.gov/assay/assay.cgi?aid=488855).
Fig 4.
When Cluster Cytometer HTS was performed in 1536-well format using the MLSMR Validation Set, 14 compounds inhibited aptamer binding to GRK2 by 25% or more (Fig. 4B and Table 2). Of these, 7 inhibited similarly in 2 separate HTS campaigns performed in the low-density, 384-well plate format (Table 2, Confirmed), first in a screen of the Validation Set (PubChem AID 488806) and subsequently in a screen of the full MLSMR library in which most of the Validation Set compounds are represented (327,943 compounds, PubChem AID 488847). There was 1 compound that had activity in the two single cytometer screens but not in the Cluster Cytometer screen; however it failed to inhibit in a follow up confirmation screen (PubChem AID 504451) and was considered a false positive (Table 2, compound ID 448222). There were 4 compounds that were apparent hits in the Cluster Cytometer screen but not in the single cytometer screen. We interpreted these hits as likely false positives since the compounds were consistently inactive in the single-cytometer screens. We have not analyzed these compounds further. Thus, HTS in high-density plate format with the Cluster Cytometer HyperCyt platform detected all compounds in the Validation Set for which activity had been previously confirmed using our conventional single cytometer HTS platform.
Table 2.
Validation of Hit Detection in HTS.
| Inhibition of Aptamer-3′-FAM Binding (%) | |||||
|---|---|---|---|---|---|
| Results in 384-Well HTS | Compound ID | AIDa 488806 | AID 488847 | AID 504451 | 1536-Well HTS |
| Confirmed (All >25%) | 16195270 | 92 | 88 | 94 | |
| 6763 | 67 | 28 | 88 | ||
| 1780 | 62 | 50 | 55 | ||
| 460749 | 45 | 34 | 66 | ||
| 5702697 | 39 | 89 | 66 | ||
| 646406 | 34 | 47 | 29 | ||
| 6420073 | 92 | NDb | 99 | ||
| False Positive (One < 25% ) | 2957802 | 86 | 11 | 75 | |
| 906542 | 61 | 0 | 82 | ||
| 2827740 | 33 | 0 | 38 | ||
| 448222 | 66 | 30 | 1 | 0 | |
| Negative (All <25%) | 2936792 | 2 | 4 | 98 | |
| 1522903 | 0 | 0 | 97 | ||
| 104871 | 5 | 2 | 57 | ||
| 12035 | 5 | 2 | 52 | ||
PubChem assay ID for 384-well plate HTS results
ND, Not done
Discussion
The concept of using multiple flow cytometers in parallel has some precedence in the recent past, applied primarily as an approach to boost throughput of cell isolation and purification operations by fluorescence-based cell sorting. An example is the commercial use of banks of high-speed cell sorters running continuously in parallel to separate X- and Y-chromosome bearing sperm for use by breeders of domestic animals (e.g., www.sexingtechnologies.com/). Cell sorting flow cytometers are now commercially available in which up to four sorting modules can be packed into the footprint of one traditional instrument and operated in parallel over remote network connections to speed purification of rare cell subpopulations (www.i-cyt.com). Our integrated, multi-flow cytometer screening platform now extends the parallel flow cytometry concept to speed performance and analysis of large numbers of discrete biological experiments involving cell and particle suspensions.
We have found cluster cytometry to offer a number of benefits as compared to the alternate approach of operating four separate flow cytometry screening platforms in parallel. 1) Screening lab space requirement is significantly reduced. 2) The close proximity of cytometers and sample delivery systems makes it more practical for the screening process to be effectively implemented and monitored by a single operator. 3) Synchronization of sample processing by the four cytometers facilitates automated “just-in-time” analysis of screening assay data. In practice, we routinely produce a complete analysis of the screening data for each plate (Z′, hit frequency, number of cells/well, etc.) within the 10–12 min time required to process the next plate. This allows rapid detection of problems that might not be obvious by visual inspection so that they can be quickly fixed. Affected plates are then placed back on the rotating suspension system for later reanalysis. 4) There is a single point of plate introduction rather than four separate locations, more efficient for interfacing with an automated plate transport system. 5) There is flexibility of operating modes. The platform can be easily switched to run 1, 2, 3 or 4 flow cytometers at a time and can accommodate microplates of various well configurations (e.g., 96, 384, 12, 24, etc.).
A potential disadvantage of the cluster cytometer approach would be the consequences of a malfunctioning flow cytometer or autosampler. One possible solution for accommodating cytometer failure would be to switch to 2-cytometer operating mode and process the two halves of a 1536-well plate in parallel. However, the modularity and moderate cost of the Accuri C6 flow cytometer lends feasibility to an alternate solution: having a backup unit available that can be quickly swapped for a malfunctioning unit in the event of an instrument problem that resists quick diagnosis and repair. We have shown this to work in practice when the red laser on one of the cytometers failed in the middle of a major screening operation. It took only ~15 min to swap in a backup unit to allow successful completion of the screen. We also have a backup autosampler (considerably less expensive than a flow cytometer) that can be swapped for the original in ~15 min in the event, rare in our experience, that the robotic sample handling component might fail. Thus, with appropriate backup infrastructure recovery from failure has similar time requirements whether operating in cluster or single cytometer mode.
An important issue for the cluster cytometer approach was the need to evaluate equivalency of fluorescence responses between flow cytometers and to have methods to correct for potential inter-unit variability. In this study we used calibration standard microspheres to evaluate FITC fluorescence response equivalency of the cytometers. All showed linear fluorescence response profiles with parallel but offset regression lines, an indication that there were differences in fluorescence response sensitivity reflecting the ability to resolve dim fluorescence in the FITC channel (Fig. 2e). It was expected that transformation of all fluorescence response data to the MESF scale would suffice to ensure equivalency. This was indeed the case for microsphere FITC fluorescence results (Supplementary Fig. 5). However, extended analysis of cell fluorescence data from control wells in the FPR ligand binding assay clearly showed that there were other sources of variability such as putative plate position effects that may not have been attributable to instrument performance differences. These contributed to apparent disparities between cytometers that could not be entirely resolved by MESF transformation (Supplementary Fig. 5). We conclude that a more robust method for achieving quantitatively comparable results is to include control wells in common for each cytometer that define assay response limits (e.g., maximum and minimum response), graded response output levels (e.g. different concentrations of a response modulating chemical). Fluorescence data from these wells can then serve as a basis for normalizing results individually for each cytometer to a common scale suitable for cross comparison such as % inhibition, % response relative to a control source of cells, % response relative to a curve produced by a control chemical, etc. The same considerations apply for any fluorescence channel in which the making of quantitative fluorescence response comparisons is of interest. If compensation is required to correct for fluorescence spillover between channels, common compensation controls are used to set up the cytometers to produce similar results prior to sample processing. Common assay control wells are then used to normalize results post-analysis. In the cluster cytometry approach, as in any studies involving a collection of flow cytometers running a common assay (e.g., multi-institutional collaborations), the low end of fluorescence intensity amenable to analysis will be governed by the least sensitive instrument. An advantage of the cluster approach is that all flow cytometers are evaluated in parallel under the same experimental conditions so that limiting conditions can be rapidly and unambiguously identified.
The present results demonstrate that bioassays involving both cell and microsphere suspensions can be successfully performed and analyzed in high-density, 1536-well format with robustness in quality and repeatability critical for the HTS environment. Since completion of these pilot studies the Cluster Cytometer HyperCyt platform has been successfully used for HTS of a multiplexed assay to detect inhibitors of 3 proteases (referenced above) against the full MLSMR library of more than 350,000 small molecules (PubChem Summary AIDs 566467, 566469 and 566470). After a preliminary series of smaller shakedown runs we routinely screened more than 51,000 compounds (60,000 wells when including controls and rinse wells) per day.
This technology complements the capabilities of imaging-based platforms that have been successfully used for high content, high throughput analyses of surface-attached cells. Moreover it is uniquely positioned to augment rapidly expanding suspension array and fluorescent cell barcoding technologies in which as many as 100 bioassays are performed in a single well (32,33). We have successfully implemented a number of such multiplexed assays for high throughput screening campaigns in single flow cytometer, 384-well plate format. Recently documented examples include a fluorescently “barcoded” 5-plex of cell strains from the Yeast-GFP collection to probe Target-of-Rapamycin (TOR) signaling pathways (34) (PubChem AID 1867) and a 6-plex of color-coded microspheres to probe BCL-2 family protein binding interactions (35,36) (PubChem AID 1908). A relatively modest 10-plex bioassay performed in high-density format can be expected to produce over 15,000 distinct and quantitative experimental measurements in less than 11 min from a single 1536-well plate. It seems likely that large-scale bioassay multiplexing capabilities will prove a useful tool for probing complex systems of biomedical importance such as signaling networks, drug selectivity and crossreactivity, etc. Of additional importance is our demonstration that such versatility can be achieved with relatively low-cost but powerful, small-footprint flow cytometers that have only recently become commercially available. The advent of the low-cost personal computer enabled the implementation of parallel computing systems as an affordable approach for resolving large scale computational problems. We anticipate that a similar trend will lead to cluster cytometry as a practical approach, affordable to a broader segment of the worldwide research community, for addressing large scale studies of biological complexity. Parallel-channel, chip-based microfluidic sorting systems are currently in development that promise to further extend the throughput capabilities of parallel flow cytometry (e.g., www.cytonome.com), and it will be of great interest to see how performance, versatility, failure mode recovery options and costs of such systems will compare with modular systems such as reported here.
Supplementary Material
Acknowledgments
Supported in part by NIH grants R01HG005066 to BSE, U54MH084690 to LAS, R03MH093184-01A1 to SWG, and R03 DA030557-01A1 and R01 HL071818 to JJGT, and by the University of New Mexico Center for Molecular Discovery and University of New Mexico Cancer Center Shared Flow Cytometry and High Throughput Screening Resource. We gratefully acknowledge Matthew Hess and Aaron Kennington of IntelliCyt Corp. for helpful discussions about software for implementing Ethernet network communications protocols. Conflict of interest statement: BSE and LAS are co-inventors of the HyperCyt high-throughput flow cytometry platform and co-founders of IntelliCyt Corporation, Albuquerque, NM, the commercial manufacturer and distributor of the HyperCyt platform.
Footnotes
This work was presented in part at the ISAC CYTO10 Congress, Seattle, WA, May, 2010, and the Biopharmaceutical Flow Cytometry and Imaging Conference, GlaxoSmithKline, Ware, UK. October, 2010.
References
- 1.Giuliano KA, DeBiasio RL, Dunlay RT, Gough A, Volosky JM, Zock J, Pavlakis G, Taylor DL. High-content screening: a new approach to easing key bottlenecks in the drug discovery process. J Biomol Screen. 1997;2:249–259. [Google Scholar]
- 2.Haney SA, LaPan P, Pan J, Zhang J. High-content screening moves to the front of the line. Drug discovery today. 2006;11:889–94. doi: 10.1016/j.drudis.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Kuckuck FW, Edwards BS, Sklar LA. High throughput flow cytometry. Cytometry. 2001;44:83–90. [PubMed] [Google Scholar]
- 4.Arterburn JB, Oprea TI, Prossnitz ER, Edwards BS, Sklar LA. Discovery of selective probes and antagonists for G-protein-coupled receptors FPR/FPRL1 and GPR30. Curr Top Med Chem. 2009;9:1227–36. doi: 10.2174/156802609789753608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sklar LA, Edwards BS. In: HTS flow cytometry, small molecule discovery, and the NIH Molecular Libraries Initative. Litvin V, Marder P, editors. Hoboken, NJ: John Wiley & Sons; 2010. pp. 71–98. [Google Scholar]
- 6.Jackson WC, Bennett TA, Edwards BS, Prossnitz E, Lopez GP, Sklar LA. Performance of in-line microfluidic mixers in laminar flow for high-throughput flow cytometry. Biotechniques. 2002;33:220–226. doi: 10.2144/02331dd06. [DOI] [PubMed] [Google Scholar]
- 7.Jackson WC, Kuckuck F, Edwards BS, Mammoli A, Gallegos CM, Lopez GP, Buranda T, Sklar LA. Mixing small volumes for continuous high-throughput flow cytometry: Performance of a mixing Y and peristaltic sample delivery. Cytometry. 2002;47:183–191. doi: 10.1002/cyto.10067. [DOI] [PubMed] [Google Scholar]
- 8.Ramirez S, Aiken CT, Andrzejewski B, Sklar LA, Edwards BS. High-throughput flow cytometry: validation in microvolume bioassays. Cytometry A. 2003;53A:55–65. doi: 10.1002/cyto.a.10035. [DOI] [PubMed] [Google Scholar]
- 9.Bartsch JW, Tran HD, Waller A, Mammoli AA, Buranda T, Sklar LA, Edwards BS. An investigation of liquid carryover and sample residual for a high-throughput flow cytometer sample delivery system. Anal Chem. 2004;76:3810–7. doi: 10.1021/ac049870f. [DOI] [PubMed] [Google Scholar]
- 10.Young SM, Curry MS, Ransom JT, Ballesteros JA, Prossnitz ER, Sklar LA, Edwards BS. High-throughput microfluidic mixing and multiparametric cell sorting for bioactive compound screening. J Biomol Screen. 2004;9:103–111. doi: 10.1177/1087057103262335. [DOI] [PubMed] [Google Scholar]
- 11.Edwards BS, Bologa C, Young SM, Balakin KV, Prossnitz ER, Savchuck NP, Sklar LA, Oprea TI. Integration of virtual screening with high-throughput flow cytometry to identify novel small molecule formylpeptide receptor antagonists. Mol Pharmacol. 2005;68:1301–1310. doi: 10.1124/mol.105.014068. [DOI] [PubMed] [Google Scholar]
- 12.Young SM, Bologa C, Prossnitz ER, Oprea TI, Sklar LA, Edwards BS. High-throughput screening with HyperCyt flow cytometry to detect small molecule formylpeptide receptor ligands. J Biomol Screen. 2005;10:374–382. doi: 10.1177/1087057105274532. [DOI] [PubMed] [Google Scholar]
- 13.Edwards BS, Young SM, Oprea TI, Bologa CG, Prossnitz ER, Sklar LA. Biomolecular screening of formylpeptide receptor ligands with a sensitive, quantitative, high-throughput flow cytometry platform. Nat Protoc. 2006;1:59–66. doi: 10.1038/nprot.2006.9. [DOI] [PubMed] [Google Scholar]
- 14.Saunders MJ, Kim H, Woods TA, Nolan JP, Sklar LA, Edwards BS, Graves SW. Microsphere-based protease assays and screening application for lethal factor and factor Xa. Cytometry A. 2006;69A:342–352. doi: 10.1002/cyto.a.20268. [DOI] [PubMed] [Google Scholar]
- 15.Edwards BS, Ivnitski-Steele I, Young SM, Salas VM, Sklar LA. High-throughput cytotoxicity screening by propidium iodide staining. Curr Protoc Cytom. 2007;Chapter 9(Unit9):24. doi: 10.1002/0471142956.cy0924s41. [DOI] [PubMed] [Google Scholar]
- 16.Simons PC, Young SM, Gibaja V, Lee WC, Josiah S, Edwards BS, Sklar LA. Duplexed, bead-based competitive assay for inhibitors of protein kinases. Cytometry A. 2007;71A:451–459. doi: 10.1002/cyto.a.20398. [DOI] [PubMed] [Google Scholar]
- 17.Dennis MK, Bowles HJ, MacKenzie DA, Burchiel SW, Edwards BS, Sklar LA, Prossnitz ER, Thompson TA. A multifunctional androgen receptor screening assay using the high-throughput Hypercyt flow cytometry system. Cytometry A. 2008;73A:390–399. doi: 10.1002/cyto.a.20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ivnitski-Steele I, Larson RS, Lovato DM, Khawaja HM, Winter SS, Oprea TI, Sklar LA, Edwards BS. High-throughput flow cytometry to detect selective inhibitors of ABCB1, ABCC1, and ABCG2 transporters. Assay Drug Dev Technol. 2008;6:263–76. doi: 10.1089/adt.2007.107. [DOI] [PubMed] [Google Scholar]
- 19.Winter SS, Lovato DM, Khawaja HM, Edwards BS, Steele ID, Young SM, Oprea TI, Sklar LA, Larson RS. High-Throughput Screening for Daunorubicin-Mediated Drug Resistance Identifies Mometasone Furoate as a Novel ABCB1-Reversal Agent. J Biomol Screen. 2008;13:185–193. doi: 10.1177/1087057108314610. [DOI] [PubMed] [Google Scholar]
- 20.Young SM, Bologa CM, Fara D, Bryant BK, Strouse JJ, Arterburn JB, Ye RD, Oprea TI, Prossnitz ER, Sklar LA, et al. Duplex high-throughput flow cytometry screen identifies two novel formylpeptide receptor family probes. Cytometry A. 2008 doi: 10.1002/cyto.a.20645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prossnitz ER. Desensitization of N-formylpeptide receptor-mediated activation is dependent upon receptor phosphorylation. J Biol Chem. 1997;272:15213–15219. doi: 10.1074/jbc.272.24.15213. [DOI] [PubMed] [Google Scholar]
- 22.Strouse JJ, Young SM, Mitchell HD, Ye RD, Prossnitz ER, Sklar LA, Edwards BS. A novel fluorescent cross-reactive formylpeptide receptor/formylpeptide receptor-like 1 hexapeptide ligand. Cytometry A. 2009;75A:264–270. doi: 10.1002/cyto.a.20670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards BS, Young SM, Oprea TI, Bologa C, Prossnitz E, Sklar LA. Biomolecular screening of formylpeptide receptor ligands with a sensitive, quantitative, high-throughput flow cytometry platform. Nat Protocols. 2006;1:59–66. doi: 10.1038/nprot.2006.9. [DOI] [PubMed] [Google Scholar]
- 24.Young SM, Bologa CM, Fara D, Bryant BK, Strouse JJ, Arterburn JB, Ye RD, Oprea TI, Prossnitz ER, Sklar LA, et al. Duplex high-throughput flow cytometry screen identifies two novel formylpeptide receptor family probes. Cytometry A. 2009;75A:253–263. doi: 10.1002/cyto.a.20645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saunders MJ, Edwards BS, Zhu J, Sklar LA, Graves SW. Microsphere-based flow cytometry protease assays for use in protease activity detection and high-throughput screening. Curr Protocols Cytometry. 2010;13.12:1–17. doi: 10.1002/0471142956.cy1312s54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saunders MJ, Graves SW, Sklar LA, Oprea TI, Edwards BS. High-throughput multiplex flow cytometry screening for botulinum neurotoxin type a light chain protease inhibitors. Assay Drug Dev Technol. 2010;8:37–46. doi: 10.1089/adt.2009.0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang JH, Chung TD, Oldenburg KR. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J Biomol Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 28.Edwards BS, Bologa C, Young SM, Balakin KV, Prossnitz E, Savchuck NP, Sklar LA, Oprea TI. Integration of virtual screening with high throughput flow cytometry to identify novel small molecule formylpeptide receptor antagonists. Mol Pharmacol. 2005;68:1301–1310. doi: 10.1124/mol.105.014068. [DOI] [PubMed] [Google Scholar]
- 29.Edwards BS, Young SM, Ivnitsky-Steele I, Ye RD, Prossnitz ER, Sklar LA. High-content screening: flow cytometry analysis. Methods Mol Biol. 2009;486:151–65. doi: 10.1007/978-1-60327-545-3_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramirez S, Aiken CT, Andrzejewski B, Sklar LA, Edwards BS. High-throughput flow cytometry: Validation in microvolume bioassays. Cytometry A. 2003;53A:55–65. doi: 10.1002/cyto.a.10035. [DOI] [PubMed] [Google Scholar]
- 31.Rockman HA, Chien KR, Choi DJ, Iaccarino G, Hunter JJ, Ross J, Jr, Lefkowitz RJ, Koch WJ. Expression of a beta-adrenergic receptor kinase 1 inhibitor prevents the development of myocardial failure in gene-targeted mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:7000–5. doi: 10.1073/pnas.95.12.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nolan JP, Sklar LA. Suspension array technology: evolution of the flat-array paradigm. Trends Biotechnol. 2002;20:9–12. doi: 10.1016/s0167-7799(01)01844-3. [DOI] [PubMed] [Google Scholar]
- 33.Krutzik PO, Nolan GP. Fluorescent cell barcoding in flow cytometry allows high-throughput drug screening and signaling profiling. Nat Methods. 2006;3:361–368. doi: 10.1038/nmeth872. [DOI] [PubMed] [Google Scholar]
- 34.Chen J, Young SM, Allen CP, Seeber A, Péli-Gulli M-P, Panchaud N, Waller A, Ursu O, Yao T, Golden JE. Identification of a small molecular inhibitor of yeast TORC1 using a flow cytometry based multiplex screen. J Biol Chem. 2012 doi: 10.1021/cb200452r. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Curpan RF, Simons PC, Zhai D, Young SM, Carter MB, Bologa CG, Oprea TI, Satterthwait AC, Reed JC, Edwards BS, et al. High-Throughput Screen for the Chemical Inhibitors of Antiapoptotic Bcl-2 Family Proteins by Multiplex Flow Cytometry. Assay and drug development technologies. 2011 doi: 10.1089/adt.2010.0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simons PC, Young SM, Carter MB, Waller A, Zhai D, Reed JC, Edwards BS, Sklar LA. Simultaneous in vitro molecular screening of protein-peptide interactions by flow cytometry, using six Bcl-2 family proteins as examples. Nature protocols. 2011;6:943–952. doi: 10.1038/nprot.2011.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.