Abstract
Associations between nicotine in cigarettes and food consumption may alter the incentive value of food such that food cue-reactivity is exaggerated during abstinence from smoking. This effect may contribute to the weight gain associated with cessation of smoking. We examined the effects of nicotine (0.4 mg/kg base SC) paired (NPD) or unpaired (NUP) with 10 % sucrose self-administration (0.2 ml/delivery, 1h/day for 10 days) on self-administration response rate and intake as well as sucrose cue-reactivity following either 1 or 30 days of forced abstinence. Rats were administered the training dose of nicotine prior to a second, consecutive cue-reactivity session. NPD rats responded at over 3 times the rate for sucrose, and earned nearly twice the number of sucrose deliveries, as NUP rats or saline controls. Sucrose cue-reactivity was greater after 30 days, vs. 1 day of forced abstinence for all groups. History of nicotine exposure had no effect on sucrose cue-reactivity. However, the subsequent injection of nicotine increased sucrose cue-reactivity only in the NPD groups. There were no abstinent-dependent effects of nicotine challenge on sucrose cue-reactivity. A study conducted in parallel with water as the reinforcer revealed a less dramatic effect of nicotine on intake. There was no history or abstinence-dependent effects of nicotine on water cue-reactivity. Nicotine increases the reinforcing effects of sucrose and sucrose-paired cues when nicotine is present. An implication of these findings is that relapse to nicotine (cigarettes) could substantially elevate food cue-reactivity.
Keywords: Addiction, craving, eating disorders, incubation, motivation, relapse
INTRODUCTION
Quitting smoking predicts weight gain and an increase in waist circumference in both men and women (Perkins 1993; Pisinger & Jorgensen 2007). There are likely several biological factors that contribute to this increase in body fat, including insulin resistance (Chiolero et al. 2008), however some of the increase may be related to changes in the reinforcing properties of foods due to their association with the nicotine in cigarettes. For example, it has been reported that nicotine can come to serve as a discriminative stimulus (Palmatier & Bevins 2008) or appetitive conditioned stimulus (CS+) (Murray & Bevins 2007) for sucrose if nicotine has been associated with sucrose availability. While exposure to nicotine has been shown to increase sucrose intake (Jias & Ellison 1990; Smith & Roberts 1995) it is not clear if nicotine paired with sucrose intake enhances subsequent sucrose cue-reactivity. Furthermore, it is not clear if exposure to nicotine following a period of abstinence from sucrose will affect sucrose seeking behavior.
One approach for examining the primary and secondary reinforcing effects of drugs and food in rats is the self-administration paradigm where rats respond for a reinforcer in daily training sessions and then respond for cues associated with the reinforcer under extinction conditions. The former (training) conditions examine primary while the latter (extinction) conditions examine secondary reinforcement. In the present study, we examined both primary and secondary reinforcing (cue-reactivity) qualities of sucrose in rats that were injected with nicotine immediately prior to sucrose self-administration sessions. To evaluate potential state-dependent or discriminative stimulus effects of nicotine, we included comparison groups that had nicotine exposure not paired with sucrose self-administration. A comparison was also made with rats given access to water self-administration to determine how nicotine might have specific effects on sucrose reinforcement. Locomotor activity was measured as an indication of conditioned locomotor activation and/or nicotine-induced locomotor activation and sensitization.
A 1 or 30 day forced-abstinence period was included as a final variable in the study. Length of forced abstinence has been found to be a key predictor of operant responding for cues previously associated with either drugs of abuse or sucrose self-administration in rats Grimm (2011). As a variable in the present study, length of forced abstinence was included to determine if this “incubation of craving” for sucrose would be affected by previous exposure to nicotine. Incubation of craving may be a critical factor in relapse for humans and in fact, has recently been observed in smokers abstaining from cigarette smoking (Bedi et al. 2011).
MATERIALS AND METHODS
Animals
148 male Long-Evans rats (3 months old at start of study; Simonsen-derived) bred in the Western Washington University vivarium were housed individually on a reverse day/night cycle (lights off at 7AM) with nutritionally-balanced Purina Mills Inc. Mazuri Rodent Pellets and water available ad libitum except as noted below. Rats were weighed the day prior to their first saline or nicotine injection (see below) and then each subsequent Monday, Wednesday, and Friday for the duration of the experiment. Immediately prior to the Training phase, the animals were deprived of water for 17 h to encourage self-administration (SA) on the first day of training. Rats were then returned to ad libitum water access. There were 12 separate groups of rats in the study created by independent variable levels defined in subsequent sections: SA (2), training drug (3), and forced-abstinence period (2). As indicated in the Figure captions, final n’s for these groups ranged from 11–13 animals each. All procedures followed the guidelines outlined in the “Principles of laboratory animal care” (NIH publication no. 86–23) and were approved by the Western Washington University Institutional Animal Care and Use Committee.
Apparatus
Operant training and testing took place in operant conditioning chambers (30 × 20 × 24 cm; Med Associates) containing two levers (one stationary and one retractable), a tone generator, a white stimulus light above the retractable lever, and a red house light on the opposite wall. An infusion pump delivered sucrose or water into a reward receptacle to the right of the active lever. Four photobeams crisscrossed the chamber. Operant conditioning chambers were enclosed in sound-attenuating cabinets with ventilation fans.
Drug
Nicotine hydrogen tartrate salt (Sigma-Aldrich, St. Louis, MO USA) was dissolved in saline to 0.4 mg/ml (free base) to be injected SC at 1 ml/kg. The pH was adjusted to 7.0 ± 0.2 with NaOH. This dose of nicotine was found previously to serve as a conditioned stimulus (Murray, Penrod & Bevins 2009). With a half-life of 45 min (Matta et al. 2007), the daily nicotine injection would be expected to be effectively cleared before the next injection.
Procedure
Nicotine administration
On the three days prior to the first day of the training phase, all rats received SC injections of saline or nicotine in their home cage to acclimate the animals to receiving injections and to develop tolerance to the acute locomotor depressant effect of nicotine (Besheer et al. 2004; Clarke & Kumar 1983). There were 3 training drug treatment groups. Saline-treated (Saline) rats were injected with saline every day of training both 5 min prior and 3 h following each SA session. The two nicotine-treated groups were injected every day of training either 3 h following each SA session, nicotine unpaired group (NUP), or 5 min prior to each SA session, nicotine paired group (NPD). For the nicotine groups, saline was injected at the other time point (5 min prior or 3 h following SA).
Training phase
Rats spent 1 h/day for 10 consecutive days in operant conditioning chambers where they were allowed to press the retractable (active) lever for a 0.2 ml delivery of either 10 % sucrose or tap water into the receptacle to the right of the lever. This response also activated a compound stimulus consisting of the tone 92 kHz, 15 dB over ambient noise) and the white light. The compound stimulus lasted for 5 s and was followed by a 40-s time out, during which presses on the active lever were recorded but had no programmed consequence. A response on the inactive (stationary) lever did not have a programmed consequence, but responses were recorded. The total number of photobeam breaks was recorded during all phases of the study. At the end of each session, rats were returned to home cages.
Forced-abstinence phase
The 1 or 30 day forced-abstinence phase began as the first day (“Day 1”) following the 10th day of the training phase. Rats were housed in home cages for the duration of forced abstinence.
Testing phase
On Day 1 or Day 30 rats were tested in the operant conditioning chambers for sucrose or water cue-reactivity. This session was identical to the 1-h training procedure, except that the sucrose or water was not delivered following a lever response. The testing phase consisted of 2, 1-h sessions separated by approximately 5 min. A saline challenge (Sal) injection preceded the first session by 5 min and a nicotine challenge (Nic) injection preceded the second session by 5 min.
Statistical analyses
Body weight
Pre-experiment body weights were compared using one-way analysis of variance (ANOVA) across independent variables DAY (1 or 30), DRUG (Saline, NUP, NPD), and SA condition (water or sucrose) to verify that groups were statistically equivalent to start with. Body weights were then compared across the Training phase (5 data points) using three-way repeated measures (RM) ANOVA with the between-group factors of DAY, DRUG, and SA. This same statistical procedure was used to compare body weights across the forced abstinence period (13 data points) for those rats to be tested on Day 30 of forced abstinence.
Training phase
Water and sucrose SA groups were analyzed separately. Active lever responses, number of reward deliveries, inactive lever responses, and photobeam breaks during operant training were analyzed separately using two-way RM ANOVA of the 10 days of training using between-group factors of DAY and DRUG. The DAY analysis was used to verify that DAY groups received equal training.
Testing phase
Water and sucrose SA groups were analyzed separately. The effects of treatments for the active lever responses, inactive lever responses, and photobeam breaks were evaluated separately using two-way RM ANOVA. Factors were TIME, DAY, and DRUG.
For all ANOVAs, post-hoc comparisons were made with LSD tests. ANOVAs were calculated using SPSS version 18.0. Descriptive statistics were calculated in Excel 2010. Group data are presented as means ± standard errors of the means (SEMs) in the text and figures. For statistical comparisons, p < 0.05 was the criterion for the statistical significance. In general, only the statistics for significant effects and interactions are indicated in the text.
RESULTS
Of 148 rats that were trained for sucrose self-administration, 6 were removed from the study because of mistakes with injections during training conditions. This left 142 subjects for analyses. There were no SA acquisition criteria for this study. Final group size ranges are indicated in the figure captions.
Body weight
Initial body weights of the rats were 390.2 ± 3.1 g. Final body weights for rats tested on Day 30 of forced abstinence (n = 70) were 456.4 ± 3.3 g. Initial body weights did not differ across the assigned conditions. Rats gained weight over the 10 days of training F(4,520) = 344.4, p < 0.001 and there were significant interactions of TIME and DRUG F(8,520) = 2.4 as well as TIME and SA F(4, 520) = 3.1, p’s < 0.05. Post-hoc evaluation of these interactions did not reveal significant effects. Visual inspection of these data revealed that nicotine-treated rats and rats pressing for water gained slightly more weight over the first few days of training, but the differences disappeared by the end of the 10-day training period (data not shown). Rats gained weight over the month of forced abstinence TIME F(12,768) = 380.1, p < 0.001 and there was a significant interaction of TIME and SA F(12,768) = 2.0, p < 0.05. Post-hoc evaluation of this interaction did not reveal significant effects.
Training phase
Water SA
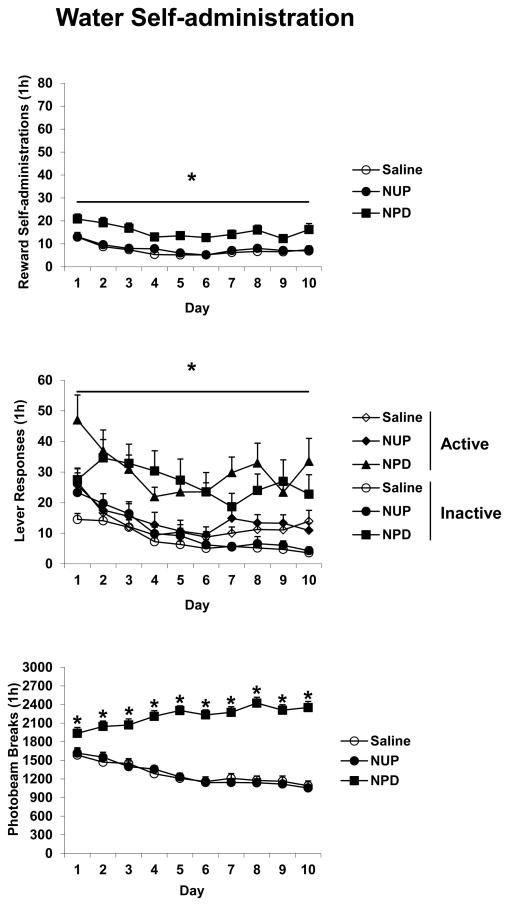
Active lever responding, number of rewards, and inactive lever responses all decreased over the Training phase with all TIME F(9,585) values > 10.0, p’s < 0.001. For each of these measures there were also significant effects of DRUG with F(2,65) values > 9.0, p’s < 0.001. Post hoc analyses indicated that the NPD group responded more than the other groups (Figure 1).
Figure 1.
The effects of nicotine injections on water self-administration. Saline rats were injected with saline (SC) 5 min prior and 3 h after each 1 h session wherein responding on the active lever resulted in a 0.2 ml delivery of water along with presentation of a tone + light stimulus. NUP and NPD rats were treated similarly except NUP rats received nicotine (0.4 mg/kg SC) 3 h after each session and NPD rats received nicotine 5 min prior to each session. Overall effects of DRUG are indicated with an underlined * where NPD rats responded more than NUP or Saline rats; individual *’s indicate significant post hoc differences where NPD rats responded more than NUP or Saline rats, p’s < 0.05. Group n’s were 22–25 animals each. Means ± SEMs are indicated on the figure.
Photobeam breaks increased over the Training phase for the NPD group but decreased over the Training phase for the other groups indicated by significant effects of TIME F(9,585) = 5.8, TIME and DRUG F(18,585) = 13.2, and DRUG F(2,65) = 75.2, all p’s < 0.001 and significant post-hoc tests (Figure 1).
Sucrose SA
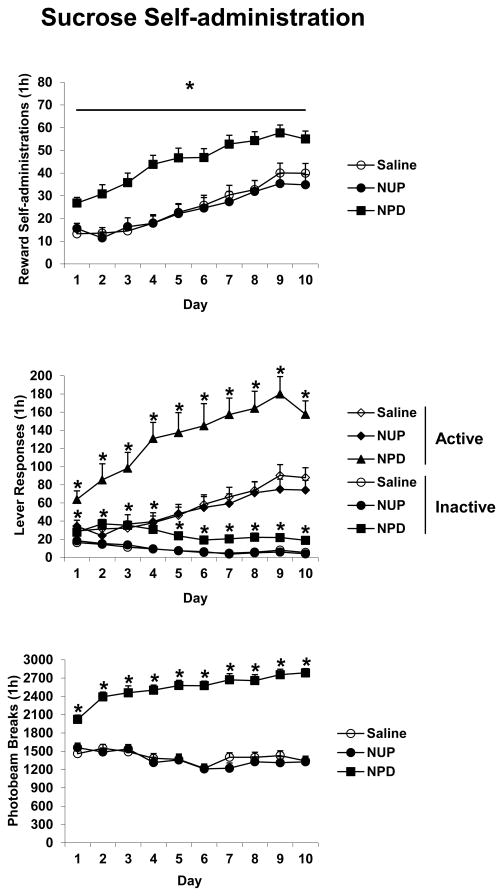
Active lever responding and number of rewards increased over the Training phase with TIME F(9,585) values of 21.8 and 43.6, respectively, p’s < 0.001. For these measures there were also significant effects of DRUG with F(2,65) values of > 15.0, p’s < 0.001. For active lever responding there was also a significant interaction of TIME and DRUG F(18,585) = 1.8, p < 0.05. Inactive lever responding decreased over the Training phase TIME F(9,585) = 22.1, p < 0.001, and there was a significant TIME and DRUG interaction F(18,585) = 1.7, p < 0.05. For all of these measures, post hoc analyses indicated that the NPD group responded more than the other groups (Figure 2).
Figure 2.
The effects of nicotine injections on sucrose self-administration. Saline rats were injected with saline 5 min prior and 3 h after each 1 h session wherein responding on the active lever resulted in a 0.2 ml delivery of sucrose along with presentation of a tone + light stimulus. NUP and NPD rats were treated similarly except NUP rats received nicotine (0.4 mg/kg SC) 3 h after each session and NPD rats received nicotine 5 min prior to each session. An overall effect of DRUG is indicated with an underlined * where NPD rats responded more than NUP or Saline rats; individual *’s indicate significant post hoc differences where NPD rats responded more than NUP or Saline rats, p’s < 0.05. Group n’s were 22–25 animals each. Means ± SEMs are indicated on the figure.
Photobeam breaks increased over the Training phase for the NPD group but decreased over the Training phase for the other groups indicated by significant effects of TIME F(9,585) = 3.2, TIME and DRUG F(18,585) = 9.3, and DRUG F(2,65) = 119.3, all p’s < 0.01 and significant post-hoc tests (Figure 2).
Testing phase
Water SA
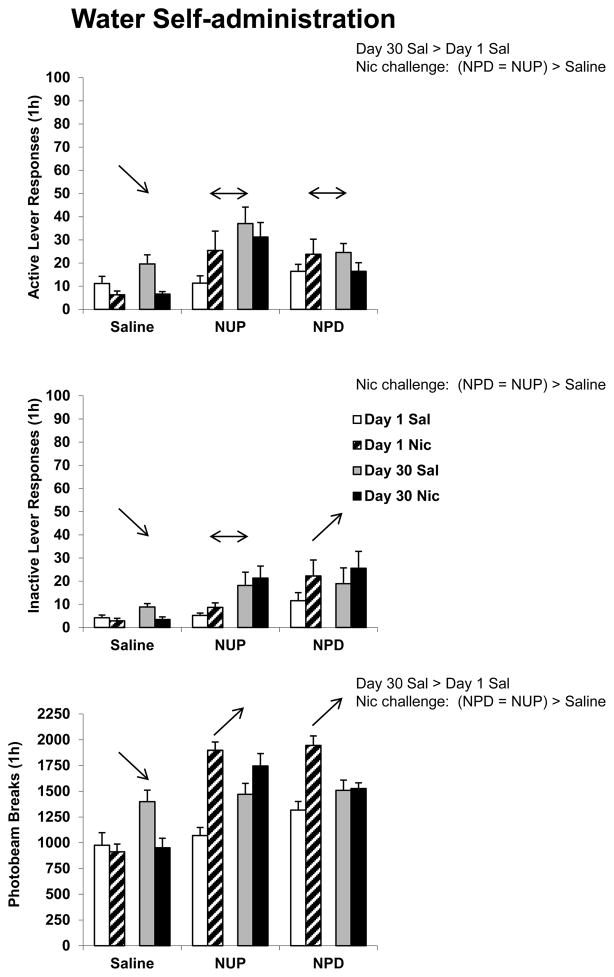
For active lever responding there were significant effects of DAY F(1,65) = 4.1, p < 0.05 and DRUG F(2,65) = 6.2, p < 0.01. There were also significant TIME and DAY and TIME and DRUG interactions [TIME and DAY F(1,65) = 15.4, p < 0.001; TIME and DRUG F(2,65) = 4.0, p < 0.05]. For inactive lever responding there were significant effects of DRUG F(2,65) = 6.1, p < 0.01, TIME F(1,65) = 4.1, p < 0.05, and a significant TIME and DRUG interaction F(2,65) = 5.9, p < 0.01. There was a significant effect of DRUG for photobeam breaks F(2,65) = 24.1, p < 0.001 and significant TIME and DAY and TIME and DRUG interactions [TIME and DAY F(1,65) = 48.6, p < 0.001; TIME and DRUG F(2,65) = 37.9, p < 0.001]. Active lever responding, inactive lever responding, and photobeam break data are indicated in Figure 3. Overall results of post-hoc tests are indicated on the figure. Briefly, active lever responding was greater following 30 days of forced abstinence, but only in the first (Sal) hour of testing. Acute nicotine (Nic) reduced active lever responding in rats with a history of saline injections, but did not alter responding of rats with a history of nicotine injections. Considered as a between-group comparison, rats with a history of nicotine injections responded more for the water-paired cue following acute nicotine than rats with a history of saline injections. Due to the large number of groups, the within-group component of the study, and the 2-way interactions, results of between-group post-hoc comparisons are summarized on the Figure as text and results of within-group comparisons as arrows.
Figure 3.
Responding during two consecutive Test sessions where active lever responses produced a tone + light stimulus previously associated with water self-administration. Rats were injected with saline (Sal) 5 min prior to the first 1-h session and nicotine (Nic) 5 min prior to the second 1-h session. Between-group comparisons (text) refer to either a significant effect of length of forced abstinence for all rats when challenged with saline on the Test day, or an effect of drug treatment history (Saline, NUP, or NPD) on the animals’ response to either Sal or Nic. A unidirectional arrow indicates a significant increase or decrease in responding following the acute nicotine challenge for that drug treatment history group (Saline, NUP, or NPD) compared to the response following saline, regardless of day of forced abstinence. A double-headed arrow indicates no significant change in responding. All post-hoc results indicated were statistically significant at p < 0.05. Group n’s were 11–13 animals each. Means ± SEMs are indicated on the figure.
Sucrose SA
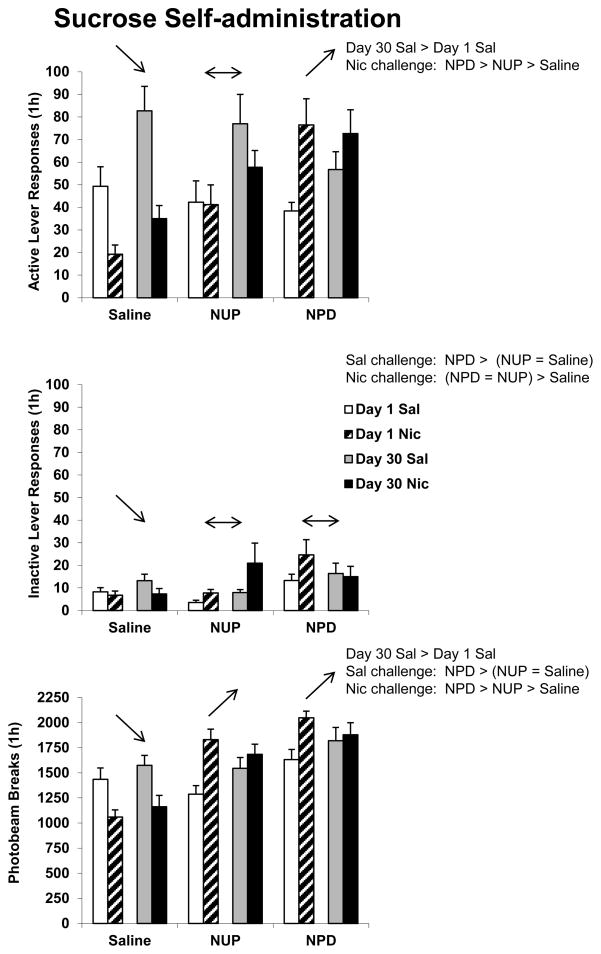
For active lever responding there was a significant effect of DAY F(1,65) = 10.3, p < 0.01. There were also significant TIME and DAY and TIME and DRUG interactions [TIME and DAY F(1,65) = 5.5, p < 0.05; TIME and DRUG F(2,65) = 21.7, p < 0.001]. For inactive lever responding there was a significant effect of DRUG F(2,65) = 3.9, p < 0.05, and a significant TIME and DRUG interaction F(2,65) = 4.0, p < 0.05. There was a significant effect of DRUG for photobeam breaks F(2,65) = 19.8, p < 0.001 and significant TIME and DAY and TIME and DRUG interactions [TIME and DAY F(1,65) = 8.7, p < 0.01; TIME and DRUG F(2,65) = 26.6, p < 0.001]. Active lever responding, inactive lever responding, and photobeam break data are indicated in Figure 4. Overall results of post-hoc tests are indicated on the figure. Briefly, active lever responding was greater following 30 days of forced abstinence, but only in the first (Sal) hour of testing. Acute nicotine (Nic) reduced active lever responding in rats with a history of saline injections, but increased responding only of rats with a history of nicotine injections that preceded sucrose self-administration sessions. Considered as a between-group comparison, rats with a history of nicotine injections preceding sucrose self-administration sessions responded more for the sucrose-paired cue following acute nicotine than rats with a history of nicotine unpaired, and those rats responded more than rats with a history of saline injections. Details of these tests are provided in the caption for Figure 4. As with Figure 3, results of between-group post-hoc comparisons are summarized on Figure 4 as text and results of within-group comparisons as arrows.
Figure 4.
Responding during two consecutive Test sessions where active lever responses produced a tone + light stimulus previously associated with sucrose self-administration. Rats were injected with saline (Sal) 5 min prior to the first 1-h session and nicotine (Nic) 5 min prior to the second 1-h session. Between-group comparisons (text) refer to either a significant effect of length of forced abstinence for all rats when challenged with saline on the Test day, or an effect of drug treatment history (Saline, NUP, or NPD) on the animals’ response to either Sal or Nic. A unidirectional arrow indicates a significant increase or decrease in responding following the acute nicotine challenge for that drug treatment history group (Saline, NUP, or NPD) compared to the response following saline, regardless of day of forced abstinence. A double-headed arrow indicates no significant change in responding. All post-hoc results indicated were statistically significant at p < 0.05. Group n’s were 11–13 animals each. Means ± SEMs are indicated on the figure.
DISCUSSION
Two main findings emerge from this study. First, nicotine 5 min prior to a self-administration Training session increased responding for water or sucrose, and the effect was paralleled by increased inactive lever responding and locomotor activity. Second, nicotine 5 min prior to a Test session enhanced responding reinforced by a sucrose-paired cue, but not a water-paired cue, significantly more in rats with a history of nicotine preceding self-administration access. This effect was similar both in rats 1 and 30 days into forced abstinence from sucrose self-administration.
Training phase
Although rats responded for either water or sucrose, the pattern of sucrose self-administration contrasted sharply with that for water self-administration. Specifically, rats did not appear to acquire water self-administration as would be typically defined. Active lever response rate actually decreased across the 10 days of Training and response rates on the active and inactive levers were similar (Figure 1). In contrast, responding for sucrose increased over training and responding on the active lever was severalfold higher than on the inactive lever (Figure 2). As nicotine preceding water or sucrose self-administration sessions enhanced responding in a non-selective manner (increased number of rewards, lever responses, photobeam breaks), these data could be interpreted as an indication of a general non-specific enhancement of activity. An alternative hypothesis is that nicotine enhanced the incentive motivational properties of the self-administration context in general. This interpretation fits with a conception of nicotine as a drug that not only has reinforcing effects of its own, but can enhance the reinforcing value of other stimuli (Caggiula et al. 2009). Nicotine has been demonstrated to enhance responding for various stimuli including non-food or drug stimuli such as a stimulus light (Palmatier et al. 2006) and alcohol (Lê et al. 2003; Bito-Onon et al. 2011), and to enhance Pavlovian discriminative approach behavior (Olausson, Jentsch & Taylor 2003). In the case of sucrose, nicotine may have further enhanced the already high incentive value of sucrose leading to the high rate of responding for sucrose in the NPD animals. This effect complements previous reports including one where rats implanted with nicotine pellets drank more sucrose than controls (Jias & Ellison 1990) and one where rats were found to respond at higher rates for a combined solution of sucrose and nicotine, as opposed to sucrose alone (Smith & Roberts 1995). The enhancement of incentive salience for environmental cues and primary reinforcers by nicotine could be due to pharmacological enhancement of neural pathways underlying incentive motivation. Nicotine has been shown to affect a presumed substrate of incentive motivation, mesolimbic dopamine (Xi, Spiller & Gardner 2009). Acute nicotine elevates dopamine levels in the nucleus accumbens (Kleijn et al. 2011; Reid, Ho & Berger 1996; Shearman et al. 2008) and in the hippocampus (Shearman et al. 2008).
Interestingly, studies with operant intake of food pellets actually reported decreases in responding following nicotine (Goldberg et al. 1989; Lau, Spear & Falk 1994; Shoaib et al. 1997). These negative findings would alone indicate that if nicotine alters operant behavior due to general rate-altering effects, the effect would be expected to be a decrease in response rate. The inconsistency between these previous findings and the present results could be due to the type of reinforcer (food pellets vs. water, alcohol, or sucrose) or some aspect of the conditioning environment or training parameters. Alternately this inconsistency, at least in the case of sucrose, may be due to anorectic effects of nicotine (Dandekar et al. 2011) overcome in the presence of a highly palatable reinforcer.
Testing phase
There was a forced-abstinence dependent increase in responding for the water or sucrose-paired cue following a saline challenge injection. While “incubation of craving” for sucrose and several abused drugs has been well-characterized, this is the first report we are aware of regarding an incubation of water craving. We have considered incubation to be reflective of enhanced motivational attribution to reinforcement-paired stimuli (Grimm et al. 2011). Given these results with a water-paired cue in rats that were not water deprived at testing (nor during the vast majority of training), it appears that even the relatively low reinforcing efficacy of water (compared to sucrose) leads to incubation of craving and/or that a component of the incubated responding is not explicitly tied to reinforcement, but to some other aspect of learning. This novel finding will be of interest in further evaluation of the generality of the incubation of craving effect.
A history of nicotine injections did not predict the active lever response rates of animals following a saline injection (Sal) in what is essentially an extinction session with access to a cue (tone + light stimulus) that was previously associated with either water or sucrose self-administration (Figures 3 and 4). The lack of effect of nicotine paired with self-administration on subsequent responding with access to the cue also previously associated with nicotine is surprising if nicotine did enhance incentive motivational properties of either the primary (water or sucrose) or secondary (tone + light cue) reinforcers during Training. It would seem that such enhancement would have carried over to the Testing phase as enhanced responding for the cue, now functioning as a conditioned reinforcer. That being stated, our results are consistent with those of Lê et al. (2003) who reported that extinction responding was similar in rats with or without a history of nicotine paired with alcohol self-administration. As noted above, nicotine was found to increase alcohol self-administration in that study.
In contrast to the lack of effect of previous exposure to nicotine on responding for water or sucrose cues in the present study, the second phase of Testing revealed a rather striking effect of nicotine history. A history of nicotine paired with sucrose, but not water, self-administration resulted in a significantly higher rate of responding following the acute nicotine challenge (Nic). This was the case when comparing the sucrose SA NPD group against either the NUP or Saline groups challenged with nicotine OR when comparing all three groups against their own responding following the saline challenge. As the arrows in Figure 4 indicate, NPD rats responded more than the NUP and Saline groups following Nic AND the NPD rats increased responding following Nic vs. Sal while the NUP group responded at a similar rate following either challenge. The Saline group actually responded less following Nic. The results with the NUP group indicate that the enhanced responding following Nic in the NPD group was not due to a pharmacological sensitization due to a history of nicotine exposure. The decreased responding by the Saline (nicotine-naïve) rats following nicotine could be indicative of an acute rate-suppressant effect of nicotine (Clarke & Kumar 1983).
The enhanced responding in the NPD group may be explained by the learning history of these animals, specifically in how nicotine came to predict and/or accompany sucrose self-administration. Nicotine has been shown to potentiate responding for conditioned reinforcement (Olausson, Jentsch & Taylor 2004a). The present study extends these findings to a potentiation of conditioned reinforcement that depends on a history of nicotine paired with primary reinforcement. This effect is reminiscent of a “state-dependence” where memory for an association is only recalled when under the same pharmacologically-induced state as was in place during conditioning (Overton 1964). Our results, where responding for the sucrose cue was no different for rats with or without a history of nicotine, but much greater following a nicotine challenge in rats with a history of nicotine paired with self-administration, could fit with a state-dependence interpretation. However, an alternate explanation would be that in this instance nicotine serves as a discriminative stimulus or “occasion-setter” such that responding on the Test day is most robust only when the complete set of predictive stimuli (nicotine + sucrose cues) are available. Nicotine has been shown to serve as a discriminative stimulus (Palmatier & Bevins 2008). Furthermore, Bevins and colleagues have demonstrated a role for nicotine as a CS+ or CS- itself (Murray & Bevins 2007; Murray et al. 2011), and that the interoceptive stimulus effects of nicotine can even overshadow a light stimulus in gaining control of conditioned responding for sucrose (Murray & Bevins 2011). Clearly, nicotine has a powerful ability to serve as an informative cue. Our findings further indicate that these properties are long-lasting as nicotine retained its ability to potentiate sucrose cue-reactivity for one month after the last exposure of rats to the drug.
The present findings and previous reports of reinforcer-enhancing and information-signal qualities of nicotine fit with the growing consensus that nicotine has more than one stimulus property (e.g. Caggiula et al. 2009). Furthermore, for our rats, the conditioned effects of nicotine were mostly limited to when nicotine was present. These different stimulus effects may be mediated by dissociable neurochemical actions of the drug. As noted above, nicotine would likely potentiate responding for incentive stimuli through mesolimbic dopamine activation (Kleijn et al. 2011; Reid et al. 1996; Shearman et al. 2008). Conditioned contextual effects (e.g. CS+ and CS−) may be due to activation of cholinergic receptors in hippocampal and frontal cortical regions (Kenney & Gould 2008; Pirch, Turco & Rucker 1992; Rosecrans 1989). The effects may combine to give nicotine its truly unique behavioral pharmacological profile. Even more, given the fact that sucrose cue-reactivity was potentiated by nicotine after either 1 or 30 days of forced abstinence from sucrose SA and nicotine exposure, learning-related neuroplastic changes due to nicotine exposure may be responsible for the persistence of nicotine addiction.
A final observation is that the effects of nicotine enhancing behavior in the present study were paralleled by development and expression of locomotor sensitization to nicotine (Clarke & Kumar 1983; Ericson, Norrsjo & Svensson 2010). NPD rats increased daily photobeam breaks across the 10 days of training. Upon testing with nicotine, rats with this history of nicotine paired with sucrose self-administration had more photobeam breaks than rats that received nicotine unpaired with the operant conditioning chamber. It is possible that sensitization of neural circuits mediating this locomotor change account for the operant behavior. For example, Reid et al. (1996) described a context-dependent nicotine locomotor sensitization that was accompanied by a preferential increase in nucleus accumbens dopamine, as compared to rats in a pseudo-conditioned group. Sensitized accumbal dopamine could mediate both elevated locomotor and operant response behaviors. However, this conclusion is tempered by observations in the literature and within the present data set. In the literature it is clear that while locomotor sensitization sometimes accompanies potentiated operant responding (e.g. Olausson, Jentsch & Taylor 2004b), locomotor sensitization does not necessarily account for potentiated operant responding. For example, incubation of craving is not accompanied by locomotor sensitization (Grimm et al. 2006). Furthermore, while locomotor responding was greatest following nicotine challenge on the Test day in groups that had a history of nicotine paired with Training (Figures 3 and 4), within-group correlations between active lever responding and photobeam breaks were not significant and nearly absent (for example the Pearson’s r for the NPD group with a history of sucrose self-administration was r = 0.2, p = 0.4 for the 1 h of responding and r = 0.03, p = 0.9 when examining the first 2 min of responding only, n = 23, data not shown). For these rats, general locomotor activation accompanied the enhancement of responding for a sucrose paired cue by nicotine, but the relationship between these measures was not consistent from animal to animal. Finally, perhaps a more convincing dissociation between locomotor sensitization and cue reactivity in the present study are the findings that acute nicotine increased locomotor activity in NUP and NPD rats with a history of water SA, but did not affect water cue reactivity (Figure 3) AND that acute nicotine selectively enhanced cue reactivity in NPD rats with a history of sucrose SA, but increased locomotor activity in both NUP and NPD groups with a history of sucrose SA (Figure 4).
Summary and conclusions
Nicotine enhanced responding for water or sucrose and then preferentially, and persistently, enhanced responding for a sucrose-paired cue in rats with a history of nicotine paired with sucrose self-administration. Essentially, nicotine enhances ongoing goal-directed behaviors. With sucrose, the memory of this enhancement is enduring over a period of extended forced abstinence. These findings contribute to the emerging picture of nicotine as a drug with complex effects on learning and motivation (Caggiula et al. 2009). This behavioral pharmacological profile indicates a challenge not only for researchers but for individuals attempting to maintain abstinence from both nicotine and excessive food consumption. Specifically, relapse to nicotine could serve as strong reminder of previous associations to foods consumed in the context of smoking. This effect may lead to increased food seeking and subsequently consumption, contributing to the increase in weight seen in smokers attempting to maintain abstinence.
Acknowledgments
This study was supported by National Institute on Drug Abuse/National Institutes of Health grant R15 DA016285-02S1 and the Western Washington University Biomedical Research Activities in Neuroscience Initiative. The authors wish to thank Addison Tice, David Goodman and Jon Koerber for helping with data collection.
Footnotes
Conflict of Interest
All authors declare that they have no conflict of interest.
Authors Contribution
JWG and CR were responsible for the study concept and design. CR, KN, JB, and SC contributed to the acquisition of the animal data. JWG, CR, and KN assisted with data analysis and interpretation of findings. JWG and CR drafted the manuscript. All authors provided critical revisions of the manuscript for important intellectual content. All authors critically reviewed the content and approved the final version for publication.
References
- Bedi G, Preston KL, Epstein DH, Heishman SJ, Marrone GF, Shaham Y, de Wit H. Incubation of cue-induced cigarette craving during abstinence in human smokers. Biol Psychiatry. 2011;69:708–711. doi: 10.1016/j.biopsych.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Palmatier MI, Metschke DM, Bevins RA. Nicotine as a signal for the presence or absence of sucrose reward: a Pavlovian drug appetitive conditioning preparation in rats. Psychopharmacology (Berl) 2004;172:108–117. doi: 10.1007/s00213-003-1621-9. [DOI] [PubMed] [Google Scholar]
- Bito-Onon JJ, Simms JA, Chatterjee S, Holgate J, Bartlett SE. Varenicline, a partial agonist at neuronal nicotinic acetylcholine receptors, reduces nicotine-induced increases in 20% ethanol operant self-administration in Sprague-Dawley rats. Addict Biol. 2011;16:440–449. doi: 10.1111/j.1369-1600.2010.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF. The role of nicotine in smoking: a dual-reinforcement model. Nebr Symp Motiv. 2009;55:91–109. doi: 10.1007/978-0-387-78748-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiolero A, Faeh D, Paccaud F, Cornuz J. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr. 2008;87:801–809. doi: 10.1093/ajcn/87.4.801. [DOI] [PubMed] [Google Scholar]
- Clarke PB, Kumar R. Characterization of the locomotor stimulant action of nicotine in tolerant rats. Br J Pharmacol. 1983;80:587–594. doi: 10.1111/j.1476-5381.1983.tb10733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandekar MP, Nakhate KT, Kokare DM, Subhedar NK. Effect of nicotine on feeding and body weight in rats: involvement of cocaine- and amphetamine-regulated transcript peptide. Behav Brain Res. 2011;219:31–38. doi: 10.1016/j.bbr.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Ericson M, Norrsjo G, Svensson AI. Behavioral sensitization to nicotine in female and male rats. J Neural Transm. 2010;117:1033–1039. doi: 10.1007/s00702-010-0449-9. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Risner ME, Stolerman IP, Reavill C, Garcha HS. Nicotine and some related compounds: effects on schedule-controlled behaviour and discriminative properties in rats. Psychopharmacology (Berl) 1989;97:295–302. doi: 10.1007/BF00439441. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Barnes J, North K, Collins S, Weber R. A general method for evaluating incubation of sucrose craving in rats. J Vis Exp. 2011;57:e3335. doi: 10.3791/3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Buse C, Manaois M, Osincup D, Fyall A, Wells B. Time-dependent dissociation of cocaine dose-response effects on sucrose craving and locomotion. Behav Pharmacol. 2006;17:143–149. doi: 10.1097/01.fbp.0000190686.23103.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW. Animal Models of Craving. In: Olmstead MC, editor. Animal Models of Drug Addiction. Springer-Verlag; New York: 2011. pp. 311–336. [Google Scholar]
- Jias LM, Ellison G. Chronic nicotine induces a specific appetite for sucrose in rats. Pharmacol Biochem Behav. 1990;35:489–491. doi: 10.1016/0091-3057(90)90192-k. [DOI] [PubMed] [Google Scholar]
- Kenney JW, Gould TJ. Modulation of hippocampus-dependent learning and synaptic plasticity by nicotine. Mol Neurobiol. 2008;38:101–121. doi: 10.1007/s12035-008-8037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleijn J, Folgering JH, van der Hart MC, Rollema H, Cremers TI, Westerink BH. Direct effect of nicotine on mesolimbic dopamine release in rat nucleus accumbens shell. Neurosci Lett. 2011;493:55–58. doi: 10.1016/j.neulet.2011.02.035. [DOI] [PubMed] [Google Scholar]
- Lau CE, Spear DJ, Falk JL. Acute and chronic nicotine effects on multiple-schedule behavior: oral and SC routes. Pharmacol Biochem Behav. 1994;48:209–215. doi: 10.1016/0091-3057(94)90518-5. [DOI] [PubMed] [Google Scholar]
- Lê AD, Wang A, Harding S, Juzytsch W, Shaham Y. Nicotine increases alcohol self-administration and reinstates alcohol seeking in rats. Psychopharmacology (Berl) 2003;168:216–221. doi: 10.1007/s00213-002-1330-9. [DOI] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- Murray JE, Bevins RA. Behavioral and neuropharmacological characterization of nicotine as a conditional stimulus. Eur J Pharmacol. 2007;561:91–104. doi: 10.1016/j.ejphar.2007.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, Bevins RA. Excitatory conditioning to the interoceptive nicotine stimulus blocks subsequent conditioning to an exteroceptive light stimulus. Behav Brain Res. 2011;221:314–319. doi: 10.1016/j.bbr.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, Penrod RD, Bevins RA. Nicotine-evoked conditioned responding is dependent on concentration of sucrose unconditioned stimulus. Behav Processes. 2009;81:136–139. doi: 10.1016/j.beproc.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, Walker AW, Li C, Wells NR, Penrod RD, Bevins RA. Nicotine trained as a negative feature passes the retardation-of-acquisition and summation tests of a conditioned inhibitor. Learn Mem. 2011;18:452–458. doi: 10.1101/lm.2177411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Repeated nicotine exposure enhances reward-related learning in the rat. Neuropsychopharmacology. 2003;28:1264–1271. doi: 10.1038/sj.npp.1300173. [DOI] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Nicotine enhances responding with conditioned reinforcement. Psychopharmacology (Berl) 2004a;171:173–178. doi: 10.1007/s00213-003-1575-y. [DOI] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Repeated nicotine exposure enhances responding with conditioned reinforcement. Psychopharmacology (Berl) 2004b;173:98–104. doi: 10.1007/s00213-003-1702-9. [DOI] [PubMed] [Google Scholar]
- Overton DA. State-Dependent or “Dissociated” Learning Produced with Pentobarbital. J Comp Physiol Psychol. 1964;57:3–12. doi: 10.1037/h0048023. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Bevins RA. Occasion setting by drug states: Functional equivalence following similar training history. Behav Brain Res. 2008;195:260–270. doi: 10.1016/j.bbr.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, Liu X, Booth S, Gharib M, Craven L, Sved AF. Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology (Berl) 2006;184:391–400. doi: 10.1007/s00213-005-0183-4. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Weight gain following smoking cessation. J Consult Clin Psychol. 1993;61:768–777. doi: 10.1037//0022-006x.61.5.768. [DOI] [PubMed] [Google Scholar]
- Pirch JH, Turco K, Rucker HK. A role for acetylcholine in conditioning-related responses of rat frontal cortex neurons: microiontophoretic evidence. Brain Res. 1992;586:19–26. doi: 10.1016/0006-8993(92)91366-m. [DOI] [PubMed] [Google Scholar]
- Pisinger C, Jorgensen T. Waist circumference and weight following smoking cessation in a general population: the Inter99 study. Prev Med. 2007;44:290–295. doi: 10.1016/j.ypmed.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Reid MS, Ho LB, Berger SP. Effects of environmental conditioning on the development of nicotine sensitization: behavioral and neurochemical analysis. Psychopharmacology (Berl) 1996;126:301–310. doi: 10.1007/BF02247381. [DOI] [PubMed] [Google Scholar]
- Rosecrans JA. Nicotine as a discriminative stimulus: a neurobehavioral approach to studying central cholinergic mechanisms. J Subst Abuse. 1989;1:287–300. [PubMed] [Google Scholar]
- Shearman E, Fallon S, Sershen H, Lajtha A. Nicotine-induced monoamine neurotransmitter changes in the brain of young rats. Brain Res Bull. 2008;76:626–639. doi: 10.1016/j.brainresbull.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Thorndike E, Schindler CW, Goldberg SR. Discriminative stimulus effects of nicotine and chronic tolerance. Pharmacol Biochem Behav. 1997;56:167–173. doi: 10.1016/s0091-3057(96)00174-8. [DOI] [PubMed] [Google Scholar]
- Smith A, Roberts DC. Oral self-administration of sweetened nicotine solutions by rats. Psychopharmacology (Berl) 1995;120:341–346. doi: 10.1007/BF02311182. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Spiller K, Gardner EL. Mechanism-based medication development for the treatment of nicotine dependence. Acta Pharmacol Sin. 2009;30:723–739. doi: 10.1038/aps.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]