Abstract
T cell development requires periodic importation of hematopoietic progenitors into the thymus. The receptor-ligand pair P-selectin and P-selectin glycoprotein ligand 1 (PSGL-1) are critically involved in this process. In this study we examined the expression of functional PSGL-1 on BM hematopoietic progenitors. We demonstrate that functional PSGL-1 is expressed at low levels on hematopoietic stem cells, but upregulated on the cell surface of progenitors that bear other homing molecules known to be important for thymic settling. We found that progenitors able to home to the thymus expressed high levels of PSGL-1 transcripts compared to hematopoietic stem cells. We further demonstrate that hematopoietic progenitors lacking Fucosyltransferase 4 and 7 do not express functional PSGL-1, and do not home efficiently to the thymus. These studies provide insight into the developmentally regulated expression of a critical determinant involved in progenitor homing to the thymus.
Keywords: Hematopoiesis, T cells development, Thymus settling, PSGL-1
Introduction
T cells develop in the thymus; however, the thymus contains no self–renewing progenitors. T lymphopoiesis depends on importation of rare bone marrow (BM) precursor cells, which express chemokine receptors required for efficient thymic homing. These thymus homing progenitors are developmentally downstream of hematopoietic stem cells (HSC) (1–5). HSC are contained within a BM population with the cell surface phenotype of Lineage-marker−Kit+Sca1+(LSK). Expression of the cytokine receptor Flt3 divides LSK cells further into populations enriched for self-renewing HSC (Flt3−LSK) (6), non-renewing multipotent progenitors (MPP) (LSKFlt3lo) (2), and lymphoid-primed multipotent progenitors (LMPP) (LSK Flt3hi). Here, LMPP that express CCR9 are referred to as CCR9+ lymphoid progenitors (CCR9+ LP); these cells co-express CCR7 and are likely to represent efficient thymic homing progenitors (7–10). Further down stream of LMPP, common lymphoid progenitors (CLP) possessing the phenotype Lin−Sca1loKitloFlt3hiIL7Rα+ have been shown to express high levels of CCR7 and a subset also expresses CCR9 (8, 10). All of these progenitor types have the potential to generate T cells (8). It has recently been demonstrated that CLP are heterogeneous, and the Ly6D− subset which expresses low level of RAG 1 has been suggested to contribute to T cell generation more efficiently compared to downstream Ly6D+ RAG1high cells (11, 12).
Recruitment of BM-derived precursors to the thymus has been suggested to depend on the multi-step engagement of adhesion molecules and chemo-attractants (13). Progenitors egress the BM, circulate in the bloodstream, and finally a select subset of these cells settles in the thymus for T cell development. Although many progenitors possess T cell potential, only a select subset with T lineage potential can physiologically settle in the thymus (8). The most efficient thymic settling progenitors (TSPs) appear to be contained within a small subset of LMPP and CLP progenitors but not HSC and MPP (8, 14). In the thymus TSPs generate early thymic progenitors or ETP, the earliest described and most efficient intrathymic T progenitors. ETPs give rise to DN2 and DN3 cells. DN3 cells differentiate into DP cells, which subsequently generate CD4, or CD8 single-positive SP cells and then exit the thymus to the periphery.
The ability of specific hematopoietic progenitors with T cell potential to settle the thymus reflects their expression of required homing molecules. Recent work has suggested that the chemokine receptors CCR7 and CCR9 each independently support the migration of progenitors into the thymus (8, 10, 15, 16). Parabiosis and competitive thymus reconstitution assays further indicate that P-selectin and its ligand, P-selectin glycoprotein ligand 1 (PSGL-1) also have a role in recruiting progenitors to the thymus (17, 18). Selectins and their oligosaccharide ligands control lymphocyte trafficking to lymph nodes in normal circumstances (19). P-selectin is an adhesion molecule expressed on the surface of activated endothelial cells (20, 21). P-selectin glycoprotein ligand 1 (PSGL-1) is a transmembrane protein that is a ligand for all 3 selectin molecules (19). PSGL-1 is a 220-kDa disulfide-linked homodimeric sialomucin expressed on the surface of most leukocytes. Post-translational modi cations of PSGL-1 are required for selectin binding. Functional PSGL-1 is a sialyated, fucosylated, core 2 O-glycan clustered with tyrosine sulfate (22). Glycans that contribute to PSGL-1 activity are constructed by aglycosylation reaction in which terminal steps are catalyzed by α (1,3) fucosyltransferase. However, whether expression of functional PSGL-1 is developmentally regulated on hematopoietic progenitors in parallel to the established regulation of CCR7 and CCR9 is unknown.
In this study, we demonstrate that functional PSGL-1 is expressed on hematopoietic stem cells at very low levels but up-regulated on downstream progenitors that are thought to home to and settle the thymus. We further show that glycosyltransferase enzymes including fucosyltransferase 7 specially regulate the formation of functional PSGL-1, thus contributing to T lymphopoiesis.
Materials and Methods
Mice
C57BL/6 (CD45.2) and B6.Ly5SJL (CD45.1) mice were purchased from the National Cancer Institute (NCI) animal facility. PSGL-1 knockout mice were purchased from The Jackson Laboratory. Rag1-Cre mice (23) were a gift of Dr Terence H. Rabbitts (University of Cambridge). Fuc-TVII/Fuc-TIV DKO (24, 25) mice were obtained from Dr. John Harris and Dr. Phillip Scott (University of Pennsylvania). Mice were used when 6–10 weeks of age. Live animal experiments were performed according to approved protocols of the Office of Regulatory Affairs of the University of Pennsylvania in accordance with NIH guidelines.
Cell preparations, flow cytometry and cell sorting
BM isolated from femurs and tibias was treated with ACK lysis buffer (Lonza) to remove RBC, and single-cell suspensions were made. Thymocytes were prepared as single-cell suspension. For thymic endothelial cell preparation, the thymus was treated with 40ng/ml liberase (Roche) and 200ng/ml DNAse (Roche) in HBSS media at 37°C for 20 minutes. mAbs in the Lineage (Lin) cocktail included anti-B220 (RA3-6B2), anti-CD19 (1D3), anti-CD11b (M1/70), anti-Gr-1 (8C5), anti-CD11c (HL3), anti-NK1.1 (PK136), anti–Ter-119 (Ter-119), anti-CD3 (2C11), anti-CD8α (53.6–7), anti-CD8β (53–5.8), anti-TCRβ (H57), and anti-TCRγδ (GL-3). Additional Abs used included anti-CD45.2 (104), anti-CD45.1 (A20), anti-Kit (2B8), anti-Sca1 (D7), anti-CD4 (GK1.5), anti-Flt3 (A2F10), anti-IL7Rα (A7R34), anti -CD25 (PC61.5), anti-Thy1.2 (53–2.1), anti PSGL-1 (2PH1), anti-Ly6D (49-H4), anti-CD150 (9D1), anti-CD34 (RAM34), anti-CCR9 (CW-1.2), anti CD62P (Psel KO2.3) and anti-CD31 (390). For P-selectin binding, a P-selectin-IgG Fc fusion protein (eBioscience) was used, followed by allophycocyanin-conjugated anti-Fc (Jackson ImmunoResearch). mAbs were directly conjugated to FITC, PE, PEcy5.5, PEcy7, APC, APCcy5.5 (or Alexa 700), APCcy7 (or APCeFluor780), Pacific Blue, or biotin and were purchased either eBioscience, BioLegend, or BD Pharmingen. Biotinylated Abs were revealed with Streptavidin PE-TexasRed (BD Pharmingen). Flow cytometric (FACS) analysis was performed on a CANTO or LSRII (BD Biosciences). Dead cells were excluded with 4,6 diamidino-2-phenylindole (DAPI). Doublets were excluded through forward scatter–height by forward scatter–width and side– scatter height by side scatter–width parameters. Data were analyzed using FlowJo version 4.6.2 (TreeStar). For cell sorting, BM was first enriched for progenitors, then stained and sorted on a FACSAria II (BD Biosciences). Aliquots of sorted cells were reanalyzed to ensure purity, which was usually greater than 90%.
Competitive adoptive transfer
To generate mixed BM chimeras, host wild-type (WT) mice (expressing CD45.1) were lethally irradiated (9.5 Gy) and subsequently injected with T cell–depleted host-type BM mixed with either WT, PSGL-1 heterozygous, PSGL-1 KO or Fuc-TVII/Fuc-TIV DKO BM (all expressing CD45.2) at a 1:2 ratio. T cells were depleted from BM by incubation with anti-CD4 and anti-CD8 Abs followed by removal of Ab-bound cells with magnetic beads (QIAGEN).
Intravenous transfers
For intravenous transfers into unirradiated mice, 1 × 105 sorted HSC, LMPP, Ly6D− CLP and Ly6D+ CLP were injected retro-orbitally. To prevent rejection, mice were given 0.1 mg of anti-CD4 (GK1.5) the day before BM transfer and every 4 days thereafter (8, 26). Previous work by our laboratory has shown that this treatment does not affect BM or thymic engraftment by donor cells (8, 10).
Real-time PCR
RNA was prepared from indicated sorted populations using the RNeasy kit (QIAGEN). cDNA was generated using the Superscript II kit (Invitrogen). Real-time PCR was performed using TaqMan Universal PCR Master Mix (Applied Biosystems) and primer/probe mixtures or Syber green PCR Master Mix (Applied Biosystems) for genes and GAPDH (Applied Biosystems) and analyzed on an ABI Step One Plus Real-Time PCR System (Applied Biosystems). Relative expression levels were normalized using GAPDH transcript levels and calculated using the 2−ΔΔCT method.
TaqMan probes used were as follows: GAPDH (Mm99999915_g1), CCR7 (Mm00432608_m1), CCR9 (Mm02620030_s1), PSGL-1 (Mm01204601_m1), Fut4 (Mm00487448_s1), C2GnT1 (Mm02010556_s1), β4galT1 (Mm00480752_m1), St3gal4 (Mm00501503_m1).
The primers were used for St3gal6 were Forward 5′-GCCCTTTCAAAACTGCAGAG -3′ and Reverse 5′-TCCCAACTTCCTCTTCATGG-3′; and for Fut7 Forward 5′-CAGTGCTGATGCTGTGGTCT-3′ and Reverse 5′-GCGACCGTAGGGTACAAAGA-3′.
OP9 and OP9-DL4 cell culture
OP9-GFP (OP9) and OP9-DL4 cells were a gift from Dr. Juan Carlos Zúñiga-Pflücker, (University of Toronto) and were used as described (27, 28). Freshly sorted LMPP either from WT or Fuc-TVII/Fuc-TIV DKO were placed onto 12 well plates containing OP9 and OP9-DL4 stromal layer in MEM α (Invitrogen), supplemented with 20% FCS, IL-7 (1ng/ml) and Flt3-L (5ng/ml). Stromal cells were plated 2 days before initiation of culture at a concentration of 20,000 cells/ml in 24-well plates for bulk cultures.
Statistical analysis
Values are expressed as mean ± S.E.M. P values were calculated using Microsoft Excel by Student t test.
Results
PSGL-1 in thymic homing
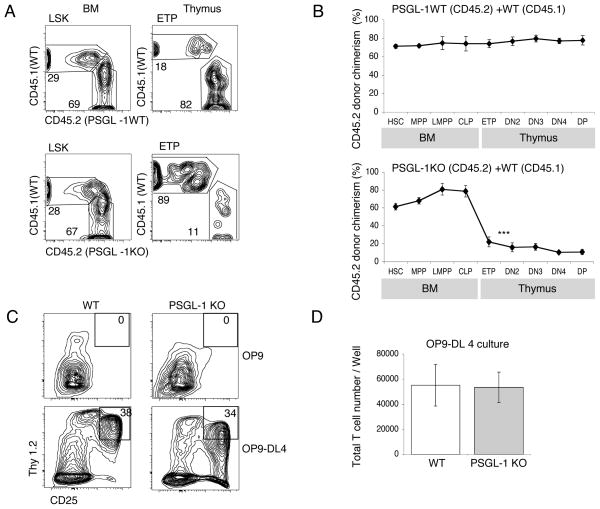
Previous work has shown the importance of PSGL-1 in T cell development (17). We wished to determine the stage at which PSGL-1 is required in T cell development. We generated competitive BM chimeras by intravenously transferring both PSGL-1 KO BM (CD45.2) and WT BM (CD45.1) at 2:1 ratio into lethally irradiated WT hosts. As a control, we injected mixtures of PSGL-1 WT BM (CD45.2) and WT BM (CD45.1) to reconstitute irradiated mice. After hematopoietic reconstitution at 10 weeks post-transplant, the BM and thymi of these mice were analyzed by flow cytometry to determine the donor-derived chimerism at successive stages of T cell development (Fig. 1, A and B). Generation of ETP was almost comparable in control chimeras to the level expected based on the level of reconstitution by BM LSK progenitors (Fig. 1B, top panel). PSGL-1 KO HSC were able to engraft in the BM and differentiate into downstream MPP, LMPP and CLP efficiently (Fig. 1B, bottom panel). However, the donor chimerism of early thymic progenitors derived from the PSGL-1 KO BM was significantly lower than the chimerism in the BM LSK (Lin−Kit+Sca1+) progenitors (Fig. 1B, bottom panel). These data confirm that the absence of PSGL-1 conferred a disadvantage in the generation of ETP in this competitive situation.
Figure 1. PSGL-1 is functionally important for homing of the BM progenitors.
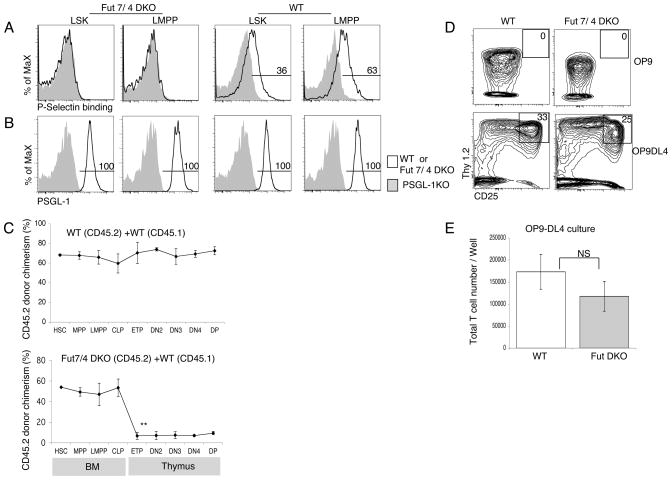
(A) Mixed BM chimeras were generated using CD45.2 PSGL-1 WT BM and CD45.1 WT BM mixtures as controls (top panels), or CD45.2 PSGL-1 KO BM and CD45.1 WT BM mixtures (bottom panels). Chimeras were analyzed by flow cytometry after 10 weeks using antibodies to CD45.1 and CD45.2 to determine donor chimerism. Representative FACS profiles of indicated progenitors in the BM and Thymus are shown. (B) Data are mean CD45.2 donor chimerism ± S.E.M. for each indicated population in mixed BM chimeras described in (A). ***P<0.001 for the CD45.2 donor chimerism of the indicated population compared with HSC CD45.2 donor chimerism. Numbers are from 5 mice per group, and are representative of 3 independent experiments that gave similar results. (C) Sorted LMPP from WT or PSGL-1 KO mice were cultured on OP9 (top panel) or OP9-DL4 (bottom panel) stromal layers. Both cells were cultured in triplicate wells. After 2 weeks, cultured cells were analyzed by flow cytometry for expression of CD25 and Thy1.2. Representative FACS plots of cells gated for CD45 expression are shown. (D) Graph show the total numbers of cells obtained from the cultures described in panel C. Data are mean ± S.E.M. of triplicate wells.
To determine whether PSGL-1 might be required for efficient responses to T-inductive Notch signaling rather than for thymic homing, sorted LMPP from PSGL-1 KO BM were cultured in vitro on OP9-DL4 stromal cells, which expresses the Notch1 ligand delta-like 4 (DL4) that supports T cell development (27). After 2 weeks of culture PSGL-1 KO LMPP and WT control LMPP generated similar numbers of progeny expressing T lineage markers CD25 and Thy1 (Fig. 1, C and D). These data suggest that the lower number of ETPs in irradiation chimeras made with PSGL-1 KO BM cells was due to impaired thymic settling rather than defective intrathymic development, consistent with previous reports (17).
Expression of functional PSGL-1 is developmentally regulated in BM progenitors
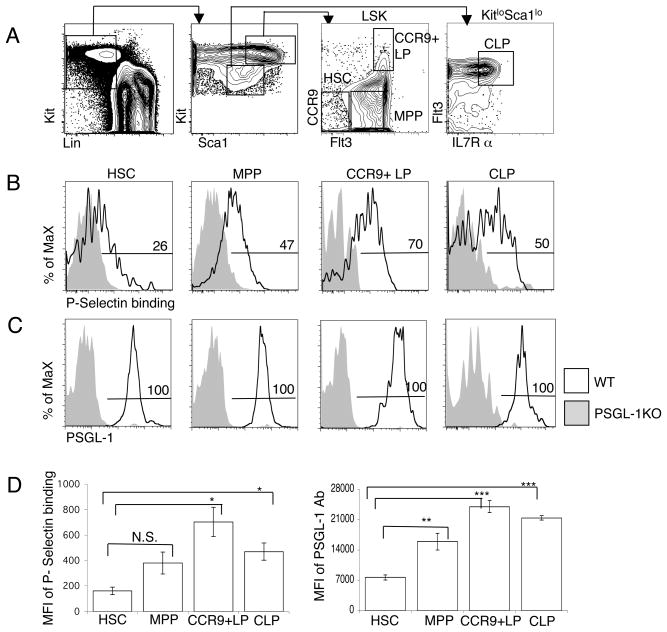
PSGL-1 is expressed by all subsets of leukocytes (19). The binding of P selectin chimera immunoglobulin (IgG) Fc fusion protein was used to measure functional PSGL-1 levels on the cell surface. BM progenitor populations were gated as indicated in Fig. 2A. Flow cytometric analysis results revealed that expression of functional PSGL-1 was increased in CCR9+LP compare to HSC populations (Fig. 2B). We found that expression of functional PSGL-1 is low in HSC, gradually increases in MPP, and is highest in CCR9+LP and CLP. Functional PSGL-1 expression appeared bimodal in CLP consistent with earlier reports of heterogeneity in this population (Fig. 2B) (11, 12) Progenitors previously suggested to settle the thymus, CCR9+LP and CLP, were those with the highest level of functional PSGL-1 on their surface. Interestingly, differences were also detected using anti PSGL-1 Ab, indicating that there may be developmental regulation of PSGL-1 itself (Fig. 2C). Calculation of the mean fluorescence intensity (MFI) for each population showed that MFI of both functional PSGL-1 (Fig. 2D, left panel) and PSGL-1 (Fig. 2D, right panel) was significantly increased in CCR9+LP and CLP, putative thymic settling progenitors, as compared with HSC (Fig. 2D). These data indicate that populations thought to settle the thymus express increased levels of functional PSGL-1.
Figure 2. PSGL-1 expression is developmentally regulated on BM progenitors.
(A) Gating strategy to identify HSC, MPP, CCR9+ lymphoid progenitor and CLP. (B) BM from wild type (WT) and PSGL-1 KO mice was analyzed by flow cytometry for P-selectin binding and (C) PSGL-1 on the indicated populations. Numbers represent the percentage of WT cells in the indicated gate. The gray histogram is from a PSGL-1 KO control sample. (D) The mean fluorescence intensity (MFI) of P-selectin fusion protein and PSGL-1 Ab on BM progenitor populations was determined by FACS which was obtained from the gates applied in (B) and (C). Data are shown as mean ± S.E.M. and are averages of three independent experiments performed on different days. The two–tailed student’s t test indicated a significant increase of function PSGL-1 and PSGL-1 protein on LMPP and CLP.
The expression of functional PSGL-1 separates common lymphoid progenitor populations
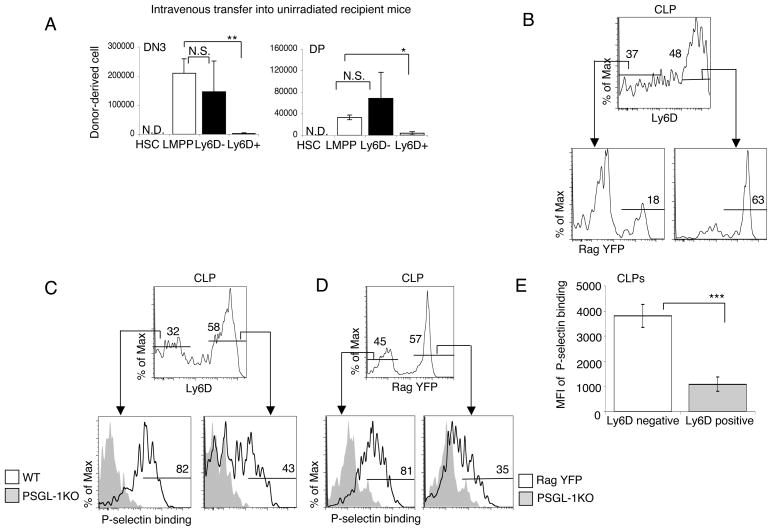
Downstream of LMPP, common lymphoid progenitors (CLP) also settle the thymus and possess T cell potentials (8, 10, 29–31). Recently it has been demonstrated that CLP in BM are a heterogeneous population, containing multiple subsets with different lineage potentials (11, 12). Previous work has assessed the in vivo T potential of CLP subsets in irradiated recipients (12). However, hematopoiesis following irradiation is far from physiologic, as irradiation has many secondary effects (8, 32, 33). To investigate the potential of CLP to undergo T cell development in physiological conditions, we assessed T cell development in unirradiated normal adult recipient mice. For these experiments, HSC, LMPP, Ly6D− CLP and Ly6D+CLP cells were sorted from the BM of B6 donors and 1×105 cells from each population were injected intravenously into each unirradiated B6.Ly5SJL congenic recipient. Two weeks after intravenous transfer of purified progenitors into mice, recipient thymi were analyzed for donor–derived DN3 and DP thymocytes (Fig. 3A). In all experiments HSC failed to produce any DN3 or DP thymocytes at this time point, as expected, consistent with earlier work (8). Both LMPP and Ly6D−CLP generated donor-derived thymocytes. The number of donor derived DN3 and DP from Ly6D+CLP progenitor was significantly lower than that generated by LMPP and Ly6D−CLP (Fig. 3A). These results established that the Ly6D+CLP subset is an inefficient T cell progenitor in the unirradiated physiological thymus environment.
Figure 3. Expression of functional PSGL-1 in Common Lymphoid Progenitor.
(A) Total number of donor-derived DN3 and DP thymocytes at 2 weeks following intravenous injection of sorted HSC, LMPP, Ly6D−CLP and Ly6D+CLP into unirradiated conjenic recipient mice. Shown are the means of three mice per group ± S.E.M. (B) BM from Rag1-Cre Rosa26-YFP reporter mice was analyzed by flow cytometry for Ly6D expression in CLP (Lin−Sca1loKitloFlt3hiIL7Rα+). (C) BM from wild type (WT) and (D) Rag1-Cre Rosa26-YFP reporter mice was analyzed for Ly6D and Rag expression in CLP subsets (Top panel). P-selectin binding was used to determine functional PSGL-1 on the indicated populations (Bottom panel). Numbers represent the percentage of WT cells in the indicated gate (Bottom panel). The gray histogram in the bottom panel is from the PSGL-1 KO control sample. (E) The mean fluorescence intensity (MFI) of P-selectin fusion protein binding on CLP sub-populations was determined by FACS which was obtained from the gates applied in Fig 3C, left panel. The two–tailed student’s t test indicated a significant decrease of function PSGL-1 protein on Ly6D+ CLP.
Because the recombinase activating gene 1 (Rag-1) is highly expressed in the CLP population (34), we next examined Ly6d expression on CLP cells in Rag-1/YFP mice, which were created by crossing Rag1/Cre mice with mice in which YFP was knocked into the Rosa26 locus. This reporter allows for a more precise analysis of CLP subpopulations (35). Flow cytometric analysis of BM from Rag1-YFP mice showed that CLP expressing high levels of Ly6D also expressed high levels of Rag1-YFP, whereas many fewer Ly6D−CLP expressed YFP (Fig. 3B). About two-thirds of CLP in these experiments were Ly6D+, and of these most expressed Rag1-YFP as expected (11). We sought to analyze the expression of functional PSGL-1 in CLP subsets in the Rag1 reporter mouse. The expression of functional PSGL-1 was decreased by about half in Ly6D+ Rag1-YFP marked cells, compared to Ly6D− Rag1 low cells (Fig. 3, C and D). The mean fluorescence intensity (MFI) seen with P-selectin fusion protein binding by flow cytometry was significantly decreased as cells progressed from Ly6D− CLP to Ly6D+ CLP (Fig. 3E). These results indicate that functional PSGL-1 expression is downregulated within CLP co-ordinately with the loss of efficient T cell potential.
Requirement of PSGL-1 in thymus settling
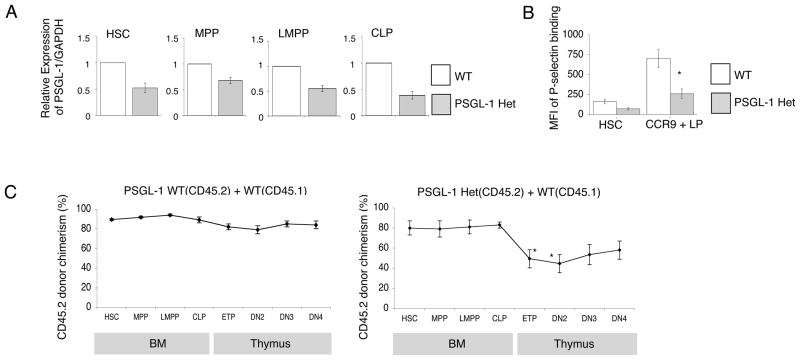
Increases in expression of functional PSGL-1 from HSC to CCR9+ LP were of the order of 4-fold. Reduction in surface expression as progenitors progressed to Ly6D+ CLP stages were similarly modest, of the order of 3–4-fold. To investigate the biological importance of modest alterations in expression of functional PSGL-1, we examined PSGL-1 heterozygous mice. We first confirmed reduced expression of PSGL-1 transcripts in bone marrow subsets from PSGL-1 heterozygous mice. Quantitative RT-PCR confirmed that PSGL-1 expression was reduced compared to the wild type in all BM progenitors examined (Fig. 4A). The mean fluorescence intensity (MFI) of binding by P-selectin fusion protein on PSGL-1 heterozygous CCR9+LP was significantly decreased from wild type CCR9+LP cells (Fig. 4B) as determined by flow cytometry. To determine if a two-fold reduction could be functionally relevant we generated competitive BM chimeras by intravenously transferring mixtures of PSGL-1 Het BM (CD45.2) and WT BM (CD45.1) into lethally irradiated WT hosts. As a control, we injected mixtures of PSGL-1 WT BM (CD45.2) and WT BM (CD45.1) to reconstitute irradiated mice. After hematopoietic reconstitution at 10 weeks post-transplant, the BM and thymi of these mice were analyzed by flow cytometry to determine the donor-derived chimerism at successive stages of T cell development (Fig. 4C). Generation of ETP in control chimeras was at the level expected based on the level of reconstitution in BM (Fig. 4C, left panel). PSGL-1 Het HSC were able to engraft in the BM and differentiate into downstream MPP, LMPP and CLP efficiently (Fig. 4C, right panel). However, the donor chimerism of early thymic progenitors derived from the PSGL-1 Het BM was significantly lower than the chimerism in the BM LSK (Lin−Kit+Sca1+) progenitors (Fig. 4C, right panel). These data indicate that a 50% reduction of PSGL-1 conferred a detectable disadvantage in the generation of ETP in this competitive situation, and suggest developmentally regulated differences in expression of functional PSGL-1 are functionally relevant.
Figure 4. Requirement for PSGL-1 in thymus settling.
(A) Quantitative PCR analysis of total cellular RNA from indicated cell fractions purified from Gr-1, CD19, Mac-1 depleted BM of PSGL-1 heterozygous mice. We identified HSC as LSKFlt3− cells among LSK BM progenitors. MPP were LSKFlt3lo, LMPP were LSKFlt3hi, CLP were Lin−Sca1loKitloFlt3hiIL7Rα+ cells in BM. Transcript levels of PSGL-1 was normalized to GAPDH mRNA levels and are indicated in arbitrary units. Data are mean and ± S.E.M. of 3 experiments. (B) BM from wild type (WT), PSGL-1 Het and PSGL-1 KO mice was analyzed by flow cytometry for P-selectin binding on the indicated populations. The gray solid histogram is from a PSGL-1 Het control sample. The mean fluorescence intensity (MFI) of P-selectin fusion protein on BM population was determined by FACS which was obtained from the open histogram in WT type and PSGL-1 Het mice. Data are shown as mean ± S.E.M. and are representative of three experiments. *P<0.05. (C) Mixed BM chimeras were generated using CD45.2 PSGL-1 WT BM and CD45.1 WT BM mixtures as controls (left panel), CD45.2 PSGL-1 Het BM and CD45.1 WT BM mixtures (right panel). Chimeras were analyzed by flow cytometry after 10 weeks using antibodies to CD45.1 and CD45.2 to determine donor chimerism. Data are mean CD45.2 donor chimerism ± S.E.M. for each indicated population in mixed BM chimeras. Numbers are from 5 mice per group.
Expression of PSGL-1 and glycosyltransferase genes
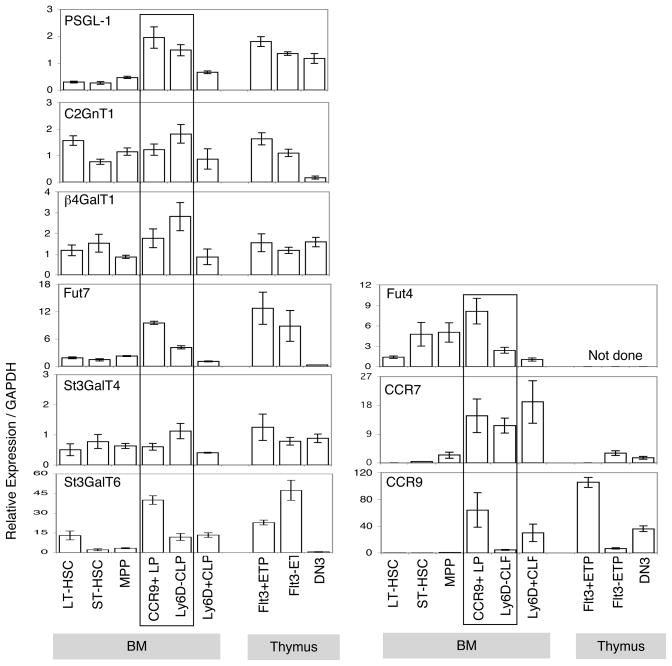
We next investigated the molecular basis of dynamic regulation of functional PSGL-1 expression on BM progenitors. It is established that glycosyltransferases regulate several key enzymatic steps for biosynthesis of functional PSGL-1. Important glycosyltransferaseses for this process include: core 2 β1,6-N-acetylglucosaminyltransferases (C2GnT1) (36); α1,3 fucosyltransferase (FucT-7) (25);β1,4-Galactosyltransferase (β1,4–GalT1) (37, 38); and α2,3 sialyltransferase (ST3GalT4) and (ST3GalT6) (39, 40). We examined the expression of glycosyltransferase enzymes in BM and early thymic progenitors. Quantitative RT-PCR confirmed that PSGL-1 and the glycosyltransferase enzyme fucosyltransferase 7 (Fut7) were expressed at higher levels in CCR9+LP cells and Ly6D− CLP (Fig. 5), in agreement with their elevated surface expression of functional PSGL-1 (Fig. 5). In contrast, reduced expression of both of these genes in Ly6D+CLP cells (Fig. 5) suggests that both PSGL-1 and Fut7 influence functional PSGL-1 levels in lymphoid progenitor cells, since functional PSGL-1 declines in the Ly6D+ CLP population. The glycosyltransferase enzyme sialyltransferase ST3GalT6 is highly expressed in CCR9+LP cells; and sialyltransferase ST3GalT4, β1,4-Galactosyltransferase β1,4-GalT1, fucosyltransferase 4 Fut4 and core 2 β1,6-N-acetylglucosaminyltransferases C2GnT1 are expressed in CCR9+LP cells and also Ly6D−CLP lymphoid progenitor cells. We also examined expression of CCR7 and CCR9; consistent with previous reports we found strong expression of CCR7 in CCR9+LP and CLP subsets (Fig. 5). Interestingly, CCR9 was highly expressed in CCR9+ LP, as expected, but also in Ly6D+ CLP (Fig. 5).
Figure 5. Glycosyltransferase enzyme gene expression profile in BM and early thymic progenitors.
Quantitative PCR analysis of total cellular RNA from indicated cell fractions purified from Gr-1, CD19, Mac-1 depleted BM and CD4, CD8 depleted thymus of C57BL/6 WT mice. We identified LT-HSC as CD150+CD34− and ST-HSC as CD150−CD34+ cells among LSK BM progenitors. MPP were LSKFlt3lo, CCR9+LP were LSKFlt3hiCCR9hi, Ly6D−CLP were Lin−Sca1loKitloFlt3hiIL7Rα+Ly6D− and Ly6D+CLP were Lin−Sca1loKitloFlt3hiIL7Rα+ Ly6D+ cells in BM. To isolate thymic progenitors, we identified Flt3+ETP as Lin−Kit+CD25−Flt3+, Flt3−ETP as Lin−Kit+CD25−Flt3−, and DN3 as Lin−KitloCD25+ cells in thymus. Transcript levels of indicated genes were normalized to GAPDH mRNA levels and are indicated in arbitrary units. Means and standard errors of the results obtained from 3 independent measurements are shown.
Fucosyltransferase enzyme 7(Fut7) regulates thymus settling through formation of functional PSGL-1
PSGL-1 becomes functional (i.e. able to bind P-selectin) only after specific α1,3 fucosylation, which is accomplished by an α1,3 fucosyltransferase (Fuc-T), Fuc-TVII, with Fuc-TIV playing a supporting role (25, 41). We analyzed progenitor populations in the BM of Fuc-TVII/Fuc-TIV double-deficient mice and found that functional PSGL-1 expression was absent on LSK and LMPP progenitor populations (Fig. 6A, left panels), whereas PSGL-1 protein continues to be expressed on the surface of the indicated populations as expected (Fig. 6B, left panels). The numbers of hematopoietic precursors were comparable in BM from WT and Fuc-TVII/Fuc-TIV DKO mice (Supplemental Fig. 1A). The absence of functional PSGL-1 on progenitor cells from Fuc-TVII/Fuc-TIV double deficient mice indicates that expression of functional P-selectin on hematopoietic progenitors is dependent on α 1,3 fucosylation catalyzed by fucosyltransferase enzymes Fut7 and/or Fut4 as has been previously described for neutrophils (41).
Figure 6.
Fucosyltransferase enzyme play role in thymus settling process. (A) BM from Fuc-TVII/Fuc-TIV DKO (left panels) and wild-type (WT) (right panels) mice was analyzed by flow cytometry for Functional PSGL-1. (B) BM from Fuc-TVII/Fuc-TIV DKO (left panels) and wild-type (WT) (right panels) mice was analyzed by flow cytometry for surface PSGL-1 protein expression using mAb to PSGL-1. Numbers represent the percentage of WT cells in the indicated gate. (C) Mixed BM chimeras were generated using CD45.2 WT BM and CD45.1 WT BM mixtures as controls (top panel), CD45.2 Fuc-TVII/Fuc-TIV DKO BM and CD45.1 WT BM mixtures (bottom panel). Chimeras were analyzed by flow cytometry after 10 weeks using antibodies to CD45.1 and CD45.2 to determine chimerism. The mean CD45.2 donor chimerism ± S.E.M. for each indicated population is shown. (D) Sorted LMPP from WT or Fuc-TVII/Fuc-TIV DKO mice was cultured on OP9 (top panel) or OP9-DL4 (bottom panel) stromal layers in triplicate. After 10 days, cultured cells were analyzed by flow cytometry for expression of CD25 and Thy1.2. Representative FACS plots of cells gated on CD45+ are shown. (E) Total numbers of cells obtained from the cultures described in panel D for OP9-DL4. Data are mean and ± S.E.M. of three wells.
It has been previously shown that inactivation of Fut 7 by homologous recombination results in more than 80% decrease in lymphocyte homing to peripheral lymph nodes (25). We tested whether diminished expression of functional PSGL-1 on the cell surface of Fuc-TVII/Fuc-TIV double-deficient BM progenitors resulted in a thymic settling defect. We generated competitive BM chimeras by intravenously transferring both Fuc-TVII/Fuc-TIV DKO BM (CD45.2) and WT BM (CD45.1) at a 2:1 ratio into lethally irradiated WT recipients. As a control, we injected mixtures of CD45.2 WT BM and CD45.1 WT BM. After 10 weeks to allow for hematopoietic reconstitution, BM and thymi were analyzed by flow cytometry to determine the donor chimerism within defined progenitor populations (Fig. 6C, upper panel). Fuc-TVII/Fuc-TIV double-deficient HSC were able to engraft in the BM and differentiate into MPP, LMPP and CLP normally, however, the donor chimerism was significantly decreased in early intrathymic T cell progenitors (Fig. 6C, bottom panel). Indeed, Fuc-TVII/Fuc-TIV progenitors showed a severe defect in thymic settling that was comparable in magnitude to the defect seen with PSGL-1−/− mixed BM chimeras, (Fig. 1B). Defects in thymic immigration and immunodeficiency have been linked to increases in thymic receptivity mediated by increased frequencies of P-selectin+ thymic endothelial cells (18). However, frequencies of P-selectin+ thymic endothelial cells appeared unaffected in Fut4/Fut7 DKO mice (Supplemental Fig. 1B, C). When cultured on OP9-DL4 stromal cells, Fuc-TVII/Fuc-TIV DKO LMPP generated similar number of CD25 and Thy1.2 double positive T cells on per cell basis compared to WT controls, indicating equivalent responses to T-inductive Notch signals (Fig. 6, D and E). These findings establish an important role for fucosylation-mediated functional PSGL-1 in thymic settling in a competitive situation.
Discussion
We have established that expression of functional PSGL-1 is regulated on BM derived hematopoietic progenitors cells, and investigated the molecular basis of this regulation. We found that functional PSGL-1 is expressed at low levels on hematopoietic stem cells but that high levels are found on downstream progenitors that are thought to home to and settle the thymus. Finally, we found that Fuc-TVII/Fuc-TIV deficient BM progenitors demonstrate thymic settling defects, due to the abrogation of fucosyltransferase-mediated modifications necessary to form functional PSGL-1.
The ability to settle the thymus is selectively achieved by progenitors downstream of HSC, and includes LMPP and CLP (7, 8, 29, 42). One molecular basis of this selectivity was previously shown to be the expression of the chemokine receptors CCR7 and CCR9 on CLP and subsets of LMPP, and progenitors lacking both of these molecules are almost completely unable to contribute to T lymphopoiesis in a competitive situation (8, 10, 15, 16). Earlier work had established that the selectin ligand PSGL-1 also plays a role in facilitating thymic settling and efficient homing of T cells to secondary lymphoid organs (17, 43). We have demonstrated that progenitors lacking PSGL-1 contribute inefficiently to the earliest thymic progenitor populations in competitive irradiation chimeras, thus confirming and extending previous work (17). Interestingly, some donor derived PSGL-1 deficient cells do still settle the thymus even in this competitive situation, suggesting that other undiscovered molecules may play a role in the absence of PSGL-1.
The formation of functional P-selectin ligand requires the orchestrated action of glycosyltransferases (25, 36, 41). Whereas there are at least five distinct α1,3 fucosyltransferase genes in the human and mouse genome, only two of these, specifically Fuc-TVII and Fuc-TIV, are expressed in leukocytes or their progenitors (38, 44, 45). In order to investigate the importance of PSGL-1 fucosylation in the migration of progenitors to the thymus, we performed competitive BM transplantation assays using BM from Fuc-TVII/Fuc-TIV double-deficient mice. In competition with wild type cells, Fuc-TVII/Fuc-TIV double-deficient cells were clearly disadvantaged in thymic settling. Thus, our data suggest that PSGL-1 is the main substrate of Fuc-TVII/Fuc-TIV involved in thymic homing. However, some progenitor cells can get into thymus without Fuc-TVII, and it remains possible that other Fut genes including Fuc-TVI (46) or residual PSGL-1 interactions with Fuc-TVII independent ligands such as E-selectin may play a role (47). Perhaps for this reason, we did not see a clear increase in frequencies of P-selectin+ thymic endothelial cells, which has been reported to underlie the increased thymic receptivity seen in PSGL-1−/− mice and IL-7R−/− mice (18). However, the phenotype of Fuc-TVII/Fuc-TIV double-deficient cells otherwise largely mirrors the phenotype of PSGL-1 deficient progenitors. The occurence of some T cell development from PSGL-1 deficient progenitors and also Fuc-TVII/Fuc-TIV double-deficient progenitors suggests that receptor ligand pairs distinct from PSGL-1 and its ligands may also exist that support thymic settling; these remain to be discovered.
In conclusion, the present study established that expression of functional PSGL-1 on BM progenitor is dynamically regulated. Together with the known selective expression of CCR7 and CCR9 chemokine receptors, selective expression of functional PSGL-1 therefore contributes to the regulated basis of selective thymic settling by T cell progenitors.
Supplementary Material
Acknowledgments
We thank Maria Elena De Obaldia, Qi Yang and Sugata Manna for critical review of this manuscript.
Abbreviations used in this paper
- BM
bone marrow
- HSC
hematopoietic stem cells
- MPP
multipotent progenitor
- LMPP
lymphoid-primed multipotent progenitors
- CLP
common lymphoid progenitor; early lymphoid progenitor
- ETP
early T lineage progenitor
- DP
CD4+CD8+ double positive
- DN
CD4−CD8− double negative
- Lin
lineage antigens
- Sca-1
stem cell antigen-1
- Flt3
fms-like tyrosine kinase receptor-3
- Flt3L
Flt3 ligand
- PSGL-1
P-selectin glycoprotein ligand 1
- Fuc-TVII
α1,3 fucosyltransferase 7
- Fuc-TIV
α 1, 3 fucosyltransferase 4
- WT
wild type
Footnotes
This work was supported by National Institutes of Health Grants HL110741 and AI059621.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 2.Adolfsson J, Borge OJ, Bryder D, Theilgaard-Monch K, Astrand-Grundstrom I, Sitnicka E, Sasaki Y, Jacobsen SE. Upregulation of Flt3 Expression within the Bone Marrow Lin(−)Sca1(+)c- kit(+) Stem Cell Compartment Is Accompanied by Loss of Self-Renewal Capacity. Immunity. 2001;15:659–669. doi: 10.1016/s1074-7613(01)00220-5. [DOI] [PubMed] [Google Scholar]
- 3.Donskoy E, Goldschneider I. Thymocytopoiesis is maintained by blood-borne precursors throughout postnatal life. A study in parabiotic mice. J Immunol. 1992;148:1604–1612. [PubMed] [Google Scholar]
- 4.Shortman K, Wu L. Early T lymphocyte progenitors. Annu Rev Immunol. 1996;14:29–47. doi: 10.1146/annurev.immunol.14.1.29. [DOI] [PubMed] [Google Scholar]
- 5.Goldschneider I, Komschlies KL, Greiner DL. Studies of thymocytopoiesis in rats and mice. I. Kinetics of appearance of thymocytes using a direct intrathymic adoptive transfer assay for thymocyte precursors. J Exp Med. 1986;163:1–17. doi: 10.1084/jem.163.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spangrude GJ, Muller-Sieburg CE, Heimfeld S, Weissman IL. Two rare populations of mouse Thy-1lo bone marrow cells repopulate the thymus. J Exp Med. 1988;167:1671–1683. doi: 10.1084/jem.167.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai AY, Kondo M. Identification of a bone marrow precursor of the earliest thymocytes in adult mouse. Proc Natl Acad Sci U S A. 2007;104:6311–6316. doi: 10.1073/pnas.0609608104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwarz BA, Sambandam A, Maillard I, Harman BC, Love PE, Bhandoola A. Selective thymus settling regulated by cytokine and chemokine receptors. J Immunol. 2007;178:2008–2017. doi: 10.4049/jimmunol.178.4.2008. [DOI] [PubMed] [Google Scholar]
- 9.Benz C, V, Martins C, Radtke F, Bleul CC. The stream of precursors that colonizes the thymus proceeds selectively through the early T lineage precursor stage of T cell development. J Exp Med. 2008;205:1187–1199. doi: 10.1084/jem.20072168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zlotoff DA, Sambandam A, Logan TD, Bell JJ, Schwarz BA, Bhandoola A. CCR7 and CCR9 together recruit hematopoietic progenitors to the adult thymus. Blood. 2009 doi: 10.1182/blood-2009-08-237784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mansson R, Zandi S, Welinder E, Tsapogas P, Sakaguchi N, Bryder D, Sigvardsson M. Single cell analysis of the common lymphoid progenitor compartment reveals functional and molecular heterogeneity. Blood. 2009 doi: 10.1182/blood-2009-08-236398. [DOI] [PubMed] [Google Scholar]
- 12.Inlay MA, Bhattacharya D, Sahoo D, Serwold T, Seita J, Karsunky H, Plevritis SK, Dill DL, Weissman IL. Ly6d marks the earliest stage of B-cell specification and identifies the branchpoint between B-cell and T-cell development. Genes Dev. 2009;23:2376–2381. doi: 10.1101/gad.1836009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scimone ML, Aifantis I, Apostolou I, von Boehmer H, von Andrian UH. A multistep adhesion cascade for lymphoid progenitor cell homing to the thymus. Proc Natl Acad Sci U S A. 2006;103:7006–7011. doi: 10.1073/pnas.0602024103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sultana DA, Bell JJ, Zlotoff DA, De Obaldia ME, Bhandoola A. Eliciting the T cell fate with Notch. Semin Immunol. 22:254–260. doi: 10.1016/j.smim.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu C, Saito F, Liu Z, Lei Y, Uehara S, Love P, Lipp M, Kondo S, Manley N, Takahama Y. Coordination between CCR7- and CCR9-mediated chemokine signals in prevascular fetal thymus colonization. Blood. 2006;108:2531–2539. doi: 10.1182/blood-2006-05-024190. [DOI] [PubMed] [Google Scholar]
- 16.Krueger A, Willenzon S, Lyszkiewicz M, Kremmer E, Forster R. CC chemokine receptor 7 and 9 double-deficient hematopoietic progenitors are severely impaired in seeding the adult thymus. Blood. 115:1906–1912. doi: 10.1182/blood-2009-07-235721. [DOI] [PubMed] [Google Scholar]
- 17.Rossi FM, Corbel SY, Merzaban JS, Carlow DA, Gossens K, Duenas J, So L, Yi L, Ziltener HJ. Recruitment of adult thymic progenitors is regulated by P-selectin and its ligand PSGL-1. Nat Immunol. 2005;6:626–634. doi: 10.1038/ni1203. [DOI] [PubMed] [Google Scholar]
- 18.Gossens K, Naus S, Corbel SY, Lin S, Rossi FM, Kast J, Ziltener HJ. Thymic progenitor homing and lymphocyte homeostasis are linked via S1P-controlled expression of thymic P-selectin/CCL25. J Exp Med. 2009;206:761–778. doi: 10.1084/jem.20082502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ley K, Kansas GS. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat Rev Immunol. 2004;4:325–335. doi: 10.1038/nri1351. [DOI] [PubMed] [Google Scholar]
- 20.Kivisakk P, Mahad DJ, Callahan MK, Trebst C, Tucky B, Wei T, Wu L, Baekkevold ES, Lassmann H, Staugaitis SM, Campbell JJ, Ransohoff RM. Human cerebrospinal fluid central memory CD4+ T cells: evidence for trafficking through choroid plexus and meninges via P-selectin. Proc Natl Acad Sci U S A. 2003;100:8389–8394. doi: 10.1073/pnas.1433000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ley K. The role of selectins in inflammation and disease. Trends Mol Med. 2003;9:263–268. doi: 10.1016/s1471-4914(03)00071-6. [DOI] [PubMed] [Google Scholar]
- 22.Li F, Wilkins PP, Crawley S, Weinstein J, Cummings RD, McEver RP. Post-translational modifications of recombinant P-selectin glycoprotein ligand-1 required for binding to P- and E-selectin. J Biol Chem. 1996;271:3255–3264. [PubMed] [Google Scholar]
- 23.McCormack MP, Forster A, Drynan L, Pannell R, Rabbitts TH. The LMO2 T-cell oncogene is activated via chromosomal translocations or retroviral insertion during gene therapy but has no mandatory role in normal T-cell development. Mol Cell Biol. 2003;23:9003–9013. doi: 10.1128/MCB.23.24.9003-9013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weninger W, Ulfman LH, Cheng G, Souchkova N, Quackenbush EJ, Lowe JB, von Andrian UH. Specialized contributions by alpha(1,3)-fucosyltransferase-IV and FucT-VII during leukocyte rolling in dermal microvessels. Immunity. 2000;12:665–676. doi: 10.1016/s1074-7613(00)80217-4. [DOI] [PubMed] [Google Scholar]
- 25.Maly P, Thall A, Petryniak B, Rogers CE, Smith PL, Marks RM, Kelly RJ, Gersten KM, Cheng G, Saunders TL, Camper SA, Camphausen RT, Sullivan FX, Isogai Y, Hindsgaul O, von Andrian UH, Lowe JB. The alpha(1,3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell. 1996;86:643–653. doi: 10.1016/s0092-8674(00)80137-3. [DOI] [PubMed] [Google Scholar]
- 26.Bhattacharya D, Rossi DJ, Bryder D, Weissman IL. Purified hematopoietic stem cell engraftment of rare niches corrects severe lymphoid deficiencies without host conditioning. J Exp Med. 2006;203:73–85. doi: 10.1084/jem.20051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmitt TM, Zuniga-Pflucker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 28.Bell JJ, Bhandoola A. The earliest thymic progenitors for T cells possess myeloid lineage potential. Nature. 2008;452:764–767. doi: 10.1038/nature06840. [DOI] [PubMed] [Google Scholar]
- 29.Serwold T, Ehrlich LI, Weissman IL. Reductive isolation from bone marrow and blood implicates common lymphoid progenitors as the major source of thymopoiesis. Blood. 2009;113:807–815. doi: 10.1182/blood-2008-08-173682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kondo M, I, Weissman L, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 31.Lai AY, Lin SM, Kondo M. Heterogeneity of Flt3-expressing multipotent progenitors in mouse bone marrow. J Immunol. 2005;175:5016–5023. doi: 10.4049/jimmunol.175.8.5016. [DOI] [PubMed] [Google Scholar]
- 32.Maillard I, Schwarz BA, Sambandam A, Fang T, Shestova O, Xu L, Bhandoola A, Pear WS. Notch-dependent T-lineage commitment occurs at extrathymic sites following bone marrow transplantation. Blood. 2006;107:3511–3519. doi: 10.1182/blood-2005-08-3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zubkova I, Mostowski H, Zaitseva M. Up-regulation of IL-7, stromal-derived factor-1alpha, thymus-expressed chemokine, and secondary lymphoid tissue chemokine gene expression in the stromal cells in response to thymocyte depletion: implication for thymus reconstitution. J Immunol. 2005;175:2321–2330. doi: 10.4049/jimmunol.175.4.2321. [DOI] [PubMed] [Google Scholar]
- 34.Igarashi H, Gregory S, Yokota T, Sakaguchi N, Kincade P. Transcription from the RAG1 Locus Marks the Earliest Lymphocyte Progenitors in Bone Marrow. Immunity. 2002;17:117–130. doi: 10.1016/s1074-7613(02)00366-7. [DOI] [PubMed] [Google Scholar]
- 35.Welner RS, Esplin BL, Garrett KP, Pelayo R, Luche H, Fehling HJ, Kincade PW. Asynchronous RAG-1 expression during B lymphopoiesis. J Immunol. 2009;183:7768–7777. doi: 10.4049/jimmunol.0902333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ellies LG, Tsuboi S, Petryniak B, Lowe JB, Fukuda M, Marth JD. Core 2 oligosaccharide biosynthesis distinguishes between selectin ligands essential for leukocyte homing and inflammation. Immunity. 1998;9:881–890. doi: 10.1016/s1074-7613(00)80653-6. [DOI] [PubMed] [Google Scholar]
- 37.Asano M, Nakae S, Kotani N, Shirafuji N, Nambu A, Hashimoto N, Kawashima H, Hirose M, Miyasaka M, Takasaki S, Iwakura Y. Impaired selectin-ligand biosynthesis and reduced inflammatory responses in beta-1,4-galactosyltransferase-I-deficient mice. Blood. 2003;102:1678–1685. doi: 10.1182/blood-2003-03-0836. [DOI] [PubMed] [Google Scholar]
- 38.Carlow DA, Gossens K, Naus S, Veerman KM, Seo W, Ziltener HJ. PSGL-1 function in immunity and steady state homeostasis. Immunol Rev. 2009;230:75–96. doi: 10.1111/j.1600-065X.2009.00797.x. [DOI] [PubMed] [Google Scholar]
- 39.Ellies LG, Sperandio M, Underhill GH, Yousif J, Smith M, Priatel JJ, Kansas GS, Ley K, Marth JD. Sialyltransferase specificity in selectin ligand formation. Blood. 2002;100:3618–3625. doi: 10.1182/blood-2002-04-1007. [DOI] [PubMed] [Google Scholar]
- 40.Ellies LG, Ditto D, Levy GG, Wahrenbrock M, Ginsburg D, Varki A, Le DT, Marth JD. Sialyltransferase ST3Gal-IV operates as a dominant modifier of hemostasis by concealing asialoglycoprotein receptor ligands. Proc Natl Acad Sci U S A. 2002;99:10042–10047. doi: 10.1073/pnas.142005099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Homeister JW, Thall AD, Petryniak B, Maly P, Rogers CE, Smith PL, Kelly RJ, Gersten KM, Askari SW, Cheng G, Smithson G, Marks RM, Misra AK, Hindsgaul O, von Andrian UH, Lowe JB. The alpha(1,3)fucosyltransferases FucT-IV and FucT-VII exert collaborative control over selectin-dependent leukocyte recruitment and lymphocyte homing. Immunity. 2001;15:115–126. doi: 10.1016/s1074-7613(01)00166-2. [DOI] [PubMed] [Google Scholar]
- 42.Benz C, Bleul CC. A multipotent precursor in the thymus maps to the branching point of the T versus B lineage decision. J Exp Med. 2005;202:21–31. doi: 10.1084/jem.20050146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Veerman KM, Williams MJ, Uchimura K, Singer MS, Merzaban JS, Naus S, Carlow DA, Owen P, Rivera-Nieves J, Rosen SD, Ziltener HJ. Interaction of the selectin ligand PSGL-1 with chemokines CCL21 and CCL19 facilitates efficient homing of T cells to secondary lymphoid organs. Nat Immunol. 2007;8:532–539. doi: 10.1038/ni1456. [DOI] [PubMed] [Google Scholar]
- 44.Satoh T, Kanai Y, Wu MH, Yokozeki H, Kannagi R, Lowe JB, Nishioka K. Synthesis of {alpha}(1,3) fucosyltransferases IV- and VII-dependent eosinophil selectin ligand and recruitment to the skin. Am J Pathol. 2005;167:787–796. doi: 10.1016/s0002-9440(10)62051-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Knibbs RN, Craig RA, Maly P, Smith PL, Wolber FM, Faulkner NE, Lowe JB, Stoolman LM. Alpha(1,3)-fucosyltransferase VII-dependent synthesis of P- and E-selectin ligands on cultured T lymphoblasts. J Immunol. 1998;161:6305–6315. [PubMed] [Google Scholar]
- 46.Schnyder-Candrian S, Borsig L, Moser R, Berger EG. Localization of alpha 1,3-fucosyltransferase VI in Weibel-Palade bodies of human endothelial cells. Proc Natl Acad Sci U S A. 2000;97:8369–8374. doi: 10.1073/pnas.97.15.8369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borges E, Pendl G, Eytner R, Steegmaier M, Zollner O, Vestweber D. The binding of T cell-expressed P-selectin glycoprotein ligand-1 to E- and P-selectin is differentially regulated. J Biol Chem. 1997;272:28786–28792. doi: 10.1074/jbc.272.45.28786. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.