Abstract
Acute inflammation and its resolution are essential processes for tissue protection and homeostasis. In this context, specialized pro-resolving mediators derived from polyunsaturated fatty acids are of interest. Here, we report that resolvin E2 (RvE2) from eicosapentaenoic acid is endogenously produced during self-limited murine peritonitis in both the initiation and resolution phases. RvE2 (1–10 nM) carries potent leukocyte-directed actions that include 1) regulating chemotaxis of human neutrophils, and 2) enhancing phagocytosis and anti-inflammatory cytokine production. These actions appear to be mediated by leukocyte G-protein coupled receptors as preparation of labeled RvE2 gave direct evidence for specific binding of radiolabeled RvE2 to neutrophils (Kd 24.7 ± 10.1 nM) and RvE1 activation of recombinant GPCRs was assessed. In addition to the murine inflammatory milieu, RvE2 was also identified in plasma from healthy human subjects. RvE2 rapidly downregulated surface expression of human leukocyte integrins in whole blood and dampened responses to platelet-activating factor. Together, these results indicate that RvE2 can stimulate host-protective actions throughout initiation and resolution in the innate inflammatory responses.
Introduction
Acute inflammation is a host defensive mechanism critical in injury, trauma and infection (1). Since these insults are continuous throughout life, both proper initiation and timely termination of inflammatory responses are crucial to maintain systemic homeostasis (2). Excessive and uncontrolled inflammation is now recognized as an underlying component in several prevalent chronic diseases such as cardiovascular, cerebrovascular, metabolic diseases and cancers (3–6).
Once thought to be a passive dissipation of inflammatory cellular and molecular factors, resolution is now recognized as the active biological response of the system to regain homeostasis (7–9). Novel cellular and molecular factors were identified to enhance resolution, which appear to be programmed responses at the tissue level in this process (8, 9) (recently reviewed in 10, 11). Notably, omega-3 polyunsaturated fatty acid-derived mediators, such as the E-series and D-series resolvins and protectins, are recognized for their resolution-enhancing actions. Hence, these families are collectively are coined as specialized pro-resolving mediators (SPMs5), along with the arachidonic acid-derived lipoxins (10).
This novel genus shares both anti-inflammatory and pro-resolving actions, yet each mediator family has a distinct chemical structure for each member, and in many cases each acts on separate tissues and cell types via cell-surface receptors specific for each SPM (12–15). Resolvins display potent actions that are pro-resolving in several animal models of diseases (for a recent review, see (10)). Among them, RvE1, founding member of the E series resolvins, regulates granulocyte trafficking (7) and stimulates non-phlogistic phagocytosis of macrophages (16). RvE1 also promotes microbial clearance (17) and stimulates tissue regeneration while reducing inflammatory response (13).
Resolvin E2 is a more recent addition to the E series resolvin family (18), and its complete structure is established and recently was confirmed by Ogawa et al. using total organic synthesis (19). Here, we report on the production and leukocyte-directed actions of RvE2 as one of the local mediators of tissue homeostasis during inflammation-resolution.
Materials and Methods
Materials
RvE1, 18-HEPE, PAF C-16 and human recombinant 5-LOX were purchased from Cayman Chemical (Ann Arbor, MI). NaB[3H]4 was purchased from American Radiolabeled Chemicals (St. Louis, MO). NAD and other miscellaneous chemicals were obtained from Sigma (St. Louis, MO). R-phycoerythrin-conjugated mouse anti–human CD18 (clone 6.7, BD Biosciences), RPE-Cy5–conjugated mouse anti–human CD14 (Gene Tex, San Antonio, TX), and PE-Cy5-conjugated mouse anti-human CD3 (BD Biosciences) were purchased; appropriately labeled, class-matchedmouse IgGs were used as negative controls for CD18 (BD Biosciences) staining. RBC Lysis buffer was purchased from eBioscience (San Diego, CA) and carboxy-H2DCF-DA from Invitrogen-Molecular Probes (Carlsbad, CA). Recombinant human P-selectin and recombinant human CXCL8/IL-8 chemoattractant were purchased from R&D Systems (Minneapolis, MN), human serum albumin and HBSS from Sigma-Aldrich (St. Louis, MO), Tygon tubing (Part # TGY-010-C) and needles (Part # NE-301PL-C) from Small Parts Inc.
Self-limited inflammation-resolution: Murine peritonitis
All animal protocols were in accordance with the Harvard Medical Area Standing Committee on Animals (Protocol # 02570). Peritonitis was initiated with intraperitoneal injection of zymosan A (1 mg/1 mL saline) to 6–8-week-old male FVB mice. At each scheduled time point (2,4,8,12,24,72 hours after injection), mice were euthanized with an overdose of isofluorane, and peritoneum was lavaged with 5 mL PBS−/− and kept at −80°C for lipidomic analysis. Small aliquots of lavage fluids were saved for differential counting and FACS analysis.
LC-UV-MS-MS-based lipid mediator (LM)-lipidomics
Qtrap 3200 (Applied Biosystems) equipped with Agilent HP1100 (Agilent) and diode array detector (DAD) or Shimadzu LC-20AD pumps and SIL-20AC autosampler were used for LC-UV-MS-MS spectrometry. All samples were analyzed by Agilent C18 (4.6 × 50 × 1.8 μm, flow rate 400 μL/min) with a gradient of 55:45:0.01 or 60:40:0.01 to 100:0:0.01 (methanol:water:acetic acid, v/v/v) as in Yang et al. (20). Multiple reaction monitoring (MRM) method was established to detect each mediator-specific ion fragment. RvE2 in plasma from healthy human donors was identified and quantified with m/z pairs of 333->199 and 333->115.
Synthesis of RvE2
RvE2 was prepared as in (18), and both 18S-and 18R-isomers were separated by chiral HPLC (17) (see HPLC isolation section in ref. (17) for further detail). Absolute stereochemistry of RvE2 was confirmed by matching with synthetic RvE2 prepared by total organic synthesis (a gift from Dr. Makoto Arita, University of Tokyo) (19). An Agilent HP 1100 system (Agilent) equipped with DAD was used for all HPLC-based separation of lipid mediators. For reverse-phase lipid separation, an Agilent C18 (150 × 4.6 × 5 μm, flow rate 1mL/min) or Phenomenex Luna C18(2) (150 × 2 × 5 μm, flow rate 200 μL/min) column was used with gradient of 65:35:0.01 to 100:0:0.01 (methanol:water:acetic acid, v/v/v) for RvE2 analysis or 80:20:0.01 to 100:0:0.01 for 18-oxo-EPE isolation. For chiral separation, Chiralpak AD-RH (150 × 2.0 × 5 μm, flow rate 200 μL/min) was used with a gradient of 95:5:0.01 to 100:0:0.01 (methanol:water:acetic acid, v/v/v).
Radiolabeled RvE2 preparation
All procedures using radiochemicals were reviewed by the Brigham and Women’s Hospital radiochemistry department (Dr. Castronovo). 18-HEPE (50 μM, pH 8 Tris buffer with 1mM NAD) was incubated with 1 μg 15-PGDH/eicosanoid oxidoreductase at 37°C for two hours. The incubation mixtures were directly taken to HPLC and 18-oxoEPE was isolated. Reductive tritiation was carried out by mixing 18-oxoEPE (50 μM in ethanol) with an excess amount of NaB[3H]4 in 1 N NaOH (apparent final pH ~10). The labeling was carried out using an apparatus equipped with a chemical trap to collect potential tritiated water. After quenching the reaction mixtures with 5 M potassium acetate (pH 4.5), the mixtures were extracted with ether, dried, and taken to chiral HPLC to separate 18S- and 18R-[3H]-HEPE.
Regulation of leukocyte surface CD18 activation
Whole blood aliquots were incubated with either increasing concentrations of RvE2 for 30 minutes or PAF for 15 minutes. In these, 30 nM RvE2 or 30 nM RvE1 was added to whole blood aliquots for 15 minutes followed by addition of 50 nM PAF for 15 more minutes. Experiments were performed in a 37°C water bath with gentle mixing every 5 minutes. Similarly, 30 nM RvE2 or 30 nM RvE1 or appropriate vehicle control and 50 nM PAF (15 min, 37°C) were added together to freshly collected human whole blood aliquots, following the same experimental conditions as for the incubation described above. Incubations were placed on ice followed by red blood cell lysis, and cells were fixed with 3% formalin (30 min, 4°C). Next, direct immunofluorescence labeling was performed using anti-human CD62L, CD18, CD3+, or a combination of anti-human CD3+ with the corresponding isotype controls for CD62L and CD18 in a 1:100 ratio in FACS buffer (30 min, 4°C). Stained cells were resuspendedin FACS buffer and analyzed using FACSort and Cellquest software (Becton Dickinson) or BD FACS Canto (BD Biosciences) and FlowJo (Tree Star). Monocytes, neutrophils and lymphocytes were gated using forward/side scatters and respective surface/intracellular stainings (21).
Human neutrophil isolation
Human whole (venous) blood (60 ml) was collected with heparin from healthy volunteers (who did not take medication for at least 2 weeks before donation) according to Partners Human Research Committee Protocol #1999-P-001297; donor identities were not linked to the analyses or stored. Fresh human neutrophils (PMNs) were isolated from the whole blood by Ficoll gradient and were enumerated (22).
Single-cell monitoring with microfluidic chambers
A microfluidic chamber was engineered for testing the actions and screening of the impact of putative novel lipid mediators on the chemotactic function(s) of neutrophils (23, 24). Briefly, two steady-state gradients of IL-8 (0–10 nM) were formed in the two gradient generators, one in buffer and one with an overlaying uniform concentration (10 nM) (23) of the putative lipid mediator for testing, and were ready to be delivered to the chemotaxis chambers. Next, 10 μl of capillary human blood was collected from healthy volunteers by finger prick (BD Biosciences). The blood was allowed to flow through the main channel for ~ 3 min, and then the valve was opened for the IL-8 gradient generation from one channel, leaving the second one closed. The IL-8 flow removed the majority of RBC and other cells that were not tethered to the chamber-coated P-selectin, thus allowing direct monitoring of the neutrophils captured on the surface of the chamber. After 15–20 min, the gradient was switched to the second gradient generator containing a uniform concentration of the compound, coming from one syringe with RvE2 or RvE1 alone and the other containing an equal concentration of RvE2 or RvE1 combined with 10 nM IL-8. Neutrophil migration in the chemokine gradient and their individual response(s) to addition of different concentrations of RvE2 or RvE1 (in separate experiments) were recorded with a video and/or digital camera mounted on the microscope (10x objective). Cell migration was analyzed using the cell tracking function in ImageJ software (Molecular Devices). A dozen cells per exposure to mediators were tracked and analyzed for displacement in the direction of the gradient and along the flow.
Actin polymerization
Freshly isolated human PMN were mixed with 15 nM LTB4, 15 nM RvE2, or a mixture of 15 nM LTB4 and RvE2. After designated time intervals (i.e., 15 or 30 s), incubations were fixed in ice-cold 3% formalin solution. Fixed cells were washed and resuspended in cell-permeabilizing buffer and stained with 1:100 FITC-labeled phalloidin for FACS analysis (25).
Human macrophages
Human PBMC-derived macrophages were prepared with human recombinant GM-CSF as in (17). Briefly, at Day 7, macrophages were washed and treated with different concentrations of RvE2 (15 min, 37°C) and then incubated with FITC-labeled zymosan A (30 min, 37°C). Non-cell-associated zymosan particles were washed and quenched with addition of trypan blue. Intracellular FITC intensity of each well was monitored by Victor3 plate reader. Macrophages (Day 7) were washed and media was replaced with serum-free RPMI 1640 and kept overnight at 37°C. Cells were incubated with vehicle or different concentrations of RvE2 for one hour, followed by LPS (100 ng/mL) treatment. At 24 hours, supernatants were collected and held at −80 degrees before determining IL-10 level.
ChemR23 and BLT1 β-arrestin systems for comparing RvE1/RvE2
Direct comparison between RvE1 and RvE2 for ChemR23 activation and BLT1 receptor antagonism were carried out using a G-protein coupled receptor (GPCR) β-arrestin system as in (17). Briefly, with the ChemR23 overexpressing system, cells were plated 24 h prior to experiments and incubated with resolvins in serum-free medium (1 h, 37 °C). With the BLT1 overexpressing system, plated cells were incubated with different concentrations of resolvins E1 and E2, each separately, for one hour, followed by 30 nM LTB4 for 90 minutes. Cells were further incubated with β-Gal substrate (PathHunther EFC Detection kit™, DiscoveRx) for 90 minutes, and ligand-dependent receptor activation was assessed by luminescence using a plate reader (EnVision, Perkin Elmer).
Statistics
All murine experimental results in the figures and text are expressed as mean ± SEM of n≥3 mice per group. All human macrophage results are n≥3 for each concentration and are representative of 4 similar results from different healthy donors. Statistical significance was determined by two-tailed Student’s t-test; p < 0.05 was considered significant.
Results
RvE2 appearance in vivo during self-limited acute inflammation
Comprehensive liquid chromatography-tandem mass spectrometry-based lipidomic profiles of E-series resolvins and their precursors in murine peritoneal exudates were obtained during zymosan-induced peritonitis (Fig 1A). Identification and profiles of 18-HEPE and RvE2 during the time course gave distinct patterns (Fig 1B). 18-HEPE levels were sharply increased and evident at the earliest time points following sterile challenge and parallel the initial PMN infiltration. RvE2 was also identified in a similar pattern after challenge, followed by a gradual decline at ~24 hours. In these experiments we used the FVB/N mouse strain, which is an inbred strain that is widely used in the laboratory, especially for transgenic manipulation (26). Although this strain is complement C5a deficient, FVB mice are still capable of mounting a robust leukocytic infiltration to the site of inflammation or infection (27) and show similar results in chronic infection to C5a-sufficient strain C57BL/6J (28). Upon challenge with zymosan or bacterial inoculation, FVB mice show reproducible neutrophil infiltration to sites of inflammation and have been used to quantitatively investigate the local responses to sterile inflammation and infection (17, 29). Hence, in the resolution phase (i.e., when PMN numbers are lost from the peritoneal exudate) of this murine (FVB/N) peritonitis time course (72 hours), both 18-HEPE and RvE2 accumulated in the peritoneal exudate lavages.
Figure 1. RvE2 identification in both initiation and resolution phases of self-limited inflammatory responses.
A) Differential counting (left axis-line graph, neutrophils (square) and monocytes/macrophages (diamond)) and quantitative lipidomic profiling (right axis-bar graph) of 18-HEPE (grey) and RvE2 (black). B) Representative MS/MS spectra of Resolvin E2 (left) and its precursor 18-HEPE (right). C) FACS plots of exudate cells during the time course. F4/80+ monocytes/macrophages and Gr-1+ neutrophils are indicated (for details, see Materials and Methods). Results are average ± SEM of 3–4 separate mice for each time point; spectra and FACS plots are representative of separate experiments.
E series resolvins stop PMN chemotaxis
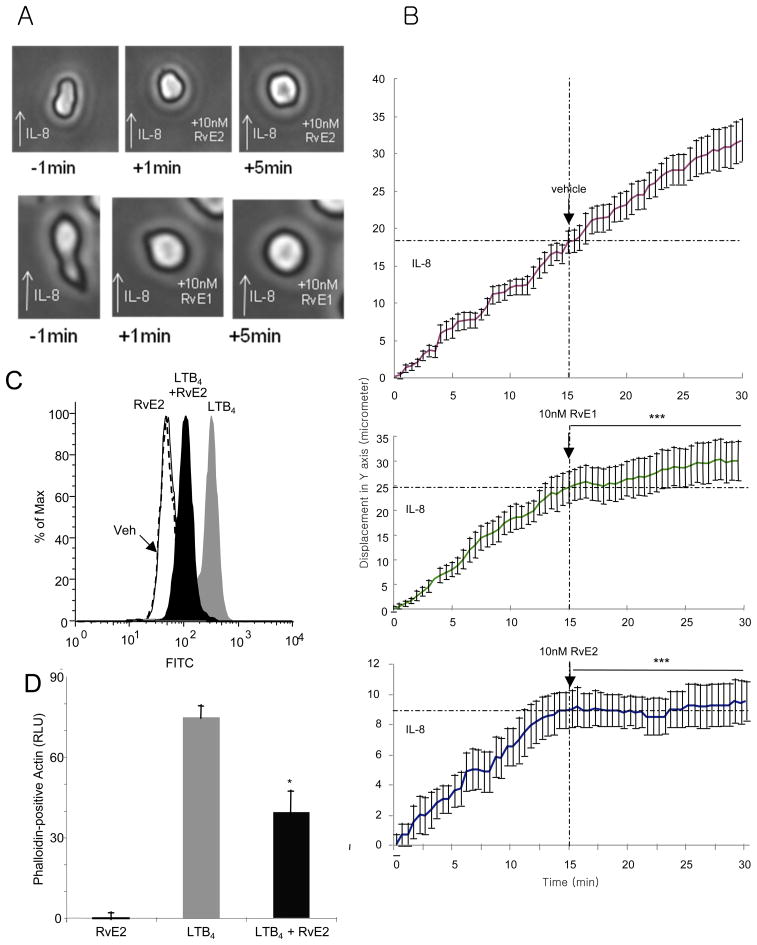
To translate and address RvE2 actions on specific human leukocytes, we next investigated human PMN. A microfluidic chamber which monitors chemotaxis at the single cell level (23, 24) was prepared and utilized to directly compare RvE2 and RvE1. PMN were isolated from a single drop of venous blood from healthy human subjects by selective adhesion to a P-selectin-coated surface. Captured PMNs migrated along the IL-8 gradient (0–10 nM) and displayed characteristic change in shape and morphology during chemotaxis (Figure 2A, left column and Supplemental Video 1). When 10 nM RvE2 was uniformly infused within the microfluidic chamber in the presence of IL-8 gradient, PMN ceased directed chemotactic movements and displayed an almost immediately visible change in shape, i.e. rounded morphology (Figure 2A, middle column). For direct comparison, RvE1 at equimolar concentration (10 nM) caused similar changes in PMN chemotaxis and morphology (Fig. 2A, right column). By tracing the displacement of each single PMN (Figure 2B) within the direction of an IL-8 chemotactic gradient with or without RvE2 or RvE1, the chemotactic velocities before and after addition of E-series resolvins were obtained and decrease of chemotaxis was calculated. RvE2, as well as RvE1, decreased chemotactic velocity in a statistically significant manner compared to the vehicle. At 10 nM, RvE2 gave 94.9% decrease and RvE1 gave 76.1% (p<0.0005 for each case; average chemotactic velocity during 0–15 min and 15–30 min are compared). These results visually demonstrate that RvE2 and RvE1 each display direct actions on PMN migration.
Figure 2. RvE2 stops neutrophil chemotaxis and stimulates rapid shape change: single cell monitoring.
A) Neutrophils with polarized morphology during exposure to IL-8 gradient quickly become rounded and were unable to regain their polarized morphology 5 minutes after exposure to 10 nM resolvin E2 or E1. B) Time-lapse single cell PMN monitoring. IL-8 gradient was applied at t=0, and RvE2, RvE1 or vehicle alone was added at the 15-minute time point (dotted line). Size of graphs was adjusted to compare deceleration ratio before and after adding resolvins. Results represent average ± SEM of PMN displacement in Y direction, n = 12. C) Regulation of human PMN actin polymerization with RvE2. Isolated human PMN were stimulated with LTB4 and coincubated with either vehicle or 30 nM RvE2. After 15 seconds, cells were fixed in formaldehyde and stained with phalloidin to measure actin polymerization. D) FACS plots and bar graphs: RvE2 reduces LTB4-mediated actin polymerization. Results are average of three separate donors. *p<0.05; ***p<0.005. See also Supplemental Videos S1A-D.
The shape change of PMN by RvE2 was further addressed by examining RvE2 action on the PMN cytoskeleton (Figure 2C). When activated, human PMN rapidly carry out actin polymerization within 15 seconds after exposure, shown by phalloidin-positive actin staining (25). RvE2 (30 nM) itself did not appear to directly affect polymerization. In contrast, when exposed at the same time, LTB4 (30 nM)-stimulated actin polymerization was reduced by 45±7% with equimolar concentration of RvE2 (p<0.05, Fig 2D).
RvE2 regulates non-phlogistic macrophage response: Enhanced phagocytosis and anti-inflammatory cytokine production
In order to investigate the actions of RvE2 in non-phlogistic leukocyte responses, we used PBMC-derived human macrophages and assessed both enhancement of phagocytic activity and anti-inflammatory cytokine production. Uptake of FITC-labeled opsonized particles by human macrophages was enhanced with increasing concentration of RvE2, showing maximum enhancement at 1 nM and 10 nM concentrations (Figure 3A). Moreover, when incubated with RvE2 and then exposed to lipopolysaccharide, RvE2 dose-dependently increased IL-10 production by macrophages (Figure 3B).
Figure 3. RvE2 enhances non-phlogistic actions of human macrophages: increases in phagocytosis and IL-10.
A) RvE2 enhances human macrophage phagocytosis. Macrophages were exposed to different concentrations of RvE2 for 15 minutes (at 37°C, pH 7.45), then FITC-labeled opsonized zymosan was added and incubated for 30 minutes. Particles that were not phagocytized were washed and quenched with trypan blue. Results are shown as % enhancement of phagocytosis above vehicle treatment. B) RvE2 enhances IL-10 production by human macrophages. Macrophages were incubated with different doses of RvE2 for one hour and then exposed to LPS (100 ng/mL). Supernatants were collected 24 hours later and IL-10 level was measured. Results are n≥3 for each concentration and are representative of 4 similar datasets from individual donors. *p<0.05.
RvE2 directly interacts with human leukocyte GPCRs
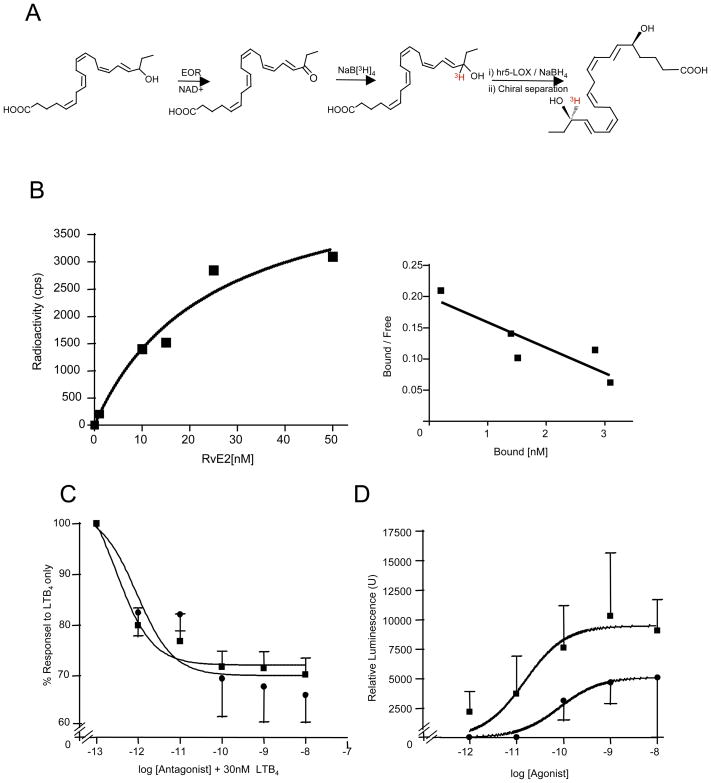
To determine whether RvE2 transmits signal via direct interactions with leukocyte membrane, radiolabeled ligand was prepared for specific binding experiments with isolated human PMN. Tritium-labeled RvE2 with sufficient specific radioactivity (15~20 Ci/mmol) was prepared by combined chemical and biogenic organic synthesis (Fig. 4A). The saturation (specific binding) curve was obtained, and Scatchard plot analysis gave a Kd of 24.7 ± 10.1 nM and Bmax of 4.9×103 ± 9.5×102 sites/cell from homoligand binding experiments with isolated human neutrophils (Fig. 4B). These values were in the range comparable to those obtained for RvE1 with human neutrophils (30, 31).
Figure 4. RvE2 specific binding and interaction with human leukocyte GPCRs.
A) Synthesis scheme of radiolabeled 18[3H]-RvE2 by chemical/biogenic approach. B) Direct binding with isolated human PMN was carried out (see Methods) and a Scatchard plot obtained (Kd = 24.7 ± 10.1 nM and Bmax = 4.9 × 103 ± 9.5 × 102/cells). Results are the average of n=3; graphs are representative of results from three separate donors. C) RvE2 competes for LTB4 with human recombinant BLT1. BLT1-overexpressing beta-arrestin cells were incubated with 30 nM LTB4 first and then increasing concentrations of RvE1 (square) or RvE2 (circle). D) RvE2 (circle) activation of human recombinant ChemR23-overexpressing beta arrestin cells (see Methods) compared to RvE1 (squares).
To test whether RvE2 interacts with the membrane GPCRs on human leukocytes as RvE1 (31), specific ligand-mediated cell activation was evaluated with a human recombinant BLT1-overexpressing beta-arrestin reporter cell system [see Materials and Methods]. In this system, RvE2 blocked LTB4-mediated tagged beta-arrestin signaling, which was comparable to that of RvE1 (Figure 4C). We next used the beta-arrestin system with overexpressed human recombinant ChemR23 receptor that binds RvE1 (31) to assess whether RvE2 directly activates this receptor compared to RvE1. RvE2 was less efficacious and less potent than RvE1 with this receptor (Figure 4D), suggesting that RvE2 is not likely a full agonist of ChemR23.
RvE2 is identified in healthy human plasma and regulates leukocyte surface integrins in human whole blood
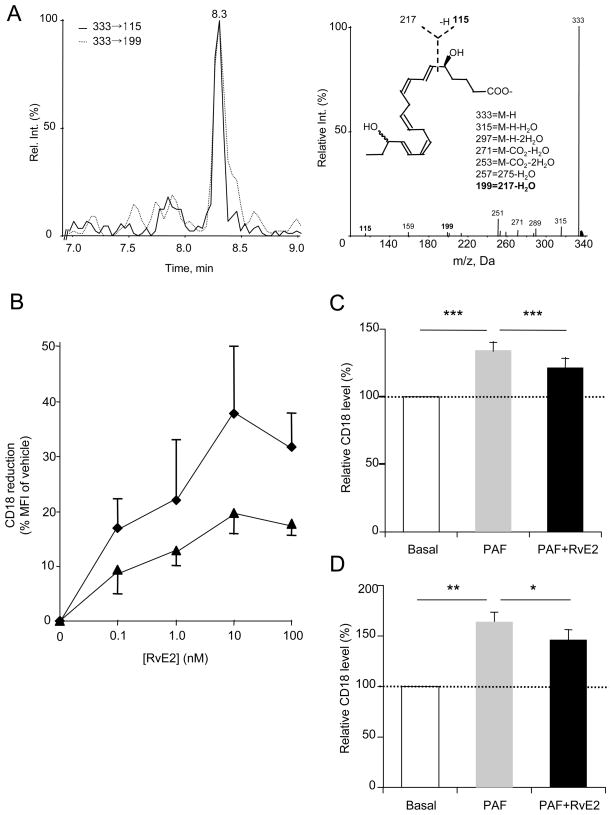
We carried out metabololipidomics of EPA-derived products from the plasma of healthy human subjects (Fig. 5A). Multiple reaction monitoring (MRM) with tandem mass spectrometry identified the E resolvins and their precursor 18-HEPE. For RvE2, signature ion pairs (333->199 and 333->115) were monitored, and LC retention time and tandem mass spectra from these peaks matched those of synthetic resolvin E2 prepared by total organic synthesis. Plasma levels of EPA-derived lipid mediators from five healthy subjects without EPA supplementation were in the range of 3.95–10.5 ng of 18-HEPE/mL of plasma (average ± SEM: 6.13 ± 1.56 ng/mL) and 0.53–3.72 ng RvE2/mL plasma (average ± SEM: 1.37 ± 0.68 ng/mL). Of interest, the 18-HEPE level is comparable to that of healthy human plasma levels with supplementation of 1 g EPA (31).
Figure 5. Resolvin E2 in human circulation and actions in whole blood.
A) RvE2 is present in human plasma from healthy subjects. MS/MS extracted ion chromatogram of m/z 333→199 and 333→115 (left) revealed one peak that matched with RvE2 synthetic and authentic standard (e.g. retention time and MS/MS spectrum, right). B) In whole blood, RvE2 regulation of CD18 in human PMN (diamond) and monocytes/macrophages (triangle) ex vivo. Vehicle or increasing concentrations of RvE2 were incubated with human whole blood (30 minutes, 37°C), fixed, RBC lysed and stained with PE-labeled CD18 antibody. Results are average +/− S.E. of four separate donors. C) RvE2 reduced PAF (50 nM)-stimulated CD18 surface expression on isolated human PMN, when exposed to RvE2 for 15 min before PAF or D) when RvE2 (30 nM) was simultaneously incubated with PAF (50 nM); see Methods. Cells were fixed and analyzed at 30 min after PAF stimulation. Results are average of n=6–8 separate donors. *p<0.05; **p<0.01; ***p<0.005 using a paired Student’s two-tailed t test.
Next, we investigated leukocyte integrin regulation in human whole blood. We assessed whether RvE2 can dampen peripheral blood leukocytes. In a dose-dependent manner, RvE2 downregulated neutrophil and monocyte/macrophage CD18 in whole blood (Figure 5B). Leukocytes exposed to resolvin E2 for 15 minutes prior to platelet-activating factor (PAF, 50 nM), gave reduced CD18 surface expression (Figure 5C). RvE2 also reduced CD18 surface expression when added together simultaneously with PAF ~49% and 31% reduction (Figure 5D). Similar reductions in CD18 expression were obtained with isolated monocytes/macrophages (data not shown).
Discussion
Acute inflammatory exudates are a complex milieu comprised of different cell types, as well as proteins and lipid-derived chemical mediators (1), which are dynamic and ideally when self limited resolve to homeostasis (2). Temporal regulation of leukocytes during acute inflammation and resolution is a crucial factor in determining the fate of acute inflammation (8). These cells are not only the targets of lipid mediators but also carry the enzymatic machinery for SPM biosynthesis as well as their metabolic inactivation. Hence, it is crucial to map the relationship(s) for specific local mediators within the onset of inflammation and its resolution.
To pinpoint the presence of RvE2 within a self-limited acute inflammation-resolution response in vivo, we carried out lipid mediator (LM)-metabololipidomic profiling during the time course with resolving peritoneal exudates. The intraperitoneal recruitment of leukocytes (e.g. PMN, monocytes) and their loss from the site is a highly ordered progression (8). This acute inflammation system is distinguished in two phases, initiation and resolution (1). RvE2 displayed biphasic production along with a sharp increase of its precursor 18-HEPE at ~2 h (Fig. 1). The RvE2 time course was distinct from that of RvE1 in that RvE1 accumulates in the resolution phase (cf. 16). RvE2 was identified when neutrophils dominate the exudate (see Fig. 1). Since PMN carry 5-LOX, they can convert 18-HEPE to 5-hydro(pero)xy intermediates (18) for both RvE1 and RvE2 biosynthesis (Fig. 1C). Considering increased permeability of the inflamed peritoneum and the polar nature of these molecules, it appears that RvE1 might be less retained in the peritoneum or more prone to rapid local metabolic inactivation and clearance than RvE2. In the resolution phase, RvE2 accumulated in vivo within the resolving exudate. At 72 h, PMN and monocytes/macrophages are present within the peritoneum, both of which can contribute to E series resolvin production, since they are well appreciated to carry 5-LOX.
To investigate RvE2 bioactions with leukocytes present during the inflammation-resolution circuit, we carried out a series of experiments with human neutrophils, which are in the first line of defense and recruited to the site of inflammation within the initiation phase, and macrophages, a key component in the resolution phase and homeostasis by uptaking apoptotic cells and debris (1, 2). We monitored chemotaxis of individual neutrophils using a microfluidic chamber (23). In this system, RvE2 potently regulated morphological changes and PMN chemotaxis, giving a rapid stop in motility of the PMN (Figure 2A); this is likely by RvE2 regulating actin polymerization (Figure 2D). RvE2 also potently enhanced non-phlogistic phagocytosis as well as increased IL-10 production by macrophages (Figure 3A, B). Together, these findings indicate that RvE2 possesses both anti-inflammatory (limiting PMN recruitment) and pro-resolving actions (enhancing nonphlogistic clearance by macrophages), in accordance and characteristic of the specialized pro-resolving mediators (SPMs) (10).
The bioactions of SPM on leukocytes are mediated via specific ligand-receptor interactions (2). In this regard, G-protein-coupled receptors on leukocytes were identified specifically for lipoxin A4 (5S,6R,15S-trihydroxy-6E,8E,10Z,12E-eicosatetraenoate) (32), RvE1 (31) and recently for RvD1 (15). Radiolabeled RvE2 (Fig. 4A), in the present study, was prepared and direct interactions of RvE2 with human neutrophils were assessed, which gave comparable Kd and Bmax values (i.e. nM) (cf. (15, 30, 31)) to other known SPM (Fig. 4B). In this regard, RvE1 binds both BLT1 and ChemR23 (30). Using a beta-arrestin tagged system, we found that RvE2, at least partially, shares receptors with RvE1 (Fig. 4C,D) and interacts with BLT1 at a potency similar to that of RvE1. These results are in accordance with earlier findings that RvE2 is essentially equipotent with RvE1 in limiting neutrophil infiltration (18) and in stimulating pro-inflammatory actions as reported in Figure 2. With the RvE1 receptor ChemR23 (31), however, RvE2 was only a weak activator of this receptor in this system. These results may explain the finding that RvE1 shows more potent actions than RvE2 in certain in vivo systems that depend on the target cell type and tissue (cf. (17, 33)). In addition to stopping neutrophil infiltration that could be mediated via regulation of BLT1, RvE2 also displays pro-resolving actions, namely enhancing phagocytosis and IL-10 production with differentiate human macrophages (Figure 3), suggesting that these RvE2 actions may be transduced by additional receptors that have yet to be defined that amplify the RvE2 signal, given its potent actions in vivo.
We showed earlier that 18-HEPE and resolvin E1 are identified from human subjects with EPA and aspirin (31). Along these lines, in the present report, we profiled plasma from healthy human subjects (without EPA supplementation). RvE2, as well as both the E-series resolvin precursors 18R- and 18S-HEPE (17), were present in considerable amounts (Figure 5A). Notably, RvE2 possesses two conjugated diene-alcohols, and it is of interest that RvE2 biosynthesis, unlike RvE1, does not require allylic epoxide formation nor its stereospecific enzymatic hydrolysis. This relatively simplified biosynthetic route may partially explain the identification of resolvins such as RvE2 in non-human sources, such as marine organisms and fish rich in omega-3 fatty acids (34, 35). Furthermore, SPM introduced via the gastrointestinal tract can be bioactive (36), as it was recently also shown that docosahexaenoic acid in 2-docosahexaenoic acid-lysophosphatidylcholine, a substrate of 15-LOX (both plant and human), produces orally active, anti-inflammatory compounds related to the resolvins (37).
Of note, in addition to the recent confirmation of 18-HEPE (38) and RvE2 (19) by total organic synthesis, RvE1, RvD1 and 17-HDHA were each identified in the human serum metabolome (39) in levels consistent with those identified in the present report for RvE2. Also, fish such as the Atlantic salmon produce resolvins de novo that are lost with cooking (40). These findings may be relevant when the impact of omega-3 fatty acid-enriched diets is considered in inflammation and in cardiovascular diseases (41).
It is well recognized now that chemical messengers play a crucial role in acute inflammation and its active resolution (2). SPMs such as RvE2, as shown in the present report, are distinct chemical mediators in both limiting inflammation and promoting resolution, namely the SPM defining biological mechanism of action (42, 43). This proresolving action is now shared with a few other molecules including peptide mediators, such as Annexin I, which is anti-inflammatory as well as enhances macrophage phagocytosis (44) and shares the ALX/FPR2 receptor with lipoxin A4 (45). Along these lines, several mediators are identified in inflammation and tissue injury, such as adenosine (46, 47), hypoxia-inducible factors (HIFs) (46, 48, 49) and others (50) that display potent anti-inflammatory and tissue protective actions. Although they are anti-inflammatory and local-acting autacoids, these molecules do not at present appear to stimulate resolution (43). Recently, intracellular signaling mechanisms of SPMs were found to involve microRNA. In this regard, resolvin D1 regulates inflammation-proresolving pathways by upregulating specific miRNA in a receptor-dependent fashion; for example, miR146b targeting NF-kB (51). Expanding such investigations to the diverse chemical mediators in inflammation and active resolution remains of interest and will likely uncover new proresolving pathways and mediators.
In the present report we also assessed the actions of RvE2 on human leukocytes in whole blood tissue where RvE2 dose-dependently downregulated leukocyte integrin activation in the nanomolar range and dampened responses to platelet-activating factor (PAF), a well appreciated and potent activator of platelets and leukocytes (1) (Figure 5B–D). In summation, the potent actions of RvE2 along with the presence of RvE2 in healthy human circulation indicate that RvE2 possesses the characteristic SPM profile of activity. Moreover, they suggest that RvE2 may contribute to homeostasis by stimulating the resolution of local inflammatory responses.
Supplementary Material
Acknowledgments
We thank Mary Halm Small for assistance with manuscript preparation and Dr. Makoto Arita (University of Tokyo) for a sample of synthetic RvE2.
Footnotes
These studies were supported in part by NIH grant nos. R01GM038765 and P01GM095467 (C.N.S.) and R01DE019938 (C.N.S., D.I.).
Abbreviations used are: EPA: eicosapentaenoic acid, HEPE: hydroxyeicosapentaenoate, 18R-HEPE, 18R-hydroxy-5Z,8Z,11Z,14Z,16E-eicosapentaenoic acid, 18S-HEPE, 18S-hydroxy-5Z,8Z,11Z,14Z,16E-eicosapentaenoic acid, HPLC: high-performance liquid chromatography, LOX: lipoxygenase, PMN: polymorphonuclear neutrophils, RvE1: resolvin E1, 5S, 12R, 18R-trihydroxy-6Z,8E,10E,14Z,16E-eicosapentaenoate, RvE2: resolvin E2, 5S,18-dihydroxy-6E,8Z,11Z,14Z,16E-eicosapentaenoate, RvD1: resolvin D1, 7S,8R,17S-trihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid, RvD2: resolvin D2, 7S,16R,17S-trihydroxy-4Z,8E,10Z,12E,14E,19Z-docosahexaenoic acid, SPM, specialized pro-resolving mediator
Conflict of Interest Disclosure
CNS is an inventor on patents [resolvins] assigned to BWH and licensed to Resolvyx Pharmaceuticals. CNS is a scientific founder of Resolvyx Pharmaceuticals and owns equity in the company. CNS’ interests were reviewed and are managed by the Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies.
References
- 1.Kumar V, Abbas AK, Fausto N, Robbins SL, Cotran RS. Robbins and Cotran pathologic basis of disease. Elsevier Saunders; Philadelphia: 2005. [Google Scholar]
- 2.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 3.Libby P, Okamoto Y, Rocha VZ, Folco E. Inflammation in atherosclerosis: transition from theory to practice. Circ J. 2010;74:213–220. doi: 10.1253/circj.cj-09-0706. [DOI] [PubMed] [Google Scholar]
- 4.Stoll G, Kleinschnitz C, Nieswandt B. Combating innate inflammation: a new paradigm for acute treatment of stroke? Ann N Y Acad Sci. 2010;1207:149–154. doi: 10.1111/j.1749-6632.2010.05730.x. [DOI] [PubMed] [Google Scholar]
- 5.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawrence T, Gilroy DW. Chronic inflammation: a failure of resolution? Int J Exp Pathol. 2007;88:85–94. doi: 10.1111/j.1365-2613.2006.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, Hong S, Serhan CN. Molecular circuits of resolution: formation and actions of resolvins and protectins. J Immunol. 2005;174:4345–4355. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- 9.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 10.Spite M, Serhan CN. Novel lipid mediators promote resolution of acute inflammation: impact of aspirin and statins. Circ Res. 2010;107:1170–1184. doi: 10.1161/CIRCRESAHA.110.223883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilroy DW. Eicosanoids and the endogenous control of acute inflammatory resolution. Int J Biochem Cell Biol. 2010;42:524–528. doi: 10.1016/j.biocel.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Campbell EL, MacManus CF, Kominsky DJ, Keely S, Glover LE, Bowers BE, Scully M, Bruyninckx WJ, Colgan SP. Resolvin E1-induced intestinal alkaline phosphatase promotes resolution of inflammation through LPS detoxification. Proc Natl Acad Sci U S A. 2010;107:14298–14303. doi: 10.1073/pnas.0914730107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasturk H, Kantarci A, Ohira T, Arita M, Ebrahimi N, Chiang N, Petasis NA, Levy BD, Serhan CN, Van Dyke TE. RvE1 protects from local inflammation and osteoclast- mediated bone destruction in periodontitis. FASEB J. 2006;20:401–403. doi: 10.1096/fj.05-4724fje. [DOI] [PubMed] [Google Scholar]
- 14.Yamada T, Tani Y, Nakanishi H, Taguchi R, Arita M, Arai H. Eosinophils promote resolution of acute peritonitis by producing proresolving mediators in mice. FASEB J. 2011;25:561–568. doi: 10.1096/fj.10-170027. [DOI] [PubMed] [Google Scholar]
- 15.Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, Petasis NA, Serhan CN. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci U S A. 2010;107:1660–1665. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong S, Porter TF, Lu Y, Oh SF, Pillai PS, Serhan CN. Resolvin E1 metabolome in local inactivation during inflammation-resolution. J Immunol. 2008;180:3512–3519. doi: 10.4049/jimmunol.180.5.3512. [DOI] [PubMed] [Google Scholar]
- 17.Oh SF, Pillai PS, Recchiuti A, Yang R, Serhan CN. Pro-resolving actions and stereoselective biosynthesis of 18S E-series resolvins in human leukocytes and murine inflammation. J Clin Invest. 2011;121:569–581. doi: 10.1172/JCI42545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tjonahen E, Oh SF, Siegelman J, Elangovan S, Percarpio KB, Hong S, Arita M, Serhan CN. Resolvin E2: identification and anti-inflammatory actions: pivotal role of human 5-lipoxygenase in resolvin E series biosynthesis. Chem Biol. 2006;13:1193–1202. doi: 10.1016/j.chembiol.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Ogawa S, Urabe D, Yokokura Y, Arai H, Arita M, Inoue M. Total synthesis and bioactivity of resolvin E2. Org Lett. 2009;11:3602–3605. doi: 10.1021/ol901350g. [DOI] [PubMed] [Google Scholar]
- 20.Yang R, Chiang N, Oh SF, Serhan CN. Metabolomics-lipidomics of eicosanoids and docosanoids generated by phagocytes. Curr Protoc Immunol. 2011;95:14.26.11–14.26.26. doi: 10.1002/0471142735.im1426s95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dona M, Fredman G, Schwab JM, Chiang N, Arita M, Goodarzi A, Cheng G, von Andrian UH, Serhan CN. Resolvin E1, an EPA-derived mediator in whole blood, selectively counterregulates leukocytes and platelets. Blood. 2008;112:848–855. doi: 10.1182/blood-2007-11-122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter pro-inflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kasuga K, Yang R, Porter TF, Agrawal N, Petasis NA, Irimia D, Toner M, Serhan CN. Rapid appearance of resolvin precursors in inflammatory exudates: novel mechanisms in resolution. J Immunol. 2008;181:8677–8687. doi: 10.4049/jimmunol.181.12.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Irimia D, Liu SY, Tharp WG, Samadani A, Toner M, Poznansky MC. Microfluidic system for measuring neutrophil migratory responses to fast switches of chemical gradients. Lab Chip. 2006;6:191–198. doi: 10.1039/b511877h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carbo C, Duerschmied D, Goerge T, Hattori H, Sakai J, Cifuni SM, White GC, 2nd, Chrzanowska-Wodnicka M, Luo HR, Wagner DD. Integrin-independent role of CalDAG-GEFI in neutrophil chemotaxis. J Leukoc Biol. 2010;88:313–319. doi: 10.1189/jlb.0110049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taketo M, Schroeder AC, Mobraaten LE, Gunning KB, Hanten G, Fox RR, Roderick TH, Stewart CL, Lilly F, Hansen CT, Overbeek PA. FVB/N: An inbred mouse strain preferable for transgenic analyses. Proc Natl Acad Sci USA. 1991;88:2065–2069. doi: 10.1073/pnas.88.6.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knott ML, Hogan SP, Wang H, Matthaei KI, Dent LA. FVB/N mice are highly resistant to primary infection withNippostrongylus brasiliensis. Parasitology. 2009;136:93–106. doi: 10.1017/S0031182008005192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mähler M, Janke C, Wagner S, Hedrich HJ. Differential susceptibility of inbred mouse strains to Helicobacter pylori infection. Scand J Gastroenterol. 2002;37:267–278. doi: 10.1080/003655202317284165. [DOI] [PubMed] [Google Scholar]
- 29.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol. 2007;178:3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- 31.Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fiore S, Maddox JF, Perez HD, Serhan CN. Identification of a human cDNA encoding a functional high affinity lipoxin A4 receptor. J Exp Med. 1994;180:253–260. doi: 10.1084/jem.180.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohira T, Arita M, Omori K, Recchiuti A, Van Dyke TE, Serhan CN. Resolvin E1 receptor activation signals phosphorylation and phagocytosis. J Biol Chem. 2009;285:3451–3461. doi: 10.1074/jbc.M109.044131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong S, Tjonahen E, Morgan EL, Lu Y, Serhan CN, Rowley AF. Rainbow trout (Oncorhynchus mykiss) brain cells biosynthesize novel docosahexaenoic acid-derived resolvins and protectins-Mediator lipidomic analysis. Prostaglandins Other Lipid Mediat. 2005;78:107–116. doi: 10.1016/j.prostaglandins.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Oh SF, Vickery TW, Serhan CN. Chiral lipidomics of E-series resolvins: aspirin and the biosynthesis of novel mediators. Biochim Biophys Acta. 2011;1811:737–747. doi: 10.1016/j.bbalip.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Serhan CN, Chiang N. Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus. Br J Pharmacol. 2008;153(Suppl 1):S200–S215. doi: 10.1038/sj.bjp.0707489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hung ND, Kim MR, Sok DE. Oral administration of 2-docosahexaenoyl lysophosphatidylcholine displayed anti-inflammatory effects on zymosan A-induced peritonitis. Inflammation. 2011;34:147–160. doi: 10.1007/s10753-010-9218-z. [DOI] [PubMed] [Google Scholar]
- 38.Krishnamurthy VR, Dougherty A, Haller CA, Chaikof EL. Total synthesis and bioactivity of 18R-hydroxy eicosapentaenoic acid. J Org Chem. 2011;76:5433–5437. doi: 10.1021/jo2002243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Psychogios N, Hau DD, Peng J, Guo AC, Mandal R, Bouatra S, Sinelnikov I, Krishnamurthy R, Eisner R, Gautam B, Young N, Xia J, Knox C, Dong E, Huang P, Hollander Z, Pedersen TL, Smith SR, Bamforth F, Greiner R, McManus B, Newman JW, Goodfriend T, Wishart DS. The human serum metabolome. PLoS One. 2011;6:e16957. doi: 10.1371/journal.pone.0016957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raatz SK, Golovko MY, Brose SA, Rosenberger TA, Burr GS, Wolters WR, Picklo MJ., Sr Baking reduces prostaglandin, resolvin, and hydroxy-fatty acid content of farm-raised Atlantic salmon (Salmo salar) J Agric Food Chem. 2011;59:11278–11286. doi: 10.1021/jf202576k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Caterina R. n-3 fatty acids in cardiovascular disease. N Engl J Med. 2011;364:2439–2450. doi: 10.1056/NEJMra1008153. [DOI] [PubMed] [Google Scholar]
- 42.Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O’Neill LAJ, Perretti M, Rossi AG, Wallace JL. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007;21:325–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Serhan CN. The resolution of inflammation: the devil in the flask and in the details. FASEB J. 2011;25:1441–1448. doi: 10.1096/fj.11-0502ufm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maderna P, Yona S, Perretti M, Godson C. Modulation of phagocytosis of apoptotic neutrophils by supernatant from dexamethasone-treated macrophages and annexin-derived peptide Ac(2–26) J Immunol. 2005;174:3727–3733. doi: 10.4049/jimmunol.174.6.3727. [DOI] [PubMed] [Google Scholar]
- 45.Perretti M, Dalli J. Exploiting the Annexin A1 pathway for the development of novel anti-inflammatory therapeutics. Br J Pharmacol. 2009;158:936–946. doi: 10.1111/j.1476-5381.2009.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Colgan SP, Eltzschig HK. Adenosine and hypoxia-inducible factor signaling in intestinal injury and recovery. Annu Rev Physiol. 2012;74:7.1–7.23. doi: 10.1146/annurev-physiol-020911-153230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haskó G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7:759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosenberger P, Schwab JM, Mirakaj V, Masekowsky E, Mager A, Morote-Garcia JC, Unertl K, Eltzschig HK. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat Immunol. 2009;10:195–202. doi: 10.1038/ni.1683. [DOI] [PubMed] [Google Scholar]
- 49.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Campbell EL, Serhan CN, Colgan SP. Antimicrobial aspects of inflammatory resolution in the mucosa: a role for proresolving mediators. J Immunol. 2011;187:3475–3481. doi: 10.4049/jimmunol.1100150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Recchiuti A, Krishnamoorthy S, Fredman G, Chiang N, Serhan CN. MicroRNAs in resolution of acute inflammation: identification of novel resolvin D1- miRNA circuits. FASEB J. 2011;25:544–560. doi: 10.1096/fj.10-169599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.