Abstract
Basophils play a key role in the development and effector phases of type 2 immune responses in both allergic diseases and helminth infections. This study shows that basophils become less responsive to IgE-mediated stimulation when mice are chronically infected with Litomosoides sigmodontis, a filarial nematode, and Schistosoma mansoni, a blood fluke. Although excretory/secretory products from microfilariae of L. sigmodontis suppressed basophils in vitro, transfer of microfilariae into mice did not result in basophil suppression. Rather, reduced basophil responsiveness, which required the presence of live helminths, was found to be dependent on host IL-10 and was accompanied by decreases in key IgE signaling molecules known to be downregulated by IL-10. Given the importance of basophils in the development of type 2 immune responses, these findings help explain the mechanism by which helminths protect against allergy and may have broad implications for understanding how helminth infections alter other disease states in people.
Introduction
Like allergic diseases, helminths induce type 2 immune responses characterized by eosinophilia, elevated IgE levels, and CD4+ T-cell production of IL-4, IL-5, and IL-13. In people with infrequent exposures to helminths, this immune response is often associated with allergic symptoms such as rash and pruritus during acute infection (1). Over time, though, allergic symptoms in patients chronically infected with helminths appear to decrease (1). The filarial nematode Loa loa, for example, causes less angioedema, hives, and itching in chronically-infected individuals living in endemic regions than in travelers with infections of shorter duration (2, 3). In addition to decreased allergic responses to the infection itself, chronic helminth infections are associated with decreased allergic responses to environmental allergens (4–9). While several studies have investigated the mechanisms by which chronic helminth infection may suppress allergic reactivity, to date no studies have evaluated the effect chronic helminth infection has on basophil responsiveness. Like mast cells, basophils function as acute effector cells in allergic disease, releasing pre-formed histamine and other inflammatory mediators within minutes of allergen exposure, primarily through cross-linking of allergen-specific IgE molecules bound to high affinity IgE receptors on the cell surface.
In addition to being effector cells of allergic disease, basophils also help development of type 2 immune responses (10–15). IL-4 is a principal driver of type 2 immunity, and studies have shown basophils to be major contributors of IL-4 in helminth and allergic diseases (16–20). Studies conducted in mice depleted of or deficient in basophils have found decreased type 2 immune responses in both allergy and helminth models (19, 21–26).
As chronic helminth infections are associated with decreases in allergic manifestations, and as basophils play important roles in the development and effector stages of allergy, in this study we tested the hypothesis that chronic helminth infection suppresses basophil responsiveness to IgE-mediated stimulation.
Materials and Methods
Animals and parasites
Female BALB/c mice (NCI Mouse Repository, Frederick, MD), BALB/c IL-10 deficient mice (Jackson Laboratory, Bar Harbor, ME), and BALB/c JH−/− mice, (Taconic, Hudson, NY) were housed at The Uniformed Services University (USU) Center for Laboratory Animal Medicine. Blood used for basophil activation studies was obtained by retroorbital bleed. At study endpoints, mice were euthanized with carbon dioxide. All experiments were performed under protocols approved by the USU Institutional Animal Care and Use Committee.
For Litomosoides sigmodontis infections, L3-stage larvae were obtained by pleural lavage of jirds (Meriones unguiculatus, obtained from TRS Laboratory Inc., Athens, GA) that had been infected 4 days earlier by the bite of infectious mites as previously described (27). Forty L3-stage larvae were collected in RPMI and injected subcutaneously in the dorsal neck region into female BALB/c mice that were 5 to 12 weeks of age.
For Schistosoma mansoni infections, 6 to 10 week old female BALB/c mice were infected by exposing the tails to 30 – 40 Schistosoma mansoni cercariae (Puerto Rican strain) obtained from infected Biomphalaria glabrata snails provided by Fred Lewis (Biomedical Research Institute, Rockville, MD).
Parasite antigens
L. sigmodontis worm antigen (LsAg) and S. mansoni worm antigen (SWAP) were prepared from the PBS-soluble fraction of homogenized male and female adult worms harvested from infected animals as previously described (26, 28).
For worm excretory/secretory (E/S) products, adult male worms, adult female worms, and microfilariae were cultured separately in RPMI 1640 supplemented with HEPES, penicillin/streptomycin and glucose. Supernatants from cultures were collected every 24 hours while worms were still living. E/S products from each day were pooled and concentrated using 3,000 Kda MWCO Amicon Ultra centrifugal filter units (Millipore) and protein concentration measured using a BCA protein assay kit (Pierce, Rockford, IL). Concentrated ES products were stored at −20°C until use.
LsAg sensitization, L. sigmodontis implantation and microfilariae injection
Mice 10 weeks of age were sensitized to LsAg by 3 intraperitoneal injections of 100 μg LsAg adsorbed to Imject Alum Adjuvant (Thermo Scientific). Injections were spaced 2 weeks apart. Three weeks after the last injection, adult worms for implantation and microfilariae were obtained from jirds that had been infected with L. sigmodontis for more than 8 weeks. After euthanizing infected jirds, adult worms were removed from the pleural cavity using dissecting probes and placed in RPMI to determine sex. Five to seven male worms or five to seven microfilaria-producing female worms were surgically implanted into the peritoneal cavity of anesthetized mice as previously described (29). Microfilariae were purified from the blood by diluting peripheral blood 1:2 with RPMI 1640 containing 25mM HEPES and 2.05mM glutamine (Mediatech, Inc.). Diluted blood was layered on top of a 25% and 30% percoll/sucrose isosmotic solution and centrifuged for 35 minutes at 400×g. Microfilariae were washed with RPMI to remove any remaining percoll. Fifty thousand microfilariae were then injected into the jugular vein of LsAg-sensitized mice. After 2 weeks, blood was obtained from mice, plasma was removed and basophil activation was measured using flow cytometry.
Antibodies
The following antibodies were used to assess basophil activation by flow cytometry: anti-CD45b/B220 PerCP (RA3-6B2), anti-CD4 PerCP (RM4–5), anti-IgE FITC (R35–72), anti-IL-4 APC (11B11), anti-CD45 FITC (30-F11), anti-CD49b PE (HM ALPHA2) and anti-CD200R PE (OX-110). Anti-IgE (R35–72) and anti-FcεRIα (MAR-1) were used for stimulation. Anti-DNP IgE (SPE-7) used to boost basophil surface IgE levels was purchased from Sigma-Aldrich. Anti-FcεRIα (MAR-1) was purchased from eBioscience, anti-CD200R PE (OX-110) was purchased from AbD SeroTec and all other antibodies were purchased from BD Biosciences.
Antibodies used to measure expression levels of basophil signaling molecules by flow cytometry: anti-Akt AF647 (5G3), anti-PKC-α, anti-phospho-Akt PE (D9E), anti-phospho-STAT5 AF647 (C71E5), and anti-rabbit F(ab') AF647 all purchased from Cell Signaling Technology. Anti-FYN PE, anti-Syk PE, anti-STAT5 and FcγRIIb/CD16-2 (2.4G2) blocking Ab purchased from Santa Cruz Biotechnology, Inc.
Antibodies used for mouse IgE ELISAs: anti-mouse IgE (R35–72) for coating, mouse IgE (27–74) used as the standard and biotinylated anti-mouse IgE (R35–118) for detection were all purchased from BD Biosciences.
Measuring Basophil Activation by Flow Cytometry
Basophil activation was determined as previously described (26, 30) with minor modifications. Briefly, after collecting whole blood in heparinized tubes, blood was centrifuged and plasma was removed. Cells were washed twice with RPMI to ensure complete removal of plasma. After resuspending blood cells back to original volume using RPMI, cells were diluted 1:2 with RPMI and aliquoted 200 μl per condition. Diluted blood cells were stimulated with media alone, 1 μg/ml ionomycin (Calbiochem) or several concentrations of either anti-IgE (0.0005, 0.002, 0.0078, 0.031, 0.125 and 0.5 μg/ml) or anti-FcεRIα (0.4, 1.6, 6.25, 25 and 100 μg/ml) for 45 minutes before adding GolgiStop (containing monensin, BD Biosciences). Stimulation proceeded for 2 more hours. At the end of the stimulation period, cells were washed with PBS, RBCs were lysed and remaining cells fixed using a whole blood lysing reagent kit (Beckman Coulter). Cells were resuspended in 1% bovine serum albumin (BSA, Cohn V fraction, Sigma)/PBS at 4°C overnight, and then surface stained with anti-IgE FITC, anti-CD4 PERCP, anti-B220 PERCP and, when CD200R was also evaluated, anti-CD200R PE. Cells were subsequently washed, permeabilized with Perm/Wash buffer (BD Biosciences), and then stained with anti-IL-4 APC. Cells were analyzed using a BD LSRII flow cytometer and FACSDiva software (BD Biosciences). In figure 1B, CD200R was measured without simultaneously measuring intracellular IL-4. For these assays, no GolgiStop was added and total stimulation time was reduced to 2 hours. Cut-off gates for CD200R-PE and IL-4-APC positivity were established using the fluorescence-minus-one (FMO) technique.
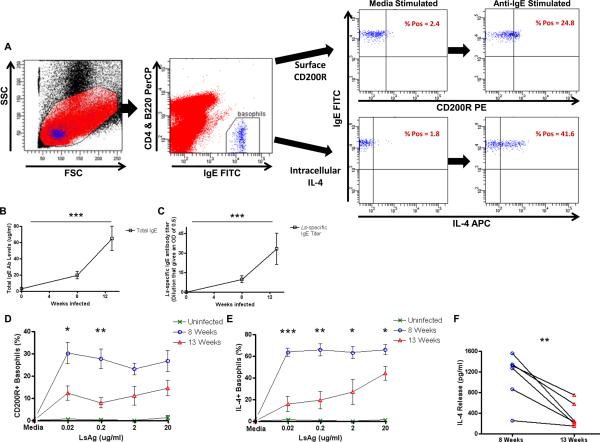
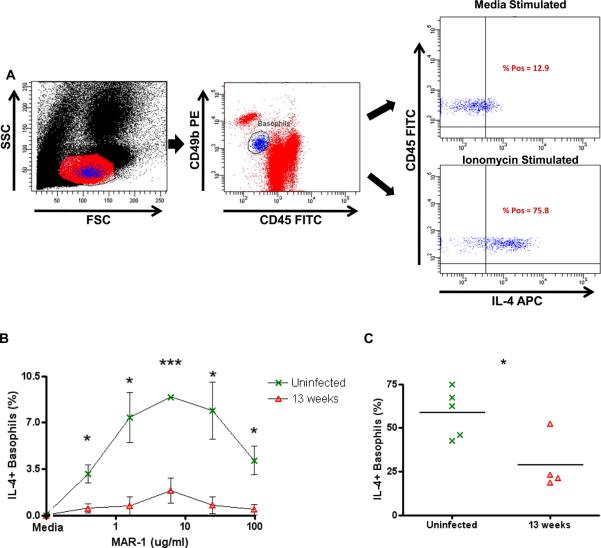
FIGURE 1.
Responsiveness of basophils from L. sigmodontis-infected mice to LsAg stimulation.
(A) Gating strategy for flow cytometric assessment of basophil activation. Initial gating by forward and side scatter (left panel). Basophils identified as CD4-B220-IgE+ peripheral blood cells (2nd panel). Right panels demonstrate CD200R and IL-4 staining after incubation of whole blood with media and 0.031 μg/ml anti-IgE. Levels of total IgE (B) and LsAg-specific IgE (C) were measured in the plasma of uninfected (n = 6), 8 week (n = 6), and 13 week (n = 6) L. sigmodontis-infected BALB/c mice. Percentages of basophils that stain positively for CD200R (D) and IL-4 (E) after stimulation with several concentrations of LsAg as measured by flow cytometry and after subtracting media levels. Each data point represents the mean of at least three independent experiments (statistically significant differences are shown between 8 week and 13 week-infected mice). (F) Supernatant IL-4 concentrations from whole blood after stimulation with 2 μg/ml LsAg for 2 hours (F). (* p < 0.05, ** p < 0.01, *** p < 0.001).
Measuring signaling molecules by flow cytometry
Blood was obtained from mice chronically infected with L. sigmodontis and age-matched uninfected controls, red blood cells were lysed and leukocytes were immediately fixed using a whole blood lysing kit (Beckman Coulter). After washing twice with PBS, cells were resuspended with 1% BSA/PBS with FcγRIIb/CD16-2 (2.4G2) blocking Ab and incubated overnight. Surface staining was performed by adding anti-IgE FITC (R35–72), anti-CD45b/B220 PerCP (RA3-6B2) and anti-CD4 PerCP (RM4–5) and incubating for 30 minutes at 4°C. After washing cells twice with PBS, they were permeabilized with Perm/Wash buffer (BD Biosciences). Antibodies to signaling molecules were added to permeabilized cells and incubated for 30 minutes at 4°C. Cells were washed 2 times with PBS before using an anti-rabbit secondary antibody for the detection of anti-stat5 and anti-PKC-α antibodies. After staining, cells were washed 2 times with PBS and fluorescence analyzed using a LSRII flow cytometer and FACSDiva 6.1.3 software (BD Biosciences). Titrations were performed to determine optimum concentrations of each antibody and BD CompBead compensation beads (BD Biosciences) were used for all colors to account for spectral overlap. Nonspecific binding of anti-rabbit secondary antibody was subtracted from MFIs obtained for stat5 and PKC-α expression. Infected and control samples were assayed at the same time to eliminate interday variability.
Worm-specific and total IgE ELISAs
ELISAs were performed using Costar half-area, high binding plates. IgG was removed from plasma using GammaBind Plus sepharose (GE Healthcare). For worm-specific IgE ELISAs, plates were coated with 20 μg/ml LsAg or SWAP diluted in PBS and incubated overnight at 4°C. Plates were blocked with 5% BSA/PBS plus 0.05% tween. Plasma was added to the plate in duplicate at a starting dilution of 1:4, followed by further 3-fold dilutions made in 1% BSA/PBS plus 0.05% tween. Bound IgE antibody was detected by adding biotinylated anti-mouse IgE diluted in 1% BSA/PBS plus 0.05% tween, followed by adding AP-conjugated streptavidin that was diluted 1:1000 in 1% BSA/PBS plus 0.05% tween. Plates were developed by the addition of 4-nitrophenyl phosphate disodium (Sigma-Aldrich) in 0.1M carbonate buffer to the plate. Absorbances were read at 405nm using a Victor3 V microplate reader from Perkin Elmer. Titers were calculated as the dilution that would yield an OD of 0.5 calculated by SoftMax Pro 4.6 (Molecular Devices, Inc). For total IgE measurements, plates were coated with 10 μg/ml anti-IgE in PBS instead of worm antigen. All subsequent steps were identical to the worm-specific IgE ELISA. Total IgE concentration was determined from the IgE standard curve using WorkOut 2.0 ELISA software (Perkin Elmer).
Passive sensitization with DNP-specific IgE
Mice were injected with 30 μg anti-DNP IgE intravenously and with 50 μg intraperitoneally two times. Injections were two weeks apart. Blood was obtained 5 days after last injection, plasma was removed and basophil activation assays by flow cytometry were performed.
Splenocyte and Whole Blood Cultures
For splenocyte cultures, after obtaining single cell suspensions of splenocytes from mice chronically infected with L. sigmodontis or age-matched uninfected controls, red blood cells were lysed with ACK lysis buffer (Quality Biological, Inc.) and cells were plated in Iscove's Dulbecco modified medium (Mediatech) containing 10% fetal calf serum (Valley Biomedical), 1% L-glutamine (Mediatech), 1% insulin-transferrin-selenium medium (Invitrogen Inc.) and 80 μg/ml gentamicin (Quality Biological, Inc.) at a concentration of 2×106/ml and were incubated with either media, 0.05 μg/ml PMA (Sigma-Aldrich) with 1 μg/ml ionomycin (Calbiochem), 5 μg/ml anti-CD3 with 2 μg/ml anti-CD28 (both from eBioscience) or 20 μg/ml LsAg and cultured at 37°C, 5% CO2, After 3 days, culture supernatants were collected and stored at −80°C until IL-10 ELISAs were performed.
For whole blood cultures, after removing plasma, blood cells were thoroughly washed 2 times with RPMI, and aliquoted 500ul per condition into 48-well cell culture plates (Corning Incorporated). Blood was stimulated with media and several concentrations of anti-IgE (0.0001, 0.0005, 0.002, 0.0078, 0.031 and 0.125 μg/ml) for 2 hours at 37°C, 5% CO2. Samples were centrifuged at 400×g for 5 minutes, supernatants collected and stored at −80 °C until IL-4 ELISAs were performed.
For whole blood cultures with worm E/S products, plasma from mouse whole blood was removed and whole blood cells were incubated with 30 μg/ml E/S products or BSA diluted in RPMI for 7 hours. Blood containing E/S products was then diluted 1:2 from the original blood volume and aliquoted 200 μl per condition. Basophil activation was performed using flow cytometry as described earlier.
Mouse IL-10 and IL-4 ELISAs
IL-10 and IL-4 ELISAs were performed using Costar half-area, high binding plates. Mouse IL-10 BD OptEIA ELISA kits and OptEIA ELISA reagents were purchased from BD Biosciences. Mouse IL-4 ELISAs were purchased from eBiosciences, Inc. ELISAs were performed using manufacturer's reagents following the protocols provided with each kit.
In Vivo Treatment with rIL-10
WT mice were IP injected twice daily with 2 μg rIL-10 (GenScript) in 100 μl of PBS or PBS alone for 5 days as previously described (31). Whole blood was obtained from injected mice and stimulated with anti-IgE and basophil activation was assessed using flow cytometry as described earlier.
In Vivo IL-10 Cytokine Capture
Using the Mouse IL-10 In Vivo Capture Assay Set (BD Biosciences), WT mice were injected with 10 μg/ml biotin-anti-IL-10 0, 2, 13, or 22 weeks after infection with L. sigmodontis. Twenty hours after injecting b-anti-IL-10 Ab, plasma was collected and stored at −80 until all timepoints were collected. IL-10 ELISA was performed according to manufacturer's instructions.
Statistical Analyses
All statistics were performed using GraphPad Prism 4.03. Statistical significance between multiple groups was determined using the Kruskal-Wallis test, followed by Dunn's multiple comparison post test. Either paired or unpaired 2-tailed Student's t test was used for comparisons between two groups. For basophil activation curves when more than two groups were compared, one-way ANOVA was performed for each stimulation concentration, followed by Bonferroni correction. For basophil activation curves when only two groups were compared, 2-tailed Student's t test was performed for each stimulation concentration. For all tests, p-values less than 0.05 were considered significant.
Results
Basophils from mice chronically infected with Litomosoides sigmodontis are less responsive to LsAg than basophils from acutely infected mice
To determine whether basophils become less responsive to worm antigen over the course of a filarial infection, BALB/c mice were infected with 40 L3-stage L. sigmodontis larvae. After obtaining whole blood from uninfected, acutely infected, and chronically infected mice, plasma was removed from blood cells by multiple washes. Blood cells were then stimulated with several concentrations of LsAg in order to develop dose-response basophil activation curves to LsAg. Basophil activation was determined by measuring surface CD200R expression and intracellular IL-4 production by flow cytometry (Fig. 1A). Consistent with prior work we have done (26), total and LsAg-specific IgE levels are low and undetectable, respectively, in uninfected animals (Figs. 1B, 1C). LsAg-specific IgE levels are measurable by 8 weeks of infection, and levels of both total and LsAg-specific IgE increase throughout the course of infection.
One of the principal mechanisms of basophil activation is by aggregation of surface high affinity IgE receptors (FcεRI), in response to cross-linking of receptor-bound IgE antibodies by specific antigen. As expected, basophils from uninfected animals, which lack specific antibodies against LsAg, do not become activated when stimulated with LsAg (Figs. 1D, 1E). The highest percentage of basophils activated in response to LsAg was observed in acutely infected mice, in which a maximal percentage of basophils became activated even at the lowest LsAg concentration tested (Figs. 1D, 1E). In contrast, basophils from chronically infected mice never achieved the same frequencies of activation, even at the highest LsAg concentration tested. Results were consistent when measuring basophil activation by either surface CD200R or intracellular IL-4 expression (Figs. 1D, 1E). Data shown for both CD200R and IL-4 demonstrates activation above basal expression levels because CD200R staining exhibited some variability when conducted on different days. For all experiments done with wild-type mice in this study, basal IL-4 expression in basophils demonstrated very little interday variability, was consistently low, and was not different between infected and uninfected groups.
Similar results were obtained when the data was analyzed by evaluating changes in CD200R and IL-4 expression on a per cell basis by MFI (Supplemental Figs. 1A, 1B). These result suggest that not only do greater percentages of basophils become activated in response to LsAg during the acute stage of infection, but that the amplitude of activation of individual basophils is greater at eight weeks than at thirteen weeks. Interestingly, the decrease in basophil responsiveness to LsAg at thirteen weeks occurred despite the fact that more Ls-specific IgE is present at that timepoint (Fig. 1C).
To confirm that the changes observed by flow cytometry reflected actual changes in basophil functionality, we assessed IL-4 release from basophils by measuring IL-4 in supernatants of whole blood cells after 2 hour in vitro stimulation with LsAg. As seen in figure 1F, there is a significant decrease in IL-4 release from whole blood of chronically infected mice after LsAg stimulation as compared to acutely infected mice (p = 0.008, Fig. 1F). Since basophils are the only circulating cells capable of releasing IL-4 within 2 hours of activation (26, 32), and as the percentages of blood cells that were basophils was equivalent at 8 and 13 weeks post-infection(1.1% vs. 0.9%, p = 0.133, data not shown), these results confirm that basophil responsiveness to worm antigen is suppressed in chronic L. sigmodontis infection.
Basophils from chronically infected mice are less responsive to anti-IgE stimulation than basophils from acutely infected mice and uninfected mice
To determine whether reduced basophil responsiveness was an antigen-specific phenomenon or if overall basophil responsiveness becomes downmodulated by chronic filaria infection, basophil activation curves in response to increasing IgE cross-linking were assessed in uninfected, acutely infected and chronically infected mice.
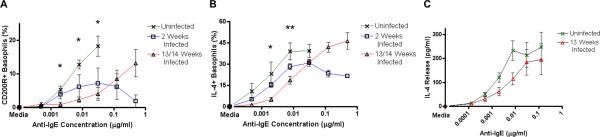
Basophils from chronically infected mice were less responsive to anti-IgE stimulation as measured by both surface CD200R (Fig. 2A) and intracellular IL-4 (Fig. 2B) expression. Significantly lower percentages of basophils from chronically infected mice expressed CD200R than uninfected mice in response to stimulation with 0.002, 0.0078 and 0.031 μg/ml anti-IgE (mean percentage of CD200R+ basophils from uninfected mice = 5.28, 12.78, 18.28 vs. 0.93, 2.33, 4.08 from chronically infected mice, p < 0.05 for all concentrations, Fig. 2A). Percentages of basophils expressing intracellular IL-4 were also significantly lower in chronically infected mice than uninfected mice when stimulating with 0.002 and 0.0078 μg/ml anti-IgE (mean percentage of IL-4+ basophils from uninfected mice = 23.12, 39.02 vs. 5.16, 18.63 from chronically infected mice, p < 0.05, p < 0.01, Fig. 2B). Basophils from two week infected mice demonstrated an intermediate basophil response phenotype, though the differences between these mice and uninfected mice were not statistically significant.
FIGURE 2.
Responsiveness of basophils from L. sigmodontis-infected mice to anti-IgE stimulation.
Percentages of basophils that stain positively for CD200R (A) and IL-4 (B) in response to increasing concentrations of anti-IgE as assessed by flow cytometry after subtracting media stimulation levels (statistical significance between uninfected and 13/14 week infected groups). (C) Supernatant IL-4 concentrations from whole blood after 2 hour stimulation with several concentrations of anti-IgE (C). Each data point represents the mean of at least four independent experiments. (* p < 0.05, ** p < 0.01).
This right shift in basophil activation curves was also observed when measuring IL-4 release from whole blood stimulated with anti-IgE (Fig. 2C), confirming the flow cytometric data. Given that blood of 13 week-infected mice had on average 1.5–2 times as many basophils as uninfected animals, the decreased IL-4 release from blood of chronically infected animals reflects a substantial decrease in basophil responsiveness.
Basophils from mice chronically infected with Schistosoma mansoni are less responsive to SWAP and anti-IgE stimulation than basophils from acutely infected and uninfected mice
To determine whether basophil suppression is filaria-specific or a phenomenon that occurs with other helminthiases, we evaluated basophil responses in mice infected with Schistosoma mansoni, a blood fluke that establishes chronic infection in BALB/c mice. Since a prior study with Schistosoma mansoni infection in mice demonstrated that it takes 16 weeks for Th2 CD4+ T-cells to develop substantial hyporesponsiveness (33), mice were infected for 16 weeks before performing basophil activation assays.
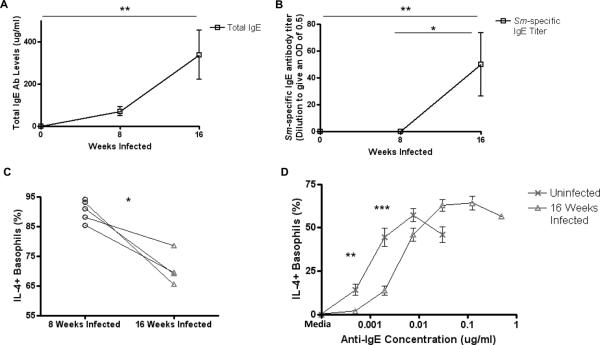
The effect of Schistosoma mansoni infection on basophil functionality mirrored that of L. sigmodontis. As with L. sigmodontis, levels of total and parasite-specific IgE were greater in chronic infection than at the 8 week timepoint (Figs. 3A, 3B). Despite the higher specific IgE concentration at 16 weeks, basophils of chronically infected mice became less activated in response to SWAP than basophils of 8-week infected mice (p = 0.017, Fig. 3C). Additionally, basophil activation curves of 16 week infected animals were significantly right-shifted in comparison to those of uninfected controls after stimulating with anti-IgE (mean percentage of IL-4+ basophils after stimulation with 0.0005 and 0.002 μg/ml anti-IgE from uninfected mice = 14.1, 44.5 vs. 1.9, 13.6 from chronically infected mice, p = 0.008, p = 0.0007, Fig. 3D).
FIGURE 3.
Responsiveness of basophils from Schistosoma mansoni-infected mice.
Levels of total IgE (A) and SWAP-specific IgE (B) measured in the plasma of uninfected (n = 5), 8 week infected (n = 5) and 16 week infected (n = 5) BALB/c mice. Percentages of basophils that stain positively for IL-4 in response to 2 μg/ml SWAP (C) and to several concentrations of anti-IgE (D) from uninfected (n = 5) and 16 week infected (n = 5) mice after subtracting media stimulation levels. (* p < 0.05; ** p < 0.01; *** p < 0.001).
These data suggest that reduced basophil responsiveness may be a phenomenon that occurs in many chronic helminth infections.
Reduced basophil responsiveness is not due to increased levels of surface IgE
Since basophils from chronically helminth-infected mice have more IgE on their surface than basophils from uninfected (p < 0.001) and even acutely infected mice (Fig. 4A), we tested whether increased surface IgE levels can account for changes in basophil responsiveness to IgE cross-linking by anti-IgE antibody. In order to increase IgE levels on the surface of basophils, uninfected BALB/c mice were injected with an IgE monoclonal antibody to DNP and basophil responsiveness to anti-IgE stimulation was compared between IgE injected and control mice.
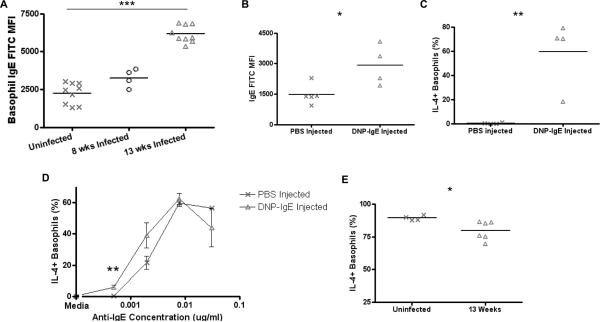
FIGURE 4.
Activation curves of basophils with different surface IgE levels.
(A) Relative surface IgE levels of uninfected, 8 week infected, and 13 week L. sigmodontis-infected BALB/c mice as assessed by mean fluorescence intensity staining of IgE on basophils. (B) Relative basophil surface IgE levels after injection of BALB/c mice with DNP IgE MAb, assessed by measuring IgE FITC MFI by flow cytometry. (C) Percentages of basophils from PBS-injected and DNP-IgE injected mice that stain positively for IL-4 after stimulation with 2 μg/ml DNP-HSA. (D) Percentages of basophils from PBS-injected (n = 5) and DNP IgE-injected (n = 4) mice that stain positively for IL-4 after stimulation with several concentrations of anti-IgE after subtracting media stimulation levels. (E) Activation of basophils from uninfected and 13 week L.s.-infected mice measured by flow cytometry after stimulation with 1 μg/ml ionomycin. (* p < 0.05, **, p < 0.01, *** p < 0.001).
Surface IgE levels were significantly increased in mice that were injected with DNP IgE (p = 0.024, Fig. 4B) and this IgE was functional and of sufficient quantity for basophils to become activated when stimulated with DNP-HSA (p = 0.0054, Fig. 4C). As seen in figure 4D, basophils with increased surface IgE levels were as sensitive to activation with anti-IgE, if not more so, than basophils from control mice (Fig. 4D).
Additionally, basophils from chronically infected mice became significantly less activated (p = 0.033) than basophils from uninfected mice when stimulated with ionomycin (Fig. 4E), a calcium ionophore that bypasses surface IgE to cause activation of the cell.
Together, these data indicate that decreases in basophil responsiveness during chronic Litomosoides infection are not due to increased surface IgE levels.
Repeated basophil activation through IgE receptors is not the cause of reduced basophil responsiveness
As previous studies have demonstrated that IgE-mediated activation of basophils reduces their responsiveness to subsequent IgE cross-linking events (34–36), we hypothesized that repeated antibody-mediated activation could be responsible for the induction of basophil hyporesponsiveness during chronic helminth infection. To test this, we measured basophil responsiveness to anti-FcεRIα and ionomycin stimulation in uninfected and chronically infected JH−/− mice. As JH−/− mice cannot generate worm-specific IgE or IgG during helminth infection (37), their basophils cannot undergo antibody mediated activation. Additionally, prior work we have conducted has shown that LsAg does not activate basophils in the absence of specific antibody (26). Because we could not use surface IgE staining as a marker for basophils in JH−/− mice, basophils were identified by flow cytometry as being CD49bhi and CD45lo (38) and then assessed for IL-4 expression by intracellular flow cytometry (Fig. 5A).
FIGURE 5.
Basophil responsiveness in L. sigmodontis-infected JH−/− BALB/c mice.
(A) Gating strategy for flow cytometric assessment of basophil activation in JH−/− BALB/c mice. Basophils identified as CD49bhi and CD45lo. Right panels demonstrate IL-4 staining after incubation of whole blood with media and 1 μg/ml ionomycin. (B) Percentages of basophils from uninfected and L. sigmodontis-infected JH−/− BALB/c mice that stain positive for IL-4 after stimulating with several concentrations of anti-FcεRIα after subtraction of media stimulation levels (n = 4 per data point) (C) Percentages of basophils from uninfected and 13 week infected JH−/− mice in response to 1 μg/ml ionomycin. (* p < 0.05, *** p < 0.001).
In contrast to studies done with wild type mice, infection of JH−/− mice increased baseline basophil IL-4 expression (mean percentage of IL-4+basophils after media stimulation = 19 % in 13 week infected JH−/− mice vs. 13 % in uninfected mice, p=0.01, data not shown). Stimulation above basal levels, however, remained suppressed in chronically infected mice. Comparing the basophil response curves to anti-FcεRIα stimulation from JH−/− chronically infected and age-matched uninfected controls revealed that basophils from chronically infected JH−/− mice still become less responsive to anti-FcεRIα when infected with L. sigmodontis despite the absence of repeated antibody-mediated activation (mean percentage of IL-4+ basophils after stimulation with 0.4, 1.6, 6.25, 25 and 100 μg/ml anti-FcεRIα from uninfected mice = 3.1, 7.4, 8.9, 7.9, 4.1 vs. 0.53, 0.7, 1.9, 1.9, 0.44 from chronically infected mice, p = 0.028, p = 0.035, p = 0.0003, p = 0.013, p = 0.013, Fig. 5B). Also, fewer basophils from chronically infected JH−/− mice became activated in response to ionomycin stimulation compared to basophils from uninfected JH−/− controls (mean percentage of IL4+ basophils after ionomycin stimulation = 28.9% vs. 58.6, p = 0.02, Fig. 5C).
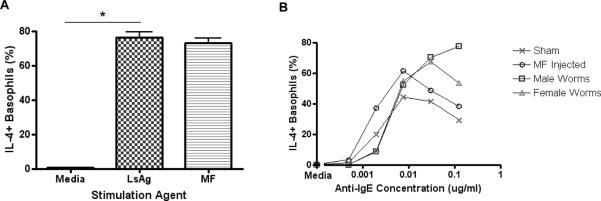
In a second experiment, BALB/c mice were sensitized to parasite antigen by three injections of LsAg adsorbed to alum. Functional evidence for specific antibody came from the observation that basophils of sensitized mice became activated when exposed to either LsAg (p < 0.05) or microfilarial antigen (Fig. 6A). To test whether basophil hyporesponsiveness is associated with chronic exposure of helminth-sensitized basophils to helminths, three weeks after sensitization mice had either adult male worms or adult female worms surgically implanted into the peritoneal cavity or had microfilariae injected into the jugular vein. Control mice had sham surgery with no worms implanted. After 14 days, blood was obtained to compare basophil responsiveness to anti-IgE stimulation. This timepoint was chosen because implantation of adult female worms, as well as direct injection of microfilariae, resulted in circulating microfilaremia for at least two weeks. Since basophil lifespan is approximately 2–3 days (39), and as even a single activation event can reduce basophil responsiveness (36), this duration should have been sufficient to observe suppressed activation curves if repeated activation was the primary mechanism decreasing basophil function.
FIGURE6.
Basophil responsiveness following adult worm implantation or microfilaria transfusion in LsAg-sensitized BALB/c mice.
BALB/c mice were sensitized to worm antigens by three injections of 100 μg LsAg+alum. (A) Percentages of basophils from LsAg-sensitized mice that are IL-4+ after stimulation with media, 2 μg/ml LsAg or 2 μg/ml microfilaria antigen three weeks after sensitization as assessed by flow cytometry. (B) Percentages of basophils that stain IL-4 positive after stimulation with several concentrations of anti-IgE as assessed by flow cytometry after subtracting media stimulation levels fourteen days after intraperitoneal implantation of 5–7 adult male worms, intraperitoneal implantation of 5–7 adult female worms, or intravenous injection of 50,000 microfilariae. Each data point represents the mean of four independent experiments. (* p < 0.05).
As seen in Figure 6B, after 14 days LsAg-sensitized basophils exposed to adult male and female worms had little or no reduced responsiveness to anti-IgE stimulation. LsAg-sensitized basophils exposed to microfilariae actually seemed to become slightly more responsive to anti-IgE stimulation (Fig. 6B).
These data indicate the reduced basophil responsiveness which develops in chronic helminth infection is not due to chronic antibody-mediated activation of basophils.
Incubation of basophils with high concentrations of microfilaria E/S products results in reduced basophil responsiveness, but in vivo microfilariae do not suppress basophil responsiveness
Since a previous study has demonstrated that a filarial excretory/secretory product from Acanthocheilonema vitae can suppress bone marrow derived mast cells (BMMCs) (40), we tested whether E/S products from L. sigmodontis could suppress basophils.
After incubating whole blood with E/S products from different life cycle stages of L. sigmodontis, basophils were stimulated with several concentrations of anti-IgE and basophil activation assessed by measuring IL-4 expression with intracellular flow cytometry.
No changes in basophil responsiveness to anti-IgE were observed after incubating whole blood with E/S products from adult male or adult female worms (Fig. 7A). However, basophil responsiveness to anti-IgE stimulation was reduced after incubating whole blood with E/S products from microfilariae (mean percentage of IL-4+ basophils after stimulation with 0.002 and 0.0078 μg/ml anti-IgE from whole blood incubated with BSA = 21.1, 33.5 vs. 3.4, 10.1 from whole blood incubated with microfilaria E/S products, p < 0.001, p < 0.01, Fig. 7A). To determine whether microfilariae were responsible for inducing basophil hyporesponsiveness in vivo, 50,000 microfilariae in RPMI were injected intravenously into BALB/c mice and, after 11 days, basophil responsiveness assessed by stimulating basophils with several concentrations of anti-IgE.
FIGURE 7.
Effects of L. sigmodontis Excretory/Secretory products on basophil responsiveness.
(A) E/S products from different life cycle stages of L. sigmodontis were incubated with whole blood containing basophils for 7 hours and then stimulated with several concentrations of anti-IgE (each data point represents mean of at least two independent experiments). Degree of basophil activation was assessed by measuring basophil IL-4 positivity by flow cytometry after subtracting media stimulation levels (only showing statistical significant difference between the BSA control and microfilaria E/S incubated conditions). (B) Percentages of basophils that stain positively for IL-4 by flow cytometry after subtracting media stimulation levels in response to several concentrations of anti-IgE eleven days after i.v. injection of 50,000 microfilairae in RPMI or RPMI only (n = 5 for each group). (C) Microfilaria levels in mice 8 weeks and 13 weeks after s.c. injection of 40 L3-stage larvae and 11 days after injection of 50,000 microfilairae (C). (** p < 0.01, *** p < 0.001).
Injection of microfilariae did not reduce basophil responsiveness to IgE cross-linking (Fig. 7B). In fact, basophils seemed more responsive to anti-IgE stimulation after microfilariae injection. Importantly, microfilaria counts when blood was obtained for basophil activation studies were equal to or higher than microfilaria counts from blood obtained from mice with chronic infections (Fig. 7C). Thus, the lack of basophil responsiveness was not due to insufficient numbers of microfilariae in the injection study. Further evidence that products secreted from microfilariae are not the main drivers of basophil hyporesponsiveness comes from the observation that in natural L. sigmodontis infections basophils are more suppressed at 13 weeks than 8 weeks (Figs. 1D, 1E), even though microfilaria counts are lower at 13 weeks than 8 weeks (Fig. 7C). In total, these results suggest that E/S products from microfilaria have the capability to suppress basophil responsiveness, but likely are not the main cause of basophil hyporesponsiveness in chronic helminth infection.
IL-10 generated during helminth infection is important for development of reduced basophil responsiveness
IL-10 is an anti-inflammatory cytokine that is produced during helminth infections and plays a role in immune and cellular hyporesponsiveness (31, 41–44). To test whether IL-10 generated during helminth infection was responsible for reduced basophil responsiveness, IL-10 deficient BALB/c mice were infected with L. sigmodontis and basophil responsiveness to anti-IgE, LsAg and ionomycin compared with uninfected IL-10 deficient BALB/c age-matched controls.
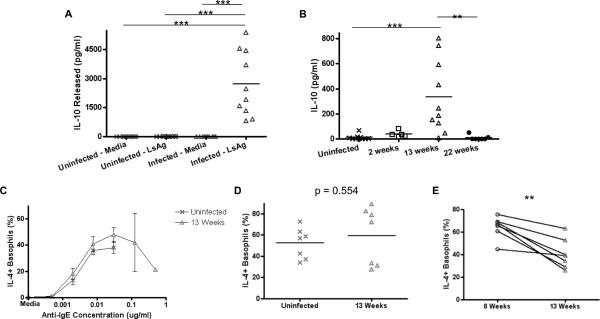
Whereas no splenocyte populations spontaneously released measurable quantities of IL-10, splenocytes from chronically infected WT BALB/c mice produced significant amounts of IL-10 (p < 0.001) in response to LsAg compared to WT uninfected controls (Fig. 8A). These splenocyte cultures also produced more IL-10 on average than cultures from uninfected mice in response to polyclonal activation (data not shown). No IL-10 was detected from splenocytes obtained from IL-10 deficient BALB/c mice under any stimulation conditions (data not shown). Using an in vivo cytokine capture kit confirmed that more circulating IL-10 was present in mice with chronic worm infections of 13 weeks duration than in uninfected mice, acutely infected mice (2 weeks), and mice that had cleared the infection (22 weeks) (Fig. 8B).
FIGURE 8.
Decreased basophil responsiveness to IgE-mediated stimulation is dependent on IL-10.
(A) IL-10 concentrations from supernatants of splenocytes from uninfected or 13 week infected WT BALB/c mice after stimulation with LsAg as measured by ELISA. (B) Circulating levels of IL-10 at different timepoints of infection as measured by in vivo cytokine capture assay. Percentages of basophils from uninfected (n = 7) and 13 week infected (n = 7) IL-10 deficient BALB/c mice that stain positively for IL-4 after stimulation with (C) several concentrations of anti-IgE, (D) ionomycin or (E) LsAg and after subtracting media stimulation levels. (* p < 0.05, ** p < 0.01, *** p < 0.001).
As seen in figures 8C and 8D, the reduced basophil responsiveness to IgE cross-linking and ionomycin stimulation previously observed in chronic helminth infections was abrogated in IL-10 deficient BALB/c mice infected with L. sigmodontis (Figs. 8C, 8D). Interestingly, basophils from chronically infected IL-10 deficient BALB/c mice still had slightly reduced responsiveness to LsAg (mean IL-4+ basophils in response to 2 μg/ml LsAg 64.3% at 8 wks vs. 40.5% at 13 wks, p = 0.003, Fig. 8E), although not to the same degree as basophils from chronically infected WT mice (mean IL-4+ basophils in response to 2 μg/ml LsAg 63.3% at 8 wks vs. 26.9% at 13 wks, p = 0.036, Fig. 1E). This finding was not due to a drop in parasite specific IgE levels at the chronic timepoint, because LsAg-specific IgE levels were greater 13 weeks post-infection than at the 8 week timepoint in IL-10-deficient mice (Supplemental Figure 2).
Because IL-10 has not been previously shown to have a suppressive effect on basophils, we assessed the effects of IL-10 on basophil function in vivo by administering IL-10 twice daily to WT mice for 5 days. As seen in supplemental figure 3, basophils of mice administered IL-10 exhibit decreased responsiveness to IgE-mediated stimulation.
These results demonstrate that IL-10 is responsible for much of the reduced basophil responsiveness observed in chronic L. sigmodontis infection.
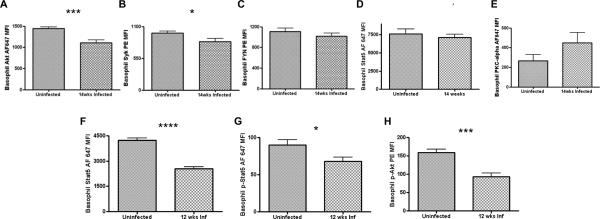
Helminth infection decreases Syk, Akt, and STAT5 expression in basophils
We assessed the expression of signal transduction molecules in basophils from WT BALB/c mice chronically infected with L. sigmodontis and age-matched uninfected controls by flow cytometry. Expression levels of Akt (p = 0.001) and Syk (p = 0.033) from mice chronically infected with L. sigmodontis were significantly lower compared to uninfected controls (Figs. 9A, 9B). Fyn expression in basophils from chronically infected mice was also slightly lower on average than uninfected controls (Fig. 9C), but this difference did not reach statistical significance. No decrease in baseline STAT5 expression (p = 0.453) could be detected in basophils of chronically infected mice (Fig. 9D). However, expression levels of STAT5 (p < 0.0001) and phosphorylated STAT5 (p = 0.049) in basophils of chronically infected mice were significantly lower than in basophils of uninfected controls after stimulation for 10 minutes with 0.0078 μg/ml anti-IgE (Figs. 9F, 9G). Basophil phosphorylated Akt expression levels (p = 0.0008) were also lower after anti-IgE stimulation (Fig. 9H).
FIGURE 9.
Alterations in signal transduction molecules in basophils of mice chronically infected with L. sigmodontis.
Expression levels of key signaling molecules were assessed by flow cytometry. After identifying basophils as CD4-B220-IgE+ peripheral blood cells, baseline (A) Akt, (B) Syk, (C) FYN, (D) STAT5, and (E) PKC-alpha MFI levels were measured from chronically infected BALB/c mice and age-matched uninfected BALB/c controls. Basophils from chronically infected mice and age-matched controls were also stimulated for 10 minutes with 0.0078μg/ml anti-IgE and then MFI levels of (F) phospho-Akt, (G) STAT5, and (H) phospho-STAT5 measured by flow cytometry. Each data point represents the mean of at least 4 independent experiments. (* p < 0.05, *** p < 0.001, **** p < 0.0001).
Because ES-62, a filarial E/S product, has been shown to suppress BMMCs in vitro by decreasing PKC-α expression (40), we also evaluated expression of PKC-α in basophils of mice chronically infected with L. sigmodontis. As seen in figure 9E, no reduction in PKC-α expression was observed in basophils of chronically infected mice (Fig. 9E).
Basophil suppression requires active helminth infection
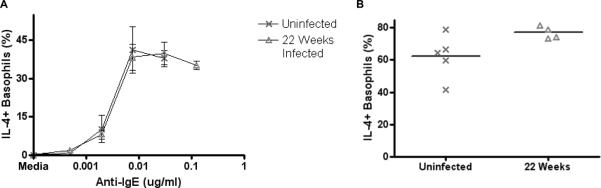
While L. sigmodontis can produce patent infections in BALB/c mice, the adult worms and microfilariae eventually die by 16–20 weeks. To determine whether helminth infection permanently reduces basophil responsiveness, basophil activation assays were performed 22 weeks post infection, a timepoint when both adult worms and microfilariae are no longer present.
No adult worms were found in the pleural cavity and no microfilariae were found in the blood of mice inoculated with L. sigmodontis 22 weeks prior. The basophil response curve to IgE cross-linking of mice that no longer had worms present was identical to that of uninfected controls (Fig. 10A). Basophils from 22 week infected mice also no longer had reduced basophil activation when stimulated with ionomycin (Fig. 10B).
FIGURE 10.
Basophil responsiveness in BALB/c mice after clearance of L. sigmodontis infection.
Basophils from mice that were infected 22 weeks prior but no longer actively infected (n = 4) and uninfected control mice (n = 5) were stimulated with several concentrations of anti-IgE (A) or 1μg/ml ionomycin (B). To assess activation, percentages of IL-4+ basophils were measured using flow cytometry after subtracting media stimulation levels.
These data suggest that active worm infections are needed to reduce basophil responsiveness and that basophil responsiveness, which is reduced in chronic helminth infections, returns to normal after death of the worms.
Discussion
This study demonstrates that chronic helminth infection suppresses basophil responsiveness to both IgE and non-IgE mediated stimuli. This phenomenon was observed with L. sigmodontis and S. mansoni infections. Given that these worms belong to different phyla (Nematoda and Platyhelminthes, respectively), these results suggest that basophil suppression may be a common immunologic phenotype in many chronic helminth infections.
Basophil suppression appeared to be due primarily to IL-10 as basophils of mice deficient in this cytokine did not exhibit alterations in their activation curves to anti-IgE stimulation. Additionally, intracellular flow cytometry revealed decreases in basophil expression of Syk and Akt as well as decreased total STAT5, phosphorylated STAT5, and phosphorylated Akt in response to IgE-mediated activation. These findings are consistent with IL-10 mediated suppression, as IL-10 suppresses IgE signaling in mast cells by decreasing expression of these same molecules (31). The reduced expression of Syk is particularly noteworthy since there is substantial evidence that Syk may be the principle signaling molecule responsible for fine-tuning basophil responsiveness (45). IL-10 production during chronic helminthiasis is well documented and has been shown to contribute to the highly immune regulated state observed in these infections (46–48). Important sources of IL-10 during filaria infection include CD4+CD25- T-cells, CD4+CD25+T-regs, CD8+ T-cells, B-cells, monocytes, and NK cells (49). Our data expands the known suppressive capabilities of IL-10 since this is the first study to find that IL-10 plays a role in basophil suppression. It is important to note that while IL-10 is an important cause of reduced basophil responses to IgE-mediated activation in the L. sigmodontis model, it is probably not the only mechanism suppressing basophil activation towards helminths since some decreased responsiveness to LsAg developed in chronically infected IL-10 deficient mice despite increases in specific IgE.
Recently, ES-62, a secreted phosphorylcholine-containing excretory/secretory product of the filarial worm Acanthocheilonema viteae, was shown to directly inhibit mast cell function in vitro by reducing intracellular levels of PKCα, a molecule implicated in a non-canonical IgE-signaling pathway (40). While we observed that E/S products from L. sigmodontis microfilariae inhibit basophil responsiveness in vitro, direct injection of microfilariae into mice did not result in basophil suppression and intracellular levels of PKCα were not diminished in basophils of chronically infected animals. These findings suggest that while E/S products of L. sigmodontis microfilariae have the capability to downmodulate basophil function, they likely do not do so in vivo during chronic infection.
Another mechanism that did not play a large role in basophil suppression was repeated IgE mediated stimulation. While IgE-mediated activation can cause negative feedback signals which suppress subsequent IgE-mediated signaling (36), this process is not required for basophil suppression during filaria infection since basophil suppression developed in chronically-infected antibody-deficient mice.
Interestingly, suppression of basophil function required the presence of living worms as basophil responsiveness returned to baseline after all worms had died. This requirement for active worm infection is consistent with studies in humans in which decreased cellular proliferation and cytokine responses to parasite antigens reverse after therapy (50, 51).
The finding that chronic helminth infections suppress basophil responsiveness has important clinical implications. Recently, basophils have become increasingly recognized as being important mediators of allergic disease. In terms of acute effector function, basophils release histamine and leukotriene C4 after becoming activated. These molecules induce classic allergy symptoms by increasing vascular permeability, mucus secretion, and smooth muscle contraction (52, 53). While the contribution basophils make to acute inflammatory responses likely varies between different diseases, a recent clinical study demonstrated that basophils may be responsible for the majority of allergic symptoms that occur after intranasal allergen challenge of individuals with cat allergy (54). Similarly, two murine studies have identified basophils as the principal effector cells of chronic allergic inflammation (18, 22). One of the mysteries regarding helminth infections and allergy has been the observation that, in contrast to its utility in developed countries, allergen-specific IgE has poor predictive value for allergic disease in developing countries with high rates of helminth disease (55). Our discovery that chronic helminth infections can suppress IgE-mediated activation of basophils provides an explanation for this finding. Given the many differences between human and mouse FcεRI expression and basophil properties (56, 57), it will be important to assess whether helminths exert a similar phenomenon in people.
In addition to contributing to acute allergic inflammation, basophils also play prominent roles in driving the type 2 responses responsible for allergic diseases. While recent studies have demonstrated that basophils play little, if any, role in initiating type 2 immune responses (21, 58), a number of studies have shown that basophils can amplify type 2 responses that are already present (21–23, 25, 26, 38, 59). Thus, suppression of basophil responsiveness may be a principal mechanism by which chronic helminth infections protect against development of allergic diseases. Interestingly, some animal studies have implicated IL-10 as being important for helminth-mediated protection against allergic disease (59, 60). Our finding that basophil suppression in chronic L. sigmodontis infection is due to IL-10 provides a downstream mechanism explaining how helminth-induced IL-10 can block allergic inflammation.
Changes in basophil responsiveness may also have important implications for susceptibility to helminths, as there is increasing evidence that basophils play a role in protective immunity against some of these infections (61, 62). Although basophils do not protect against primary murine filariasis (26), basophil depletion or deficiency results in impaired expulsion of the intestinal nematode Trichuris muris (24) and inhibits protection against reinfection by the hookworm Nippostrongylus brasiliensis (22, 23). Thus, it is possible that in certain helminth infections reduced basophil responsiveness may play a role in enabling parasite survival.
Finally, it is interesting to note that basophils have recently been implicated as contributing to the pathogenesis of lupus nephritis. In that study, basophils were shown to contribute to disease by amplifying production of autoantibodies in an IL-4 and IgE-dependent manner (63). As with allergy, worm infections are associated with protection against autoimmune disease in both human studies and animal models. If basophils play a role in mediating pathology in certain autoimmune diseases, then it is possible that basophil suppression could be one of the mechanisms by which helminths protect against autoimmunity.
In summary, this study demonstrates that chronic helminth infections reduce basophil responsiveness in an IL-10-dependent manner through reduction of key IgE signaling molecules. Given the prominent roles basophils play in the development and effector phases of type 2 responses, the protective role they may have against certain helminth infections, and their possible contribution to autoimmune disease pathogenesis, this finding has broad implications for understanding how helminth infections alter disease manifestations in people.
Supplementary Material
Acknowledgments
We thank Dr. Cara Olsen for help with statistical analyses and Karen Wolcott and Kateryna Lund at the Uniformed Services University Biomedical Instrumentation Center for valuable assistance with flow cytometry.
1This work was supported by National Institutes of Allergy and Infectious Diseases/National Institutes of Health Grant R01AI076522.
References
- 1.Cooper PJ. Interactions between helminth parasites and allergy. Curr Opin Allergy Clin Immunol. 2009;9:29–37. doi: 10.1097/ACI.0b013e32831f44a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klion AD, Massougbodji A, Sadeler BC, Ottesen EA, Nutman TB. Loiasis in endemic and nonendemic populations: immunologically mediated differences in clinical presentation. J Infect Dis. 1991;163:1318–1325. doi: 10.1093/infdis/163.6.1318. [DOI] [PubMed] [Google Scholar]
- 3.Nutman TB, Miller KD, Mulligan M, Ottesen EA. Loa loa infection in temporary residents of endemic regions: recognition of a hyperresponsive syndrome with characteristic clinical manifestations. J Infect Dis. 1986;154:10–18. doi: 10.1093/infdis/154.1.10. [DOI] [PubMed] [Google Scholar]
- 4.Cooper PJ, Chico ME, Rodrigues LC, Ordonez M, Strachan D, Griffin GE, Nutman TB. Reduced risk of atopy among school-age children infected with geohelminth parasites in a rural area of the tropics. J Allergy Clin Immunol. 2003;111:995–1000. doi: 10.1067/mai.2003.1348. [DOI] [PubMed] [Google Scholar]
- 5.Endara P, Vaca M, Chico ME, Erazo S, Oviedo G, Quinzo I, Rodriguez A, Lovato R, Moncayo AL, Barreto ML, Rodrigues LC, Cooper PJ. Long-term periodic anthelmintic treatments are associated with increased allergen skin reactivity. Clin Exp Allergy. 2010;40:1669–1677. doi: 10.1111/j.1365-2222.2010.03559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flohr C, Tuyen LN, Lewis S, Quinnell R, Minh TT, Liem HT, Campbell J, Pritchard D, Hien TT, Farrar J, Williams H, Britton J. Poor sanitation and helminth infection protect against skin sensitization in Vietnamese children: A cross-sectional study. J Allergy Clin Immunol. 2006;118:1305–1311. doi: 10.1016/j.jaci.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 7.Hartgers FC, Obeng BB, Kruize YC, Duijvestein M, de Breij A, Amoah A, Larbi IA, van Ree R, Wilson MD, Rodrigues LC, Boakye DA, Yazdanbakhsh M. Lower expression of TLR2 and SOCS-3 is associated with Schistosoma haematobium infection and with lower risk for allergic reactivity in children living in a rural area in Ghana. PLoS Negl Trop Dis. 2008;2:e227. doi: 10.1371/journal.pntd.0000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodrigues LC, Newcombe PJ, Cunha SS, Alcantara-Neves NM, Genser B, Cruz AA, Simoes SM, Fiaccone R, Amorim L, Cooper PJ, Barreto ML. Early infection with Trichuris trichiura and allergen skin test reactivity in later childhood. Clin Exp Allergy. 2008;38:1769–1777. doi: 10.1111/j.1365-2222.2008.03027.x. [DOI] [PubMed] [Google Scholar]
- 9.van den Biggelaar AH, van Ree R, Rodrigues LC, Lell B, Deelder AM, Kremsner PG, Yazdanbakhsh M. Decreased atopy in children infected with Schistosoma haematobium: a role for parasite-induced interleukin-10. Lancet. 2000;356:1723–1727. doi: 10.1016/S0140-6736(00)03206-2. [DOI] [PubMed] [Google Scholar]
- 10.Gibbs BF, Streatfield C, Falcone FH. Basophils as critical orchestrators of Th2-type immune responses. Expert Rev Clin Immunol. 2009;5:725–734. doi: 10.1586/eci.09.47. [DOI] [PubMed] [Google Scholar]
- 11.Karasuyama H, Mukai K, Tsujimura Y, Obata K. Newly discovered roles for basophils: a neglected minority gains new respect. Nat Rev Immunol. 2009;9:9–13. doi: 10.1038/nri2458. [DOI] [PubMed] [Google Scholar]
- 12.Min B, Paul WE. Basophils and type 2 immunity. Curr Opin Hematol. 2008;15:59–63. doi: 10.1097/MOH.0b013e3282f13ce8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakanishi K. Basophils as APC in Th2 response in allergic inflammation and parasite infection. Curr Opin Immunol. 2010;22:814–820. doi: 10.1016/j.coi.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Siracusa MC, Comeau MR, Artis D. New insights into basophil biology: initiators, regulators, and effectors of type 2 inflammation. Ann N Y Acad Sci. 2011;1217:166–177. doi: 10.1111/j.1749-6632.2010.05918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sullivan BM, Locksley RM. Basophils: a nonredundant contributor to host immunity. Immunity. 2009;30:12–20. doi: 10.1016/j.immuni.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Min B, Prout M, Hu-Li J, Zhu J, Jankovic D, Morgan ES, Urban JF, Jr., Dvorak AM, Finkelman FD, LeGros G, Paul WE. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J Exp Med. 2004;200:507–517. doi: 10.1084/jem.20040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitre E, Taylor RT, Kubofcik J, Nutman TB. Parasite antigen-driven basophils are a major source of IL-4 in human filarial infections. J Immunol. 2004;172:2439–2445. doi: 10.4049/jimmunol.172.4.2439. [DOI] [PubMed] [Google Scholar]
- 18.Mukai K, Matsuoka K, Taya C, Suzuki H, Yokozeki H, Nishioka K, Hirokawa K, Etori M, Yamashita M, Kubota T, Minegishi Y, Yonekawa H, Karasuyama H. Basophils play a critical role in the development of IgE-mediated chronic allergic inflammation independently of T cells and mast cells. Immunity. 2005;23:191–202. doi: 10.1016/j.immuni.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Panhuys N, Prout M, Forbes E, Min B, Paul WE, Le Gros G. Basophils are the major producers of IL-4 during primary helminth infection. J Immunol. 2011;186:2719–2728. doi: 10.4049/jimmunol.1000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammad H, Plantinga M, Deswarte K, Pouliot P, Willart MA, Kool M, Muskens F, Lambrecht BN. Inflammatory dendritic cells--not basophils--are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med. 2010;207:2097–2111. doi: 10.1084/jem.20101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohnmacht C, Schwartz C, Panzer M, Schiedewitz I, Naumann R, Voehringer D. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity. 2010;33:364–374. doi: 10.1016/j.immuni.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 23.Ohnmacht C, Voehringer D. Basophils protect against reinfection with hookworms independently of mast cells and memory Th2 cells. J Immunol. 2010;184:344–350. doi: 10.4049/jimmunol.0901841. [DOI] [PubMed] [Google Scholar]
- 24.Perrigoue JG, Saenz SA, Siracusa MC, Allenspach EJ, Taylor BC, Giacomin PR, Nair MG, Du Y, Zaph C, van Rooijen N, Comeau MR, Pearce EJ, Laufer TM, Artis D. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang H, Cao W, Kasturi SP, Ravindran R, Nakaya HI, Kundu K, Murthy N, Kepler TB, Malissen B, Pulendran B. The T helper type 2 response to cysteine proteases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nat Immunol. 2010;11:608–617. doi: 10.1038/ni.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torrero MN, Hubner MP, Larson D, Karasuyama H, Mitre E. Basophils amplify type 2 immune responses, but do not serve a protective role, during chronic infection of mice with the filarial nematode Litomosoides sigmodontis. J Immunol. 2010;185:7426–7434. doi: 10.4049/jimmunol.0903864. [DOI] [PubMed] [Google Scholar]
- 27.Hubner MP, Torrero MN, McCall JW, Mitre E. Litomosoides sigmodontis: a simple method to infect mice with L3 larvae obtained from the pleural space of recently infected jirds (Meriones unguiculatus) Exp Parasitol. 2009;123:95–98. doi: 10.1016/j.exppara.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Oliveira Fraga LA, Torrero MN, Tocheva AS, Mitre E, Davies SJ. Induction of type 2 responses by schistosome worms during prepatent infection. J Infect Dis. 2010;201:464–472. doi: 10.1086/649841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hubner MP, Stocker JT, Mitre E. Inhibition of type 1 diabetes in filaria-infected non-obese diabetic mice is associated with a T helper type 2 shift and induction of FoxP3+ regulatory T cells. Immunology. 2009;127:512–522. doi: 10.1111/j.1365-2567.2008.02958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torrero MN, Larson D, Hubner MP, Mitre E. CD200R surface expression as a marker of murine basophil activation. Clin Exp Allergy. 2009;39:361–369. doi: 10.1111/j.1365-2222.2008.03154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kennedy Norton S, Barnstein B, Brenzovich J, Bailey DP, Kashyap M, Speiran K, Ford J, Conrad D, Watowich S, Moralle MR, Kepley CL, Murray PJ, Ryan JJ. IL-10 suppresses mast cell IgE receptor expression and signaling in vitro and in vivo. J Immunol. 2008;180:2848–2854. doi: 10.4049/jimmunol.180.5.2848. [DOI] [PubMed] [Google Scholar]
- 32.Gibbs BF, Haas H, Wolff HH, Grabbe J. Early IgE-dependent release of IL-4 and IL-13 from leukocytes is restricted to basophils: a comparison with other granulocytes and mononuclear cells. Inflamm Res. 2000;49(Suppl 1):S9–10. doi: 10.1007/PL00000197. [DOI] [PubMed] [Google Scholar]
- 33.Taylor JJ, Krawczyk CM, Mohrs M, Pearce EJ. Th2 cell hyporesponsiveness during chronic murine schistosomiasis is cell intrinsic and linked to GRAIL expression. J Clin Invest. 2009;119:1019–1028. doi: 10.1172/JCI36534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gimborn K, Lessmann E, Kuppig S, Krystal G, Huber M. SHIP down-regulates FcepsilonR1-induced degranulation at supraoptimal IgE or antigen levels. J Immunol. 2005;174:507–516. doi: 10.4049/jimmunol.174.1.507. [DOI] [PubMed] [Google Scholar]
- 35.MacGlashan D, Jr., Vilarino N. Nonspecific desensitization, functional memory, and the characteristics of SHIP phosphorylation following IgE-mediated stimulation of human basophils. J Immunol. 2006;177:1040–1051. doi: 10.4049/jimmunol.177.2.1040. [DOI] [PubMed] [Google Scholar]
- 36.Macglashan D, Miura K. Loss of syk kinase during IgE-mediated stimulation of human basophils. J Allergy Clin Immunol. 2004;114:1317–1324. doi: 10.1016/j.jaci.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 37.Perona-Wright G, Mohrs K, Taylor J, Zaph C, Artis D, Pearce EJ, Mohrs M. Cutting edge: Helminth infection induces IgE in the absence of mu- or delta-chain expression. J Immunol. 2008;181:6697–6701. doi: 10.4049/jimmunol.181.10.6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Denzel A, Maus UA, Rodriguez Gomez M, Moll C, Niedermeier M, Winter C, Maus R, Hollingshead S, Briles DE, Kunz-Schughart LA, Talke Y, Mack M. Basophils enhance immunological memory responses. Nat Immunol. 2008;9:733–742. doi: 10.1038/ni.1621. [DOI] [PubMed] [Google Scholar]
- 39.Ohnmacht C, Voehringer D. Basophil effector function and homeostasis during helminth infection. Blood. 2009;113:2816–2825. doi: 10.1182/blood-2008-05-154773. [DOI] [PubMed] [Google Scholar]
- 40.Melendez AJ, Harnett MM, Pushparaj PN, Wong WS, Tay HK, McSharry CP, Harnett W. Inhibition of Fc epsilon RI-mediated mast cell responses by ES-62, a product of parasitic filarial nematodes. Nat Med. 2007;13:1375–1381. doi: 10.1038/nm1654. [DOI] [PubMed] [Google Scholar]
- 41.Fallon PG, Mangan NE. Suppression of TH2-type allergic reactions by helminth infection. Nat Rev Immunol. 2007;7:220–230. doi: 10.1038/nri2039. [DOI] [PubMed] [Google Scholar]
- 42.Cooper PJ, Mancero T, Espinel M, Sandoval C, Lovato R, Guderian RH, Nutman TB. Early human infection with Onchocerca volvulus is associated with an enhanced parasite-specific cellular immune response. J Infect Dis. 2001;183:1662–1668. doi: 10.1086/320709. [DOI] [PubMed] [Google Scholar]
- 43.King CL, Mahanty S, Kumaraswami V, Abrams JS, Regunathan J, Jayaraman K, Ottesen EA, Nutman TB. Cytokine control of parasite-specific anergy in human lymphatic filariasis. Preferential induction of a regulatory T helper type 2 lymphocyte subset. J Clin Invest. 1993;92:1667–1673. doi: 10.1172/JCI116752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sher A, Fiorentino D, Caspar P, Pearce E, Mosmann T. Production of IL-10 by CD4+ T lymphocytes correlates with down-regulation of Th1 cytokine synthesis in helminth infection. J Immunol. 1991;147:2713–2716. [PubMed] [Google Scholar]
- 45.Macglashan D. Human Basophil Phenotypes and the Associated Signaling Mechanisms. The Open Allergy Journal. 2010;3:13. [Google Scholar]
- 46.Marechal P, Le Goff L, Hoffman W, Rapp J, Oswald IP, Ombrouck C, Taylor DW, Bain O, Petit G. Immune response to the filaria Litomosoides sigmodontis in susceptible and resistant mice. Parasite Immunol. 1997;19:273–279. doi: 10.1046/j.1365-3024.1997.d01-209.x. [DOI] [PubMed] [Google Scholar]
- 47.McKee AS, Pearce EJ. CD25+CD4+ cells contribute to Th2 polarization during helminth infection by suppressing Th1 response development. J Immunol. 2004;173:1224–1231. doi: 10.4049/jimmunol.173.2.1224. [DOI] [PubMed] [Google Scholar]
- 48.Simons JE, Gray CA, Lawrence RA. Absence of regulatory IL-10 enhances innate protection against filarial parasites by a neutrophil-independent mechanism. Parasite Immunol. 2010;32:473–478. doi: 10.1111/j.1365-3024.2010.01210.x. [DOI] [PubMed] [Google Scholar]
- 49.Mitre E, Chien D, Nutman TB. CD4(+) (and not CD25+) T cells are the predominant interleukin-10-producing cells in the circulation of filaria-infected patients. J Infect Dis. 2008;197:94–101. doi: 10.1086/524301. [DOI] [PubMed] [Google Scholar]
- 50.Greene BM, Fanning MM, Ellner JJ. Non-specific suppression of antigen-induced lymphocyte blastogenesis in Onchocerca volvulus infection in man. Clin Exp Immunol. 1983;52:259–265. [PMC free article] [PubMed] [Google Scholar]
- 51.Grogan JL, Kremsner PG, Deelder AM, Yazdanbakhsh M. Antigen-specific proliferation and interferon-gamma and interleukin-5 production are down-regulated during Schistosoma haematobium infection. J Infect Dis. 1998;177:1433–1437. doi: 10.1086/517832. [DOI] [PubMed] [Google Scholar]
- 52.Galli SJ. Mast cells and basophils. Curr Opin Hematol. 2000;7:32–39. doi: 10.1097/00062752-200001000-00007. [DOI] [PubMed] [Google Scholar]
- 53.Mitre E, Nutman TB. Basophils, basophilia and helminth infections. Chem Immunol Allergy. 2006;90:141–156. doi: 10.1159/000088886. [DOI] [PubMed] [Google Scholar]
- 54.Eckman JA, Sterba PM, Kelly D, Alexander V, Liu MC, Bochner BS, Macglashan DW, Jr., Saini SS. Effects of omalizumab on basophil and mast cell responses using an intranasal cat allergen challenge. J Allergy Clin Immunol. 2010;125:889–895. e887. doi: 10.1016/j.jaci.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490–494. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- 56.de Andres B, Rakasz E, Hagen M, McCormik ML, Mueller AL, Elliot D, Metwali A, Sandor M, Britigan BE, Weinstock JV, Lynch RG. Lack of Fc-epsilon receptors on murine eosinophils: implications for the functional significance of elevated IgE and eosinophils in parasitic infections. Blood. 1997;89:3826–3836. [PubMed] [Google Scholar]
- 57.Sokol CL, Medzhitov R. Emerging functions of basophils in protective and allergic immune responses. Mucosal Immunol. 2010;3:129–137. doi: 10.1038/mi.2009.137. [DOI] [PubMed] [Google Scholar]
- 58.Sullivan BM, Liang HE, Bando JK, Wu D, Cheng LE, McKerrow JK, Allen CD, Locksley RM. Genetic analysis of basophil function in vivo. Nat Immunol. 2011;12:527–535. doi: 10.1038/ni.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bashir ME, Andersen P, Fuss IJ, Shi HN, Nagler-Anderson C. An enteric helminth infection protects against an allergic response to dietary antigen. J Immunol. 2002;169:3284–3292. doi: 10.4049/jimmunol.169.6.3284. [DOI] [PubMed] [Google Scholar]
- 60.Mangan NE, van Rooijen N, McKenzie AN, Fallon PG. Helminth-modified pulmonary immune response protects mice from allergen-induced airway hyperresponsiveness. J Immunol. 2006;176:138–147. doi: 10.4049/jimmunol.176.1.138. [DOI] [PubMed] [Google Scholar]
- 61.Karasuyama H, Wada T, Yoshikawa S, Obata K. Emerging roles of basophils in protective immunity against parasites. Trends Immunol. 2011;32:125–130. doi: 10.1016/j.it.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 62.Voehringer D. The role of basophils in helminth infection. Trends Parasitol. 2009;25:551–556. doi: 10.1016/j.pt.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 63.Charles N, Hardwick D, Daugas E, Illei GG, Rivera J. Basophils and the T helper 2 environment can promote the development of lupus nephritis. Nat Med. 2010;16:701–707. doi: 10.1038/nm.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.