Abstract
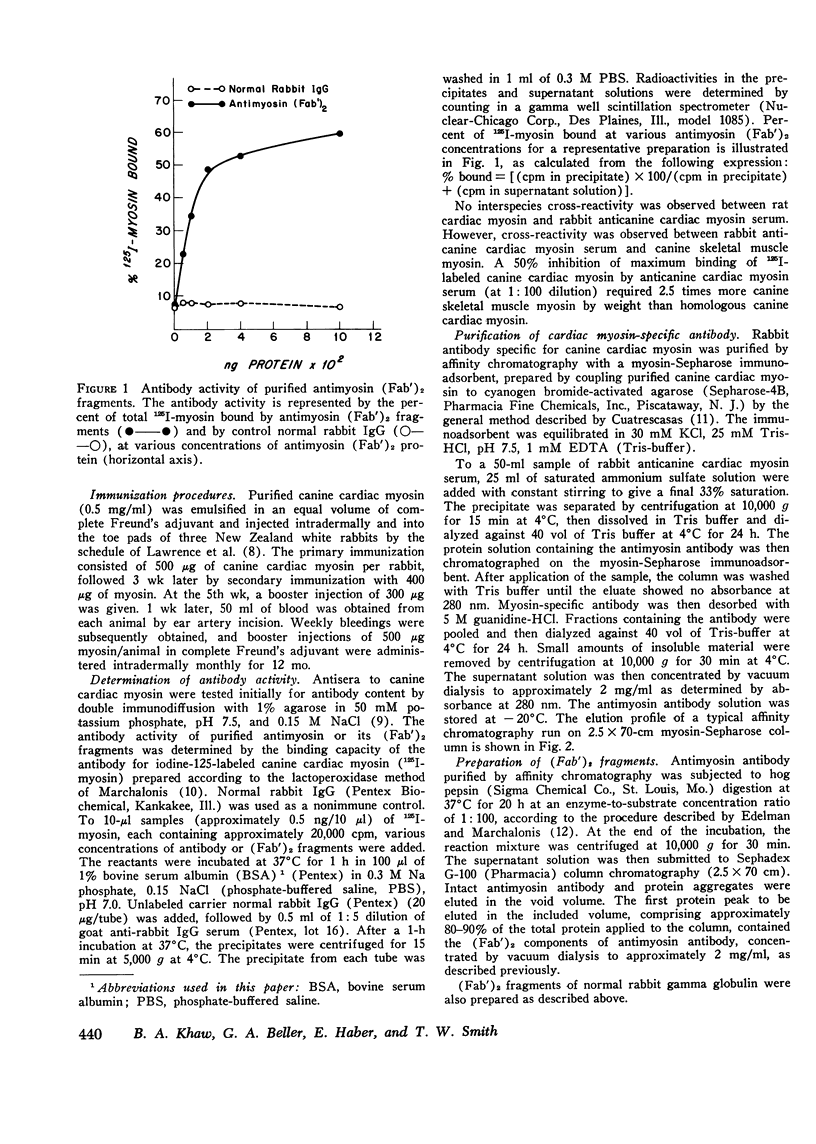
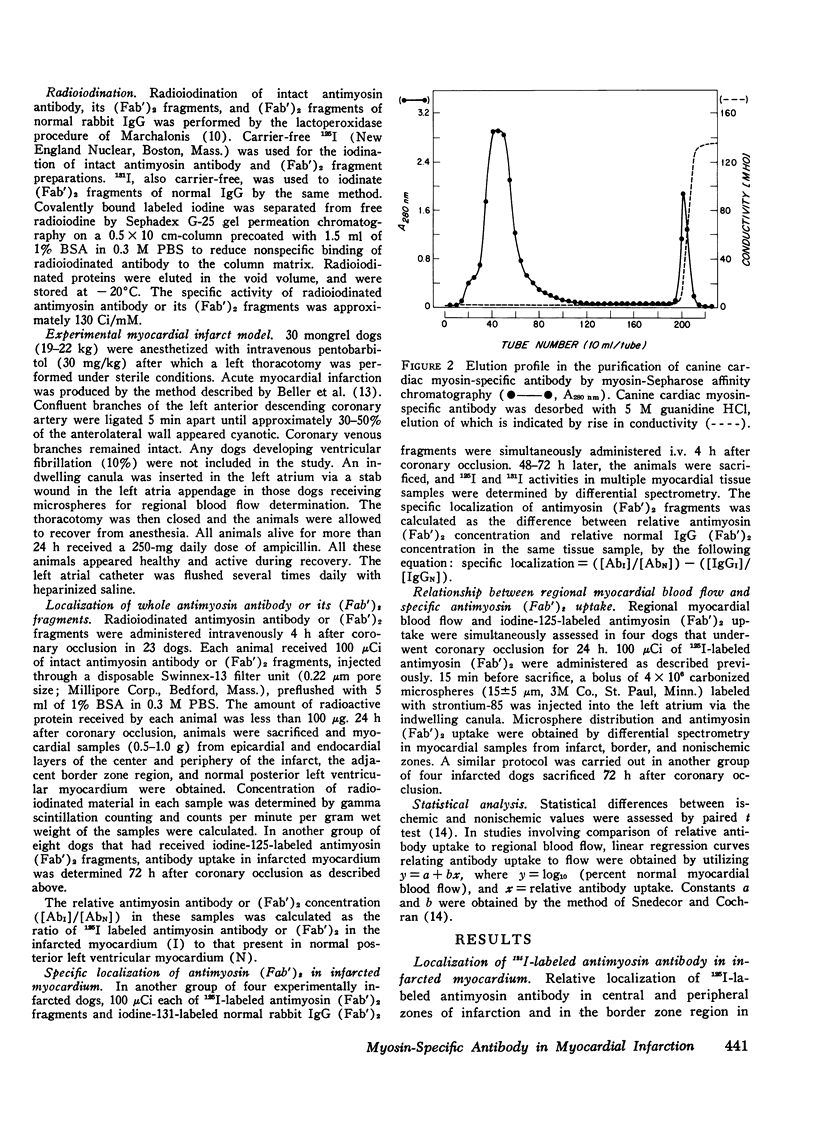
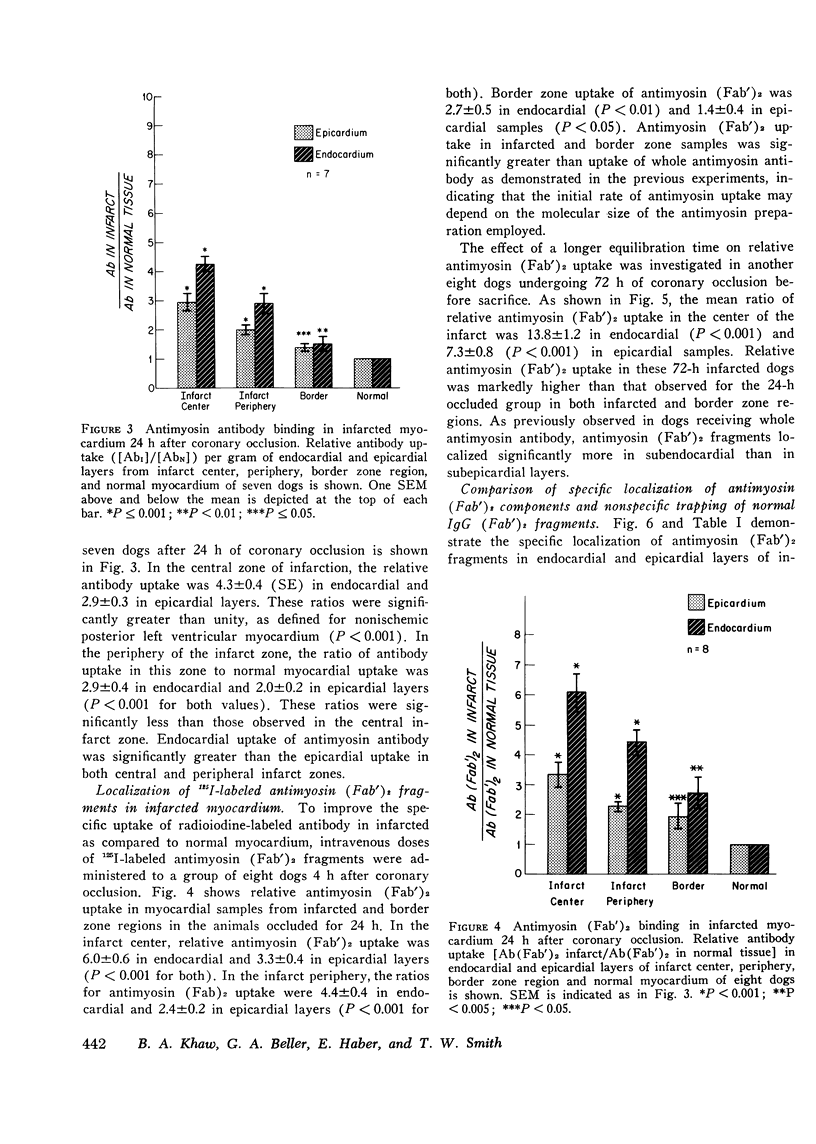
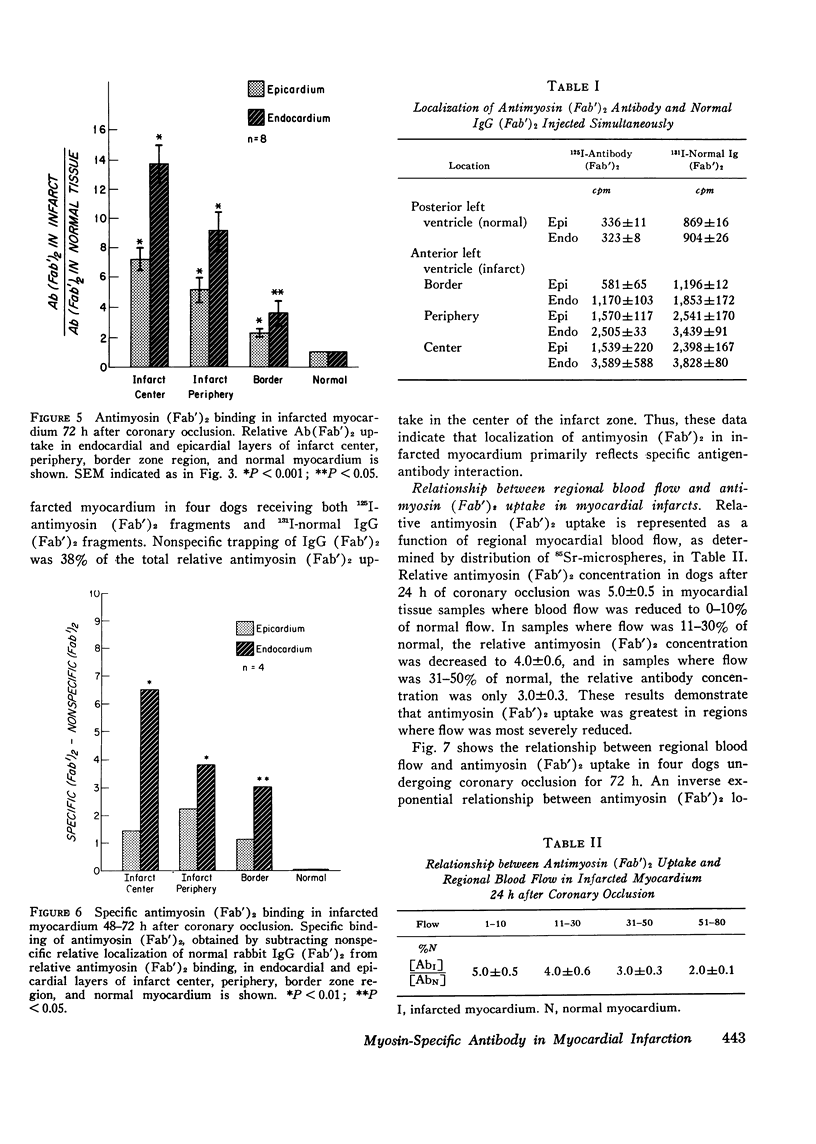
Specific localization of purified antibody against cardiac myosin has been demonstrated in areas of altered myocardial membrane permeability after experimental myocardial infarction. Intravenously administered radioiodine-labeled antimyosin was selectively localized in infarcted myocardium of seven dogs 24 h after coronary occlusion. The mean ratio (+/-SE) of antimyosin antibody in infarcted to normal myocardium in the center of the infarct was 4.2+/-0.4 for endocardial and 2.9+/-0.3 for epicardial layers. By utilizing (Fab')2 fragments of antimyosin obtained by pepsin digestion of purified antibody, the ratio of uptake was increased in eight dogs to 6.1+/-0.6 in the endocardial and 3.3+/-0.4 in the epicardial layers at the infarct center 24 h after occlusion. These ratios were further increased in the infarct center to 13.8+/-1.2 in the endocardial and 7.3+/-0.8 in the epicardial layers when eight dogs were sacrificed 72 h after coronary occlusion. The specificity of antimyosin (Fab')2 localization in infarcted myocardium was demonstrated in four dogs by simultaneous intravenous administration of 125I-labeled antimyosin (Fab')2 and 131I-labeled normal rabbit gamma globulin (Fab')2. Nonspecific trapping of normal rabbit IgG (Fab')2 was observed to be about 38% of total antimyosin (Fab')2 uptake in the central zone of infarction. Regional blood flow was related to antimyosin (Fab')2 uptake in infarcted myocardium by utilizing simultaneous administration of 85Sr-labeled microspheres. An inverse exponential relationship between antimyosin (Fab')2 uptake and regional blood flow was observed (r=0.85). The specific localization of antimyosin antibody or its (Fab')2 components in infarcted myocardium suggests a conceptually new approach to myocardial infarct localization and sizing.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker L. C., Ferreira R., Thomas M. Mapping of left ventricular blood flow with radioactive microspheres in experimental coronary artery occlusion. Cardiovasc Res. 1973 May;7(3):391–400. doi: 10.1093/cvr/7.3.391. [DOI] [PubMed] [Google Scholar]
- Beller G. A., Smith T. W., Hood W. B., Jr Altered distribution of tritiated digoxin in the infarcted canine left ventricle. Circulation. 1972 Sep;46(3):572–579. doi: 10.1161/01.cir.46.3.572. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Finck H. Immunochemical studies on myosin. 3. Immunochemical comparison of myosins from chicken skeletal heart and smooth muscles. Biochim Biophys Acta. 1965 Nov 15;111(1):231–238. [PubMed] [Google Scholar]
- Fortuin N. J., Kaihara S., Becker L. C., Pitt B. Regional myocardial blood flow in the dog studied with radioactive microspheres. Cardiovasc Res. 1971 Jul;5(3):331–336. doi: 10.1093/cvr/5.3.331. [DOI] [PubMed] [Google Scholar]
- Hoffstein S., Gennaro D. E., Fox A. C., Hirsch J., Streuli F., Weissmann G. Colloidal lanthanum as a marker for impaired plasma membrane permeability in ischemic dog myocardium. Am J Pathol. 1975 May;79(2):207–218. [PMC free article] [PubMed] [Google Scholar]
- JAQUET H., CEBRA J. J. COMPARISON OF TWO PRECIPITATING DERIVATIVES OF RABBIT ANTIBODY: FRAGMENT I DIMER AND THE PRODUCT OF PEPSIN DIGESTION. Biochemistry. 1965 May;4:954–963. doi: 10.1021/bi00881a024. [DOI] [PubMed] [Google Scholar]
- JENNINGS R. B., CROUT J. R., SMETTERS G. W. Studies on distribution and localization to potassium in early myocardial ischemic injury. AMA Arch Pathol. 1957 Jun;63(6):586–592. [PubMed] [Google Scholar]
- Katz A. M. Contractile proteins of the heart. Physiol Rev. 1970 Jan;50(1):63–158. doi: 10.1152/physrev.1970.50.1.63. [DOI] [PubMed] [Google Scholar]
- Katz A. M., Repke D. I., Rubin B. B. Adenosinetriphosphatase activity of cardiac myosin. Comparison of the enzymatic activities and activation by actin of dog cardiac, rabbit cardiac, rabbit white skeletal and rabbit red skeletal muscle myosins. Circ Res. 1966 Sep;19(3):611–621. doi: 10.1161/01.res.19.3.611. [DOI] [PubMed] [Google Scholar]
- LUCHI R. J., KRITCHER E. M., CONN H. L., Jr MOLECULAR CHARACTERISTICS OF CANINE CARDIAC MYOSIN. Circ Res. 1965 Jan;16:74–82. doi: 10.1161/01.res.16.1.74. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Weber K. Actin antibody: the specific visualization of actin filaments in non-muscle cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2268–2272. doi: 10.1073/pnas.71.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NYDICK I., WROBLEWSKI F., LADUE J. S. Evidence for increased serum glutamic oxalacetic transaminase (SGO-T) activity following graded myocardial infarcts in dogs. Circulation. 1955 Aug;12(2):161–168. doi: 10.1161/01.cir.12.2.161. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- OUCHTERLONY O. Antigen-antibody reactions in gels. IV. Types of reactions in coordinated systems of diffusion. Acta Pathol Microbiol Scand. 1953;32(2):230–240. [PubMed] [Google Scholar]
- Shell W. E., Kjekshus J. K., Sobel B. E. Quantitative assessment of the extent of myocardial infarction in the conscious dog by means of analysis of serial changes in serum creatine phosphokinase activity. J Clin Invest. 1971 Dec;50(12):2614–2625. doi: 10.1172/JCI106762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel B. E., Shell W. E. Serum enzyme determinations in the diagnosis and assessment of myocardial infarction. Circulation. 1972 Feb;45(2):471–482. doi: 10.1161/01.cir.45.2.471. [DOI] [PubMed] [Google Scholar]
- TARANTA A., FRANKLIN E. C. Complement fixation by antibody fragments. Science. 1961 Dec 15;134(3494):1981–1982. doi: 10.1126/science.134.3494.1981. [DOI] [PubMed] [Google Scholar]
- Wikman-Coffelt J., Zelis R., Fenner C., Mason D. T. Myosin chains of myocardial tissue. I. Purification and immunological properties of myosin heavy chains. Biochem Biophys Res Commun. 1973 Apr 16;51(4):1097–1104. doi: 10.1016/0006-291x(73)90040-5. [DOI] [PubMed] [Google Scholar]
- Zucker-Franklin D., Berney S. Electron microscope study of surface immunoglobulin-bearing human tonsil cells. J Exp Med. 1972 Mar 1;135(3):533–548. doi: 10.1084/jem.135.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]



