Abstract
During the process of speciation, diverging taxa often hybridize and produce offspring wherein the heterogametic sex (i.e., XY or ZW) is unfit (Haldane's rule). Dominance theory seeks to explain Haldane's rule in terms of the difference in X-linked dominance regimes experienced by the sexes. However, X inactivation in female mammals extends the effects of hemizygosity to both sexes. Here, we highlight where the assumptions of dominance theory are particularly problematic in marsupials, where X inactivation uniformly results in silencing the paternal X. We then present evidence of Haldane's rule for sterility but not for viability in marsupials, as well as the first violations of Haldane's rule for these traits among all mammals. Marsupials represent a large taxonomic group possessing heteromorphic sex chromosomes, where the dominance theory cannot explain Haldane's rule. In this light, we evaluate alternative explanations for the preponderance of male sterility in interspecific hybrids, including faster male evolution, X–Y interactions, and genomic conflict hypotheses.
Keywords: hybrid sterility, metatheria, reproductive isolation, speciation, X inactivation
Haldane's Rule Is a Pattern Based on Sex Chromosomes, Not Sex
Of the few major patterns recognized in speciation, perhaps none has enjoyed as much attention as Haldane's rule. First noted by Haldane (1922), Haldane's rule describes the phenomenon that whenever divergent taxa produce hybrid offspring, the heterogametic (XY or ZW) sex suffers a reduction in fitness more often than the homogametic (XX or WW) sex. Several studies now demonstrate that Haldane's rule is a preliminary, though perhaps not requisite, stage of speciation (Coyne and Orr 1989a; Sasa et al. 1998; Presgraves 2002; Demuth and Wade 2007; Malone and Fontenot 2008) and has come to be known as one of the “rules of speciation” (Coyne and Orr 2004; Demuth and Wade 2007; Turelli and Moyle 2007).
The study of Haldane's rule has shaped our understanding of speciation by providing a broad pattern in which to study the mechanisms of population divergence and fixation of reproductive isolating barriers. Importantly, this pattern holds across the majority of taxa studied, with examples of male and female heterogamety both conforming to Haldane's rule (Haldane 1922, 1932; Gray 1954, 1958; Hillis and Green 1990; Schilthuizen et al. 2011). For this reason, it is often noted that Haldane's rule cannot simply be explained by the general sensitivity of one sex over the other (Coyne and Orr 1989b). Instead, Haldane's rule is thought to be the result of genetic incompatibilities that are exaggerated in the genome of the heterogametic sex.
Dominance Theory and the Role of X Chromosomes in the Expression of Haldane's Rule
Among the early genetic explanations for Haldane's rule, Muller (1940, 1942) put forth the X-autosome interaction hypothesis noting that, owing to hemizygosity, hybrid males suffer from both dominant and recessive X-linked incompatibilities, whereas females only suffer from dominant incompatibilities (for convenience, we use male for the heterogametic sex and female for the homogametic sex). Orr (1993a) formalized Muller's theory mathematically and added that hemizygosity is a “double-edged sword”: although males express every X-linked incompatibility, on average females contain twice as many because they possess 2 X chromosomes. If dominant and recessive incompatibilities are equally likely, these 2 factors cancel each other and cannot explain the consistently lower fitness of males (Orr 1993a). However, if recessive alleles are more likely to produce severe incompatibilities, Haldane's rule will result (Muller and Pontecarvo 1942). Thus, somewhat ironically, the “dominance theory” relies on genetic incompatibilities being recessive on average. To further complicate matters, “dominance,” in the context of Haldane's rule, refers only to the X-chromosome component of what is more precisely an epistatic interaction between X-linked and other (perhaps multiple) loci (Demuth and Wade 2007).
Beginning in the 1980s, studies extending the dynamics of hemizygosity to females began to suggest forces in addition to dominance might contribute to Haldane's rule. For instance, when females carrying both X chromosomes from one parent in an otherwise hybrid genome (unbalanced females) were made from Drosophila species pairs that normally obey Haldane's rule for sterility, the unbalanced female hybrids remained fertile. However, if the species pair normally obey Haldane's rule for viability, unbalanced females became inviable (Coyne 1985; Orr 1993b). Later observations exploring Aedes mosquitoes that lack hemizygous sex chromosomes found related results. Hybrids follow Haldane's rule for sterility, but not viability (Presgraves and Orr 1998). Although the mechanisms by which dominance effects in females are made equivalent to males is different in the unbalance female and Aedes studies, the conclusions are the same; when both sexes have the same dominance effects, fertility conforms to Haldane's rule, but viability does not.
The unbalanced female and Aedes studies are instructive to the situation in mammals because female X-chromosome inactivation (XCI) results in only one X chromosome being expressed. Genetic explanations invoking dominance assume that chromosomal hemizygosity is equivalent to functional hemizygosity in terms of gene expression (Turelli and Orr 1995). Although this assumption is valid for Drosophila, where X-chromosome dosage compensation is achieved by the hypertranscription of the hemizygous X in males to equal the dosage expected of diploid autosomes and/or female Xs (Lucchesi 1973), it is clear that dosage compensation is not similarly achieved in other taxa (e.g., Caenorhabditis elegans and therian mammals—Xiong et al. 2010; Anopheles—Hahn and Lanzaro 2005; birds—Itoh et al. 2010; Lepidoptera—Zha et al. 2009; stickleback—Leder et al. 2010; platypus—Deakin et al. 2009; Tribolium—Prince et al. 2010; reviewed in Mank et al. 2011). Indeed, in their original formulation of dominance theory, Turelli and Orr (1995) asked, “Does the dominance theory work given mammalian dosage compensation?” Dosage compensation in mammals is particularly problematic for dominance theory because it involves XCI in females wherein one X chromosome is transcriptionally silenced (i.e., females are functionally hemizygous). Consequently, the average transcript ratio from XX:AA females is approximately 0.5—equal to the ratio in X:AA males (Gupta et al. 2006; but see Nguyen and Disteche 2006; Xiong et al. 2010). Following, we revisit Turelli and Orr's question highlighting data from marsupial hybrids that have not previously been appreciated for what they may tell us about the genetic mechanisms underlying Haldane's rule.
Dosage Compensation, X Inactivation, and the Role of Dominance in Haldane's Rule
XCI is achieved by different means in metatherian (marsupial) and eutherian (placental) mammals. In placental mammals, one copy of the X is randomly inactivated, forming a mosaic of maternal and paternal X-chromosome expression (Lyon 1961). In marsupial cells, males and females, both experience functional hemizygosity of the same set of alleles because it is always the paternal X chromosome that is inactivated (Cooper et al. 1971; Richardson et al. 1971; Al Nadaf et al. 2010). The consequences of mosaic XCI for dominance theory and Haldane's rule in placentals depend on the degree of autonomy among cells, which is largely unknown in mammals. However, the consistent hemizygous expression of only the maternal X chromosome in marsupials has clear implications, providing a situation where, if Haldane's rule is observed, dominance theory cannot be the explanation.
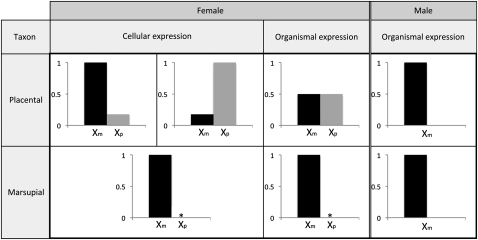
First, and perhaps more importantly, dominance theory assumes that all loci have diploid expression in F1 females, as is the case in Drosophila (Turelli and Orr 1995; Orr and Turelli 1996; Turelli and Orr 2000). Because hemizygous expression of the maternal X chromosome is shared in both sexes in marsupials (Figure 1), dominance effects are the same in males and females. Hence, under dominance theory, hybrid males and females are expected to suffer the same expected reduction of fitness due to X-linked incompatibilities, and Haldane's rule is not expected to consistently result. Additionally, the idea that females should suffer twice the average number of X-linked incompatibilities of males (Orr 1993a) is moot, if both sexes express the same X-chromosome complement. In sum, because X-linked alleles with strict paternal XCI are never functionally heterozygous, there is no X-linked dominance in either sex, and dominance theory predicts that Haldane's rule should not hold in marsupial hybrids.
Figure 1.
Expression of X-linked genes in therian mammals. Dosage inequality between the sexes is compensated in mammals by the inactivation of one X chromosome in females. Placental mammals inactivate either the maternal or paternal X chromosome at random, forming a mosaic of X-linked expression. Marsupial mammals inactivate only the paternal X chromosome, so that males and females only express the maternal X chromosome. Asterisk (*) indicates leaky expression.
Haldane's Rule for Sterility and Inviability in Hybrid Marsupials
Despite the prediction from dominance theory, published accounts show that Kangaroos (Macropus), Rock Wallabies (Petrogale), and Pademelons (Thyogale) obey Haldane's rule for fertility in most cases (Close and Lowry 1990 and references therein; Sharman et al. 1990; Eldridge and Close 1993). In 10 of 11 species pairs, males are sterile while females are fertile. However, in the remaining species pair, females are sterile while males remain fertile, representing the only reported exception to Haldane's rule for fertility among mammals (Table 1). When corrected for nonindependence among species pairs, the number obeying Haldane's rule becomes 6 of 7 for sterility. Incomplete data for an additional 12 species pairs also suggest that fertility is more frequently disturbed in males than in females (Supplementary Table 1).
Table 1.
Summary of studies of Haldane's rule in mammals
| Group | Asymmetric phenotype | Crosses with asymmetric effects | Crosses obeying Haldane's rule | Percentage of obeying |
| Eutheria | Sterility | 34 | 34 | 100.0 |
| Inviability | 5 | 5 | 100.0 | |
| Metatheria | Sterility | 7 | 6 | 85.7 |
| Inviability | 2 | 1 | 50.0 | |
| Combined | Sterility | 41 | 40 | 97.6 |
| Inviability | 7 | 6 | 85.7 |
Data for eutherian mammals are from Schilthuizen et al. (2011). For a full list, see Supplementary Table 1.
Despite the paucity of the female viability data reported, it remains possible to draw conclusions about Haldane's rule for inviability. The probability of having exactly k offspring of a particular sex is , where n is the total number of offspring observed and p = 0.5. Two species pairs exhibit a >95% chance that one sex is rare or absent (Petrogale assimilis × P. penicillata, and P. i. mareeba × P. assimilis). The first species pair produced 9 females and 1 male, conforming to Haldane's rule for inviability, whereas the second species pair produced 8 males and no females—a violation of Haldane's rule for inviability (Sharman et al. 1990). Interestingly, the reciprocal to this cross produced 6 females and 1 male, following Haldane's rule, although the result is nonsignificant (p = 0.05469). The majority of other interspecific crosses do not show significant asymmetry in viability between sexes (0.5 ≤ p ≤ 0.05469).
In placental mammals, Haldane's rule is obeyed in all 34 species pairs that produce sterile hybrids and in all 5 species pairs that produce only viable female hybrids (Laurie 1997; Schilthuizen et al. 2011). Marsupial crosses (reviewed in Close and Lowry 1990; Sharman et al. 1990; Eldridge and Close 1993) bring the overall therian mammal total for hybrid sterility to 40 of 41 species pairs and potentially add support for an additional 12 species pairs where data are limited (Supplementary Table 1). Although marsupials contain the only exceptions to Haldane's rule for both sterility and inviability among mammals, 52 of 53 hybridizations obeying the rule is still overwhelming support. If, as we propose, dominance theory cannot explain Haldane's rule in marsupials, why does the pattern still hold so regularly?
Alternative Hypotheses for Haldane's Rule in Marsupials
A key insight following the unbalanced female experiments pointed out that the genetic basis for viability is likely to involve the same set of loci in both sexes, whereas the loci governing fertility are probably different in males and females (Wu and Davis 1993). Since then, many evolutionary biologists have viewed Haldane's rule as a composite phenomenon (Coyne 1992; Johnson et al. 1992; Orr 1993b; Wu and Davis 1993). This recognition, along with the observation that male sterility evolves faster than female sterility and faster than inviability in both sexes despite male fertility being less sensitive than viability to mutagenic disruption, led Wu and colleagues to propose that either sexual selection may drive rapid evolution of genes that contribute to male sterility or spermatogenesis may be inherently more sensitive than oogenesis to perturbation (Wu and Davis 1993, Wu et al. 1996). This so called “faster male” hypothesis has since been supported by diverse lines of evidence in plants and animals (Brothers and Delph 2010; Schilthuizen et al. 2011), including rapid evolution of male reproductive proteins by positive selection in placental mammals (Torgerson et al. 2002; Swanson et al. 2003; Clark and Swanson 2005; Good and Nachman 2005; Khaitovich et al. 2005). The data for marsupials is thus far consistent with the composite view of Haldane's rule. Evidence for Haldane's rule for viability is lacking, as expected under dominance theory with strict paternal XCI. Evidence for Haldane's rule for sterility is abundant, which is consistent with faster male evolution. Future studies of marsupial reproductive protein evolution may provide additional support for faster male evolution.
Importantly, dominance and faster male theories need not be mutually exclusive. Indeed, Turelli and Orr (2000) discuss their relative roles under the same mathematical framework, where the influence of dominance scales with the proportion of the genome that is X linked, and the role of faster male evolution scales as the relative number and severity of male versus female incompatibilities. In marsupials, this interplay between dominance and faster male theories may remain, depending on the degree to which paternal X inactivation is leaky. Unfortunately, detailed mechanistic understanding of paternal XCI is still poorly understood. The most detailed study to date shows that paternal alleles escape XCI in 5–65% of cell lines (Al Nadaf et al. 2010). However, it remains unclear what proportion of transcripts at the surveyed loci belonged to the paternal X (i.e., it is unknown whether paternal alleles ever attain full expression) and furthermore, escape from inactivation may be stochastic (Al Nadaf et al. 2010).
An additional source of incompatibilities that may explain Haldane's rule in marsupials includes X–Y interactions, which have been suggested as a possible cause of male sterility in marsupials (Graves 1996; Graves and ONeill 1997). In placental mammals, proper meiotic pairing of the X and Y is facilitated by pseudoautosomal regions (PARs). Disruption of pairing in the PAR blocks meiosis and results in male infertility due to abnormal sperm development (Burgoyne et al. 1992) and is a suggested explanation for Haldane's rule in placental mammals (Graves 1996). However, because marsupials do not possess a PAR region (X and Y pair at the tips in the absence of homology), X–Y interactions are suggested to be genic (Sharp 1982; Graves and ONeill 1997). In hybrid males, the X and Y chromosomes are derived from different species, and heterospecific interactions or loss of gene complement may contribute to Haldane's rule for fertility in marsupials.
Additionally, genomic conflict, in the form of competition among oötids for inclusion into the pronucleus, potentially plays a role in Haldane's rule in marsupials (reviewed in McDermott and Noor 2010). Centromeric sequences involved in spindle fiber attachment have been shown to be involved in such competition in mammals (Henikoff et al. 2001; Pardo-Manuel de Villena and Sapienza 2001). Separated by 1–2 My (Gifford et al. 2005), 2 wallaby sister species, Macropus rufogriseus and M. eugenii, differ greatly in the repeat content of their centromeric sequences (O'Neill et al. 1998; Metcalfe et al. 2007). Interspecific crosses between these species produce infertile male and female hybrids that display extensive chromosomal remodeling and genomic instability, for example, changes in chromatin structure and the amplification of satellite repeats and transposable elements (Metcalfe et al. 2007). The effects of genomic instability may contribute to Haldane's rule in marsupials if centromeric misalignment of the X and Y chromosomes during metaphase in hybrids leads to the failure of spermatogenesis (McKee 1997; Zwick et al. 1999; Henikoff et al. 2001). Furthermore, meiotic inactivation of sex chromosomes in male hybrids, a process crucial for male fertility in mammals (Royo et al. 2010), could be delayed or derailed by the decondensation or amplification of centromeric regions. In marsupials, meiotic sex chromosome inactivation occurs before the X–Y associations that lead to the formation of the sex chromatin beginning at midpachytene (Namekawa et al. 2007). A delay in the formation of the sex chromatin may trigger the late-pachytene meiotic checkpoint and lead to spermatocyte apoptosis and reduced fertility, a phenomenon attributable to chromosomal asynapsis in placental mammals (Luan et al. 2001). If true, this mechanism would be consistent with more general “faster heterogametic sex” hypotheses that propose the XY sex evolves faster because of the conflicting pressures that the X and Y chromosomes experience to distort the sex ratio (Frank 1991; Hurst and Pomiankowski 1991; Tao and Hartl 2003).
Prospects for Future Research
In marsupial mammals, nature provides us with a system analogous to the unbalanced female experiments in Drosophila (Coyne 1985; Orr 1993b) and Aedes mosquito lacking hemizygous sex chromsomes (Presgraves and Orr 1998). In each case, the X-chromosome contribution to reproductive isolation is the same in males and females. In most species, dominance theory and faster male theory cannot be disentangled to reveal the cause of hybrid male sterility where they act simultaneously (Wu et al. 1996; Coyne and Orr 2004). However, because dominance theory cannot explain Haldane's rule in marsupials, understanding the genetics of hybrid male sterility and the evolutionary dynamics of sex chromosomes in this large group of diverse organisms will provide useful insight into one of the most sweeping empirical observations in evolutionary biology.
A major current limitation is that no records exist for hybridizations in non-macropodid marsupials such as opossums and possums (Didelphidae and Caenolestidae), gliders (Petauridae), bandicoots (Peramelemorphidae), and marsupial moles (Notoryctidae). While macropods, such as kangaroos, typically produce one offspring per season, many species in these families are highly fecund and produce anywhere from 4 to 10 offspring in a litter making them highly amenable to studying biases in sex ratio. Future research involving marsupials with high fecundity will potentially provide excellent candidates for studying the evolution of hybrid male sterility in nonplacental mammals.
Supplementary Material
Supplementary material can be found at http://www.jhered.oxfordjournals.org/.
Funding
National Institutes of Health (2R01GM065414-05A1); the University of Texas at Arlington startup funding to J.P.D.
Supplementary Material
Acknowledgments
We thank Maeli Mellotto, Esther Betrán, and Heath Blackmon for their discussion and assistance in improving the manuscript.
References
- Al Nadaf S, Waters PD, Koina E, Deakin JE, Jordan KS, Graves JAM. Activity map of the tammar X chromosome shows that marsupial X inactivation is incomplete and escape is stochastic. Genome Biol. 2010;11(12):1–18. doi: 10.1186/gb-2010-11-12-r122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers AN, Delph LF. Haldane's rule is extended to plants with sex chromosomes. Evolution. 2010;64:3643–3648. doi: 10.1111/j.1558-5646.2010.01095.x. [DOI] [PubMed] [Google Scholar]
- Burgoyne PS, Mahadevaiah SK, Sutcliffe MJ, Palmer SJ. Fertility in mice requires X–Y pairing and a Y-chromosomal “spermiogenesis” gene mapping to the long arm. Cell. 1992;71(3):391–398. doi: 10.1016/0092-8674(92)90509-b. [DOI] [PubMed] [Google Scholar]
- Clark NL, Swanson WJ. Pervasive adaptive evolution in primate seminal proteins. PLoS Genet. 2005;1(3):e35. doi: 10.1371/journal.pgen.0010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R, Lowry P. Hybrids in marsupial research. Aust J Zool. 1990;37(2–4):259–267. [Google Scholar]
- Cooper D, Vandeberg J, Sharman G, Poole W. Phosphoglycerate kinase polymorphism in kangaroos provides further evidence for paternal X-inactivation. Nature. 1971;230(13):155–157. doi: 10.1038/newbio230155a0. [DOI] [PubMed] [Google Scholar]
- Coyne JA. The genetic basis of Haldane's rule. Nature. 1985;314(6013):736–738. doi: 10.1038/314736a0. [DOI] [PubMed] [Google Scholar]
- Coyne JA. Genetics and speciation. Nature. 1992;355(6360):511–515. doi: 10.1038/355511a0. [DOI] [PubMed] [Google Scholar]
- Coyne JA, Orr HA. Patterns of speciation in Drosophila. Evolution. 1989a;43(2):362–381. doi: 10.1111/j.1558-5646.1989.tb04233.x. [DOI] [PubMed] [Google Scholar]
- Coyne JA, Orr HA. Two rules of speciation. In: Otte D, Endler JA, editors. Speciation and its consequences. Sunderland (MA): Sinauere Associates, Inc; 1989b. [Google Scholar]
- Coyne JA, Orr HA. Speciation. Sunderland (MA): Sinauer Associates, Inc; 2004. [Google Scholar]
- Deakin J, Chaumeil J, Hore T, Marshall J. Unravelling the evolutionary origins of X chromosome inactivation in mammals: insights from marsupials and monotremes. Chromosome Res. 2009;17:671–685. doi: 10.1007/s10577-009-9058-6. [DOI] [PubMed] [Google Scholar]
- Demuth JP, Wade MJ. Population differentiation in the beetle Tribolium castaneum. II. Haldane's rule and incipient speciation. Evolution. 2007;61(3):694–699. doi: 10.1111/j.1558-5646.2007.00049.x. [DOI] [PubMed] [Google Scholar]
- Eldridge MD, Close RL. Radiation of chromosome shuffles. Curr Opin Genet Dev. 1993;3(6):915–922. doi: 10.1016/0959-437x(93)90014-g. [DOI] [PubMed] [Google Scholar]
- Frank SA. Divergence of meiotic drive-suppression systems as an explanation for sex-biased hybrid sterility and inviability. Evolution. 1991;45:262–267. doi: 10.1111/j.1558-5646.1991.tb04401.x. [DOI] [PubMed] [Google Scholar]
- Gifford R, Kabat P, Martin J, Lynch C, Tristem M. Evolution and distribution of class II-related endogenous retroviruses. J Virol. 2005;79(10):6478–6486. doi: 10.1128/JVI.79.10.6478-6486.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good JM, Nachman MW. Rates of protein evolution are positively correlated with developmental timing of expression during mouse spermatogenesis. Mol Biol Evol. 2005;22(4):1044–1052. doi: 10.1093/molbev/msi087. [DOI] [PubMed] [Google Scholar]
- Graves J. Mammals that break the rules: genetics of marsupials and monotremes. Annu Rev Genet. 1996;30:233–260. doi: 10.1146/annurev.genet.30.1.233. [DOI] [PubMed] [Google Scholar]
- Graves J, ONeill R. Sex chromosome evolution and Haldane's rule. J Hered. 1997;88(5):358–360. doi: 10.1093/oxfordjournals.jhered.a023118. [DOI] [PubMed] [Google Scholar]
- Gray AP. Mammal hybrids. Farnham Royal (UK): Commonwealth Agricultural Bureaux; 1954. [Google Scholar]
- Gray AP. Bird hybrids. Farnham Royal (UK): Commonwealth Agricultural Bureaux; 1958. [Google Scholar]
- Gupta V, Parisi M, Sturgill D, Nuttall R, Doctolero M, Dudko OK, Malley JD, Eastman PS, Oliver B. Global analysis of X-chromosome dosage compensation. J Biol. 2006;5(1):1–22. doi: 10.1186/jbiol30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MW, Lanzaro GC. Female-biased gene expression in the malaria mosquito Anopheles gambiae. Curr Biol. 2005;15(6):R192–R193. doi: 10.1016/j.cub.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Haldane JBS. Sex ratio and unisexual sterility in hybrid animals. J Genet. 1922;12:101–109. [Google Scholar]
- Haldane JBS. The causes of evolution. London: Longmans, Green & Co; 1932. [Google Scholar]
- Henikoff S, Ahmad K, Malik HS. The centromere paradox: stable inheritance with rapidly evolving DNA. Science. 2001;293(5532):1098–1102. doi: 10.1126/science.1062939. [DOI] [PubMed] [Google Scholar]
- Hillis DM, Green DM. Evolutionary changes of heterogametic sex in the phylogenetic history of amphibians. J Evol Biol. 1990;3(1–2):49–64. [Google Scholar]
- Hurst LD, Pomiankowski A. Causes of sex ratio bias may account for unisexual sterility in hybrids: a new explanation of Haldane's rule and related phenomena. Genetics. 1991;128:841–858. doi: 10.1093/genetics/128.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y, Replogle K, Kim Y-H, Wade J, Clayton DF, Arnold AP. Sex bias and dosage compensation in the zebra finch versus chicken genomes: general and specialized patterns among birds. Genome Res. 2010;20(4):512–518. doi: 10.1101/gr.102343.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NA, Perez DE, Cabot EL, Hollocher H, Wu CI. A test of reciprocal X–Y interactions as a cause of hybrid sterility in Drosophila. Nature. 1992;358(6389):751–753. doi: 10.1038/358751a0. [DOI] [PubMed] [Google Scholar]
- Khaitovich P, Hellmann I, Enard W, Nowick K, Leinweber M, Franz H, Weiss G, Lachmann M, Pääbo S. Parallel patterns of evolution in the genomes and transcriptomes of humans and chimpanzees. Science. 2005;309(5742):1850–1854. doi: 10.1126/science.1108296. [DOI] [PubMed] [Google Scholar]
- Laurie CC. The weaker sex is heterogametic: 75 years of Haldane's rule. Genetics. 1997;147(3):937–951. doi: 10.1093/genetics/147.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder EH, Cano JM, Leinonen T, O'Hara RB, Nikinmaa M, Primmer CR, Merila J. Female-biased expression on the X chromosome as a key step in sex chromosome evolution in threespine sticklebacks. Mol Biol Evol. 2010;27(7):1495–1503. doi: 10.1093/molbev/msq031. [DOI] [PubMed] [Google Scholar]
- Luan L, Liu JG, Hoja MR, Lightfoot DA, Hoog C. The checkpoint monitoring chromosomal pairing in male meiotic cells is p53-independent. Cell Death Differ. 2001;8(3):316–317. doi: 10.1038/sj.cdd.4400828. [DOI] [PubMed] [Google Scholar]
- Lucchesi JC. Dosage compensation in Drosophila. Annu Rev Genet. 1973;7(1):225–237. doi: 10.1146/annurev.ge.07.120173.001301. [DOI] [PubMed] [Google Scholar]
- Lyon MF. Gene action in the X chromosome of the mouse (Mus musculus L.) Nature. 1961;190:372–373. doi: 10.1038/190372a0. [DOI] [PubMed] [Google Scholar]
- Malone JH, Fontenot BE. Patterns of reproductive isolation in toads. PLoS One. 2008;3(12):1–11. doi: 10.1371/journal.pone.0003900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mank JE, Hosken DJ, Wedell N. Some inconvenient truths about sex chromosome dosage compensation and the potential role of sexual conflict. Evolution. 2011;65(8):2133–2144. doi: 10.1111/j.1558-5646.2011.01316.x. [DOI] [PubMed] [Google Scholar]
- McDermott SR, Noor MAF. The role of meiotic drive in hybrid male sterility. Philos Trans R Soc Lond B Biol Sci. 2010;365:1265–1272. doi: 10.1098/rstb.2009.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee B. Pairing sites and the role of chromosome pairing in meiosis and spermatogenesis in male Drosophila. Curr Top Dev Biol. 1997;37:77–115. doi: 10.1016/s0070-2153(08)60172-6. [DOI] [PubMed] [Google Scholar]
- Metcalfe CJ, Bulazel KV, Ferreri GC, Schroeder-Reiter E, Wanner G, Rem W, Obergfell C, Eldridge MDB, O'Neill RJ. Genomic instability within centromeres of interspecific marsupial hybrids. Genetics. 2007;177(4):2507–2517. doi: 10.1534/genetics.107.082313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller HJ. Bearing of the Drosophila work on systematics. In: Huxley JS, editor. 1940 The new systematics. Oxford: Clarendon Press. p. 185–268. [Google Scholar]
- Muller HJ. Isolating mechanisms, evolution, and temperature. Biol Symp. 1942;6:71–125. [Google Scholar]
- Muller HJ, Pontecarvo G. Recessive genes causing interspecific sterility and other disharmonies between Drosophila melanogaster and simulans. Genetics. 1942;27:157. [Google Scholar]
- Namekawa SH, VandeBerg JL, McCarrey JR, Lee JT. Sex chromosome silencing in the marsupial male germ line. Proc Natl Acad Sci U S A. 2007;104(23):9730–9735. doi: 10.1073/pnas.0700323104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DK, Disteche CM. Dosage compensation of the active X chromosome in mammals. Nat Genet. 2006;38(1):47–53. doi: 10.1038/ng1705. [DOI] [PubMed] [Google Scholar]
- O'Neill RJ, O'Neill MJ, Graves JA. Undermethylation associated with retroelement activation and chromosome remodelling in an interspecific mammalian hybrid. Nature. 1998;393(6680):68–72. doi: 10.1038/29985. [DOI] [PubMed] [Google Scholar]
- Orr HA. A mathematical model of Haldane's rule. Evolution. 1993a;47(5):1606–1611. doi: 10.1111/j.1558-5646.1993.tb02179.x. [DOI] [PubMed] [Google Scholar]
- Orr HA. Haldane's rule has multiple genetic causes. Nature. 1993b;361(6412):532–533. doi: 10.1038/361532a0. [DOI] [PubMed] [Google Scholar]
- Orr HA, Turelli M. Dominance and Haldane's rule. Genetics. 1996;143(1):613–616. doi: 10.1093/genetics/143.1.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo-Manuel de Villena F, Sapienza C. Female meiosis drives karyotypic evolution in mammals. Genetics. 2001;159(3):1179–1189. doi: 10.1093/genetics/159.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presgraves DC. Patterns of postzygotic isolation in Lepidoptera. Evolution. 2002;56(6):1168–1183. doi: 10.1111/j.0014-3820.2002.tb01430.x. [DOI] [PubMed] [Google Scholar]
- Presgraves DC, Orr HA. Haldane's rule in taxa lacking a hemizygous X. Science. 1998;282(5390):952–954. doi: 10.1126/science.282.5390.952. [DOI] [PubMed] [Google Scholar]
- Prince EG, Kirkland D, Demuth JP. Hyperexpression of the X chromosome in both sexes results in extensive female bias of X-linked genes in the flour beetle. Genome Biol Evol. 2010;2:336–346. doi: 10.1093/gbe/evq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson BJ, Czuppon AB, Sharman GB. Inheritance of glucose-6-phosphate dehydrogenase variation in Kangaroos. Nature. 1971;230(13):154–155. doi: 10.1038/newbio230154a0. [DOI] [PubMed] [Google Scholar]
- Royo H, Polikiewicz G, Mahadevaiah SK, Prosser H, Mitchell M, Bradley A, de Rooij DG, Burgoyne PS, Turner JMA. Evidence that meiotic sex chromosome inactivation is essential for male fertility. Curr Biol. 2010;20(23):2117–2123. doi: 10.1016/j.cub.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Sasa M, Chippindale P, Johnson N. Patterns of postzygotic isolation in frogs. Evolution. 1998;52(6):1811–1820. doi: 10.1111/j.1558-5646.1998.tb02258.x. [DOI] [PubMed] [Google Scholar]
- Schilthuizen M, Giesbers MC, Beukeboom LW. Haldane's rule in the 21st century. Heredity. 2011;107:95–102. doi: 10.1038/hdy.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharman G, Close R, Maynes G. Chromosome evolution, phylogeny, and speciation of rock wallabies (Petrogale, Macropodidae) Aust J Zool. 1990;37(2–4):351–363. [Google Scholar]
- Sharp P. Sex chromosome pairing during male meiosis in marsupials. Chromosoma. 1982;86(1):27–47. doi: 10.1007/BF00330728. [DOI] [PubMed] [Google Scholar]
- Swanson WJ, Nielsen R, Yang Q. Pervasive adaptive evolution in mammalian fertilization proteins. Mol Biol Evol. 2003;20(1):18–20. doi: 10.1093/oxfordjournals.molbev.a004233. [DOI] [PubMed] [Google Scholar]
- Tao Y, Hartl DL. Genetic dissection of hybrid incompatibilities between Drosophila simulans and D. mauritiana. III. Heterogeneous accumulation of hybrid incompatibilities, degree of dominance, and implications for Haldane's Rule. Evolution. 2003;57:2580–2598. doi: 10.1111/j.0014-3820.2003.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Torgerson DG, Kulathinal RJ, Singh RS. Mammalian sperm proteins are rapidly evolving: evidence of positive selection in functionally diverse genes. Mol Biol Evol. 2002;19(11):1973–1980. doi: 10.1093/oxfordjournals.molbev.a004021. [DOI] [PubMed] [Google Scholar]
- Turelli M, Moyle LC. Asymmetric postmating isolation: Darwin's corollary to Haldane's rule. Genetics. 2007;176(2):1059–1088. doi: 10.1534/genetics.106.065979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turelli M, Orr HA. The dominance theory of Haldane's rule. Genetics. 1995;140(1):389–402. doi: 10.1093/genetics/140.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turelli M, Orr HA. Dominance, epistasis and the genetics of postzygotic isolation. Genetics. 2000;154(4):1663–1679. doi: 10.1093/genetics/154.4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Johnson N, Palopoli M. Haldane's rule and its legacy: why are there so many sterile males? Trends Ecol Evol. 1996;11:281–284. doi: 10.1016/0169-5347(96)10033-1. [DOI] [PubMed] [Google Scholar]
- Wu C-I, Davis A. Evolution of postmating reproductive isolation: the composite nature of Haldane's rule and its genetic basis. Am Nat. 1993;142(2):187–212. doi: 10.1086/285534. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Chen X, Chen Z, Wang X, Shi S, Wang X, et al. RNA sequencing shows no dosage compensation of the active X-chromosome. Nat Genet. 2010;42(12):1043–1049. doi: 10.1038/ng.711. [DOI] [PubMed] [Google Scholar]
- Zha X, Xia Q, Duan J, Wang C, He N, Xiang Z. Dosage analysis of Z chromosome genes using microarray in silkworm, Bombyx mori. Insect Biochem Mol Biol. 2009;39:315–321. doi: 10.1016/j.ibmb.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Zwick ME, Salstrom JL, Langley CH. Genetic variation in rates of nondisjunction: association of two naturally occurring polymorphisms in the chromokinesin nod with increased rates of nondisjunction in Drosophila melanogaster. Genetics. 1999;152(4):1605–1614. doi: 10.1093/genetics/152.4.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.