Summary
We characterized leukemia stem cells (LSC) in chronic phase chronic myelogenous leukemia (CML) using a transgenic mouse model. LSC were restricted to cells with long-term hematopoietic stem cell (LTHSC) phenotype. CML LTHSC demonstrated reduced homing and retention in the bone marrow (BM), related to decreased CXCL12 expression in CML BM, resulting from increased G-CSF production by leukemia cells. Altered cytokine expression in CML BM was associated with selective impairment of normal LTHSC growth and a growth advantage to CML LTHSC. Imatinib (IM) treatment partially corrected abnormalities in cytokine levels and LTHSC growth. These results were validated using human CML samples and provide improved understanding of microenvironmental regulation of normal and leukemic LTHSC and their response to IM in CML.
Introduction
Chronic myelogenous leukemia (CML) is a lethal hematological malignancy resulting from the transformation of a primitive hematopoietic cell by the BCR-ABL oncogene (Sawyers, 1999). CML leukemia stem cells (LSCs) retain the ability to regenerate multilineage hematopoiesis (Fialkow et al., 1977), and generate a vast expansion of malignant myeloid cells which retain differentiating capacity and displace residual normal hematopoiesis. CML cells also demonstrate altered trafficking resulting in increased numbers of circulating progenitors, extramedullary hematopoiesis and massive splenomegaly (Petzer et al., 1996). Leukemic cells acquire additional genetic abnormalities over time leading to disease progression from an initial chronic phase (CP) to advanced accelerated phase (AP) and blast crisis (BC) (Perrotti et al., 2011).
BCR-ABL tyrosine kinase inhibitors (TKI) are effective in inducing remission and prolonging survival in CP CML patients (Druker et al., 2006; Kantarjian et al., 2010; Saglio et al., 2010). However TKI are less effective in targeting CML stem cells compared to more mature leukemia cells (Corbin et al., 2011; Graham et al., 2002; Holtz et al., 2002). As a result LSC persist despite TKI treatment (Chomel et al., 2011), and there is a high frequency of leukemia relapse on discontinuation of treatment. Although a small subset of patients can discontinue TKI treatment without disease recurrence, most patients need to take TKIs indefinitely to prevent relapse (Mahon et al.,2011; Michor et al., 2005), with associated risk of non-compliance, toxicity, and considerable expense (Cortes et al., 2011). Improved characterization of LSC is critical to better understanding of CML pathogenesis and development of effective therapeutic strategies.
In vitro analysis of progenitor cells from CML patients has revealed enhanced proliferation, reduced apoptosis and increased differentiation in culture compared to normal progenitors. However, in vivo characterization of LSC from CP CML patients in immunodeficient mouse hosts is limited by low levels of engraftment and lack of leukemia development. Expression of BCR-ABL in murine cells using retroviral vectors followed by transplantation into irradiated hosts results in development of a myeloproliferative disorder (Li et al., 1999; Pear et al., 1998). This model has been useful to study molecular mechanisms contributing to leukemia development. However, leukemia in this model is quite fulminant, associated with pulmonary hemorrhage and death within 4 weeks, and may be more representative of advanced phase CML. In addition, although LSC are known to be present within Lin−Sca-1+c-Kit+ (LSK) cells in this model they have not been characterized with a higher degree of resolution.
An inducible transgenic mouse model of CML in which the BCR-ABL gene is expressed under control of a Tet-regulated 3′ enhancer of the murine stem cell leukemia (SCL) gene allows targeted BCR-ABL expression in stem and progenitor cells (Koschmieder et al., 2005; Schemionek et al., 2010). Induction of BCR-ABL expression by tetracycline withdrawal results in a chronic myeloproliferative disorder with neutrophilic leukocytosis and splenomegaly that resembles CP CML. Leukemia is reversed on re-introduction of tetracycline. This model allows study of leukemogenesis in vivo under steady-state conditions. In the current study, we used the transgenic BCR-ABL model to study leukemia-induced alterations in the BM microenvironment and their contributions to normal and CML LTHSC cell growth and localization, and to determine the effect of TKI treatment on these alterations.
Results
Development of CP CML-like myeloproliferative disease in BCR-ABL mice
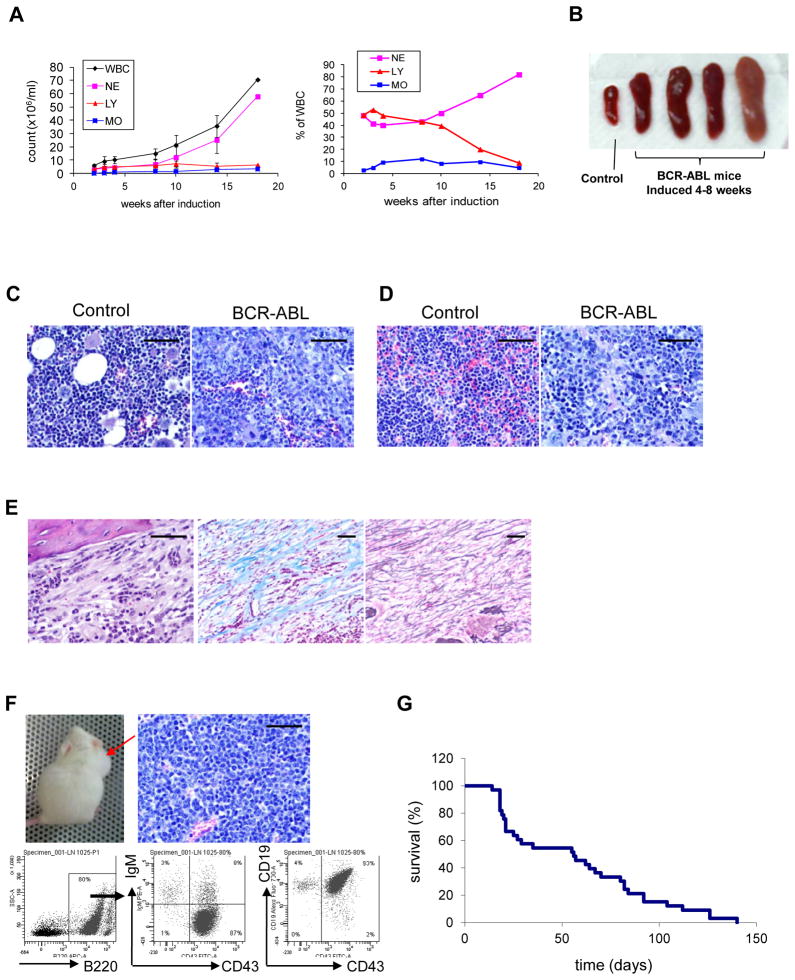
Induction of BCR-ABL expression in SCL-tTA/BCR-ABL transgenic mice (FVB/N background) by tetracycline withdrawal resulted in 4 to 6 weeks in development of a CML-like myeloproliferative disorder characterized by neutrophilic leukocytosis (Figure 1A), splenomegaly (Figure 1B), BM hypercellularity (Figure 1C), and spleen infiltration with myeloid cells (Figure 1D). A subset of mice developed BM fibrosis after 8 or more weeks (Figure 1E). Approximately 20% of mice developed lymph node enlargement, with infiltration of lymph nodes, BM and spleen with pro-B lymphoblastic cells (B220+CD43+CD19+IgM−) (Figure 1F). The median survival after induction of BCR-ABL expression was 56 days (Figure 1G). These observations suggest that the BCR-ABL mouse model is representative of CP CML, and develops features of advanced phase CML with prolonged BCR-ABL expression.
Figure 1. Development of CML-like myeloproliferative disorder in SCL-tTA/BCR-ABL transgenic mice.
Development of a myeloproliferative disorder in SCL-tTA/BCR-ABL transgenic mice within 4–6 weeks after induction of BCR-ABL expression by withdrawal of tetracycline, characterized by (A) neutrophilic leukocytosis and (B) splenomegaly (n=20). Histopathological evaluation of (C) BM and (D) spleen tissue obtained 4 weeks after induction of BCR-ABL expression showing leukocytic infiltration. (E) Development of BM fibrosis 10 weeks after induction of BCR-ABL expression as seen with H & E (left), Trichrome (center), and reticulin (right) staining. (F) Development of pro-B lymphoblastic leukemia/lymphoma with lymph node enlargement (upper left panel), lymphoblastic infiltration of lymph nodes (upper right panel), and expression of pro-B cell markers on flow cytometry (lower panel). (G) Survival curve of SCL-tTA/BCR-ABL mice after BCR-ABL induction (n=33). All scale bars represent a size of 100μm.
Changes in stem and progenitor cell populations in BCR-ABL mice
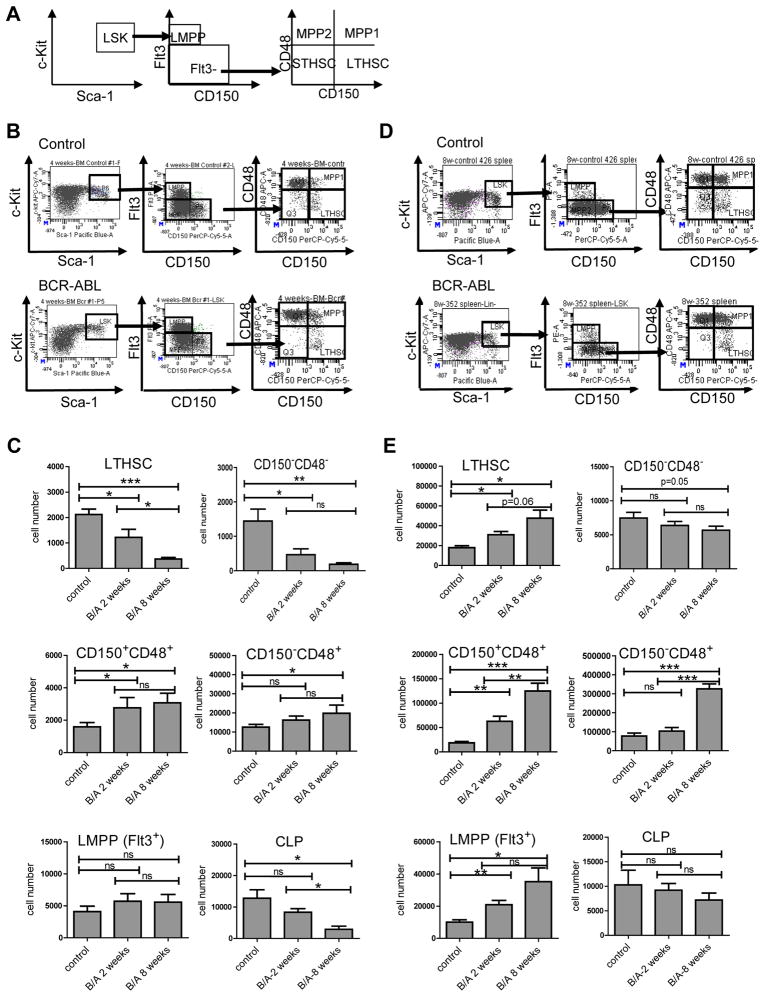
Changes in distribution of stem and progenitor populations in the BM and spleen were observed within 2 weeks after induction of BCR-ABL expression, and were more prominent after 8 weeks. Primitive LSK cells and granulocyte-macrophage progenitors (GMP) were increased in the BM of BCR-ABL mice compared to controls, whereas megakaryocyte-erythrocyte progenitors (MEP) and common myeloid progenitors (CMP) were decreased (Figure S1A-C). In normal hematopoiesis, LTHSC capacity is restricted to the Flt3−CD150+CD48−LSK subpopulation, whereas Flt3−CD150−CD48−, Flt3−CD150+CD48+ and Flt3−CD150−CD48+ LSK cells represent multipotent progenitors (MPP) lacking extensive self-renewal and long-term engrafting capacity (Figure 2A) (Akala et al., 2008; Kiel et al., 2005). Although total LSK cell numbers were increased, LTHSC numbers were markedly reduced in the BM of BCR-ABL mice (Figure 2B, C). CD150−CD48− MPP were also reduced in the BM of BCR-ABL mice. Increased LSK cells represented mainly Flt3−CD48+ MPP and Flt3+ LSK cells representing lymphoid-primed MPP (LMPP) (Mansson et al., 2007). IL-7Rα expressing common lymphoid progenitors (CLP) (Kondo et al., 1997) were reduced in BCR-ABL mice. In summary, reduction in LTHSC is associated with increase in CD48+ MPP, LMPP and GMP in the BM of BCR-ABL mice. BCR-ABL mice demonstrated increased LTHSC numbers in the spleen compared with controls (Figure 2D, E), together with a vast increase in MPP, LMPP, CMP and GMP, consistent with increased extramedullary hematopoiesis (Figure 2D, E and Figure S1D, E). Reduced BM and increased splenic LTHSC in BCR-ABL mice suggest altered LTHSC trafficking and/or niche requirements.
Figure 2. Changes in stem and progenitor cell populations in BCR-ABL expressing mice.
The populations analyzed are shown in (A) and included Lin−Sca-1+c-Kit+ (LSK) cells which were further subdivided into Flt3−CD150+CD48− (LTHSC); Flt3−CD150−CD48−, Flt3−CD150+CD48+ and Flt3−CD150−CD48+ (MPP); and Flt3+CD150− cells (LMPP). (B) Representative flow cytometry plots of LTHSC, MPP and LMPP populations in the BM of control and BCR-ABL expressing mice. (C) Total numbers of LTHSC, CD150−CD48−, CD150+CD48−, CD150−CD48+ MPP, LMPP, and CLP (common lymphoid progenitor, Lin−Sca-1Lowc-KitLowFlt3HighIL-7RαHigh) in the BM (per femur) of control and SCL-tTA/BCR-ABL mice at 2 and 8 weeks after BCR-ABL induction (n=6–8). (D) Representative flow cytometry plots of LTHSC, MPP and LMPP in the spleen of control mice and BCR-ABL expressing mice. (E) Total numbers of LTHSC, CD150−CD48−, CD150+CD48−, CD150−CD48+ MPP, LMPP, and CLP in the spleen of control mice and SCL-tTA/BCR-ABL mice at 2 and 8 weeks after BCR-ABL induction. Results represent mean±SEM. Significance values: *, p<0.05; **, p<0.01; ***, p<0.001; ns, not significant, n=6–8. See also Figure S1.
Long-term engrafting and leukemia-initiating capacity is restricted to BCR-ABL+ LTHSC
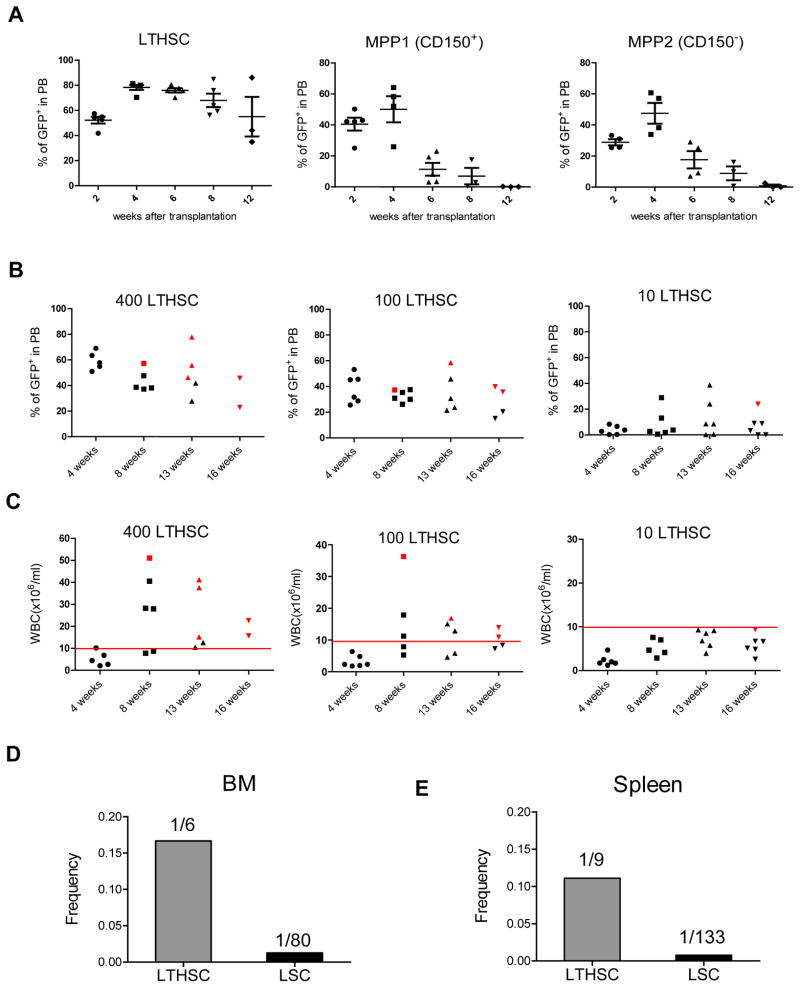
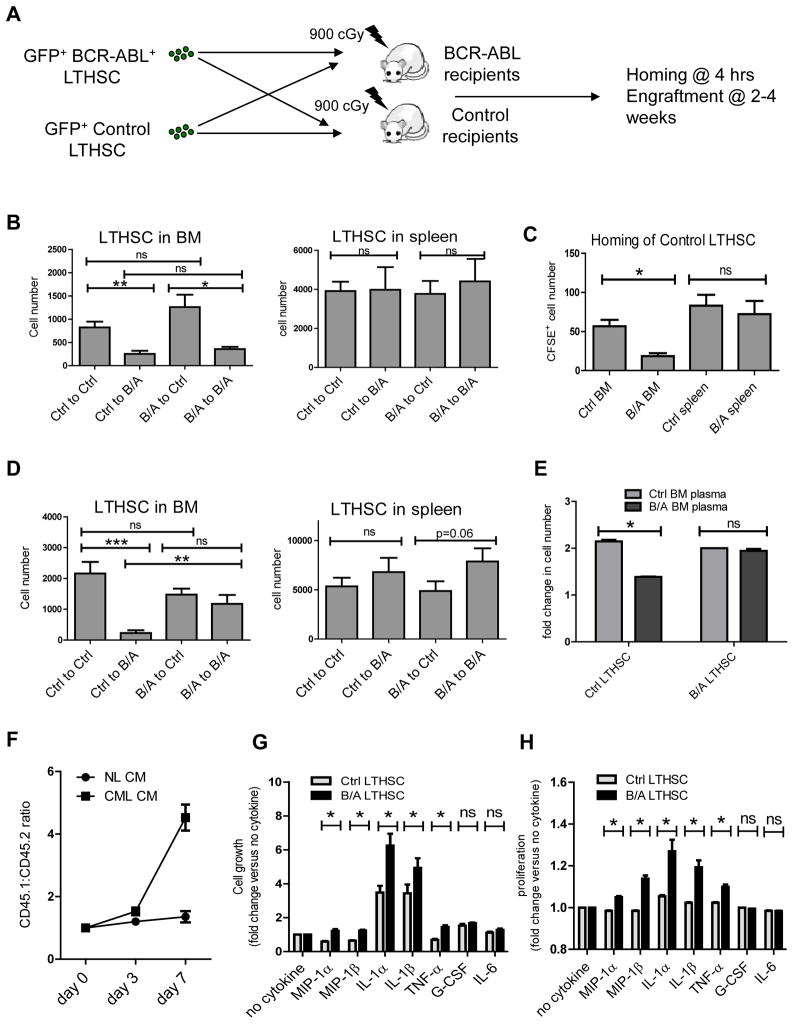
SCL-tTA/BCR-ABL mice were crossed with transgenic GFP-expressing mice to facilitate detection of donor cells after transplantation. BCR-ABL expression was induced for 4 weeks and GFP+ BM cells selected by flow cytometry and transplanted into irradiated congenic recipient mice. We observed robust engraftment of GFP+ donor cells and development of myeloproliferative disease in recipient mice (Figure S2A). Leukemia usually developed within 8 weeks post-transplant. Leukemia developing in recipient mice had a similar phenotype to that in donor mice, and was transplantable to secondary (Figure S2B) and tertiary recipients (not shown). To identify subpopulations of cells capable of long-term engraftment and leukemia-initiation, GFP+ GMP, CMP and LSK cells from BM of leukemic mice were transplanted into irradiated congenic recipients. Transplantation of LSK cells, but not CMP and GMP, resulted in long-term engraftment and leukemia development in recipient mice (Figure S2C). LSK cells from the spleen also engrafted and generated leukemia in recipient mice (Figure S2D). The frequency of functional long-term engrafting cells within the BM LSK population measured using limiting dilution analysis was significantly reduced in BCR-ABL compared to control mice (Figure S2E), consistent with reduced numbers of cells with LTHSC phenotype. Cells with LTHSC phenotype generated robust long-term engraftment with predominantly myeloid cells and development of CML-like myeloproliferative disease (Figure 3A). In contrast CD48+ MPP generated short-term engraftment with predominantly myeloid cells for up to 8 weeks but did not sustain long-term engraftment. Sufficient numbers of CD150−CD48− MPP were not obtained from CML mice for transplantation experiments. Transplantation of Flt3+ LMPP frequently resulted in development of lymphoid tumors (not shown). Tumor cells did not engraft after transplantation into secondary recipient mice. In mice that did not develop tumors, Flt3+LMPP generated short-term engraftment of lymphoblastic and myeloid cells, which was lost after 12 weeks. These observations suggest a hierarchical organization of leukemic LTHSC, MPP, LMPP within LSK cells in BCR-ABL mice as with normal hematopoiesis, with self-renewal and long-term engraftment capacity restricted to LTHSC, which was lost after differentiation to MPP. Despite having both myeloid and lymphoid capacity, leukemic LTHSC generated predominantly myeloid lineage cells. Interestingly, transplantation of limiting numbers of CML LTHSC often resulted in long-term engraftment with BCR-ABL+ cells without development of leukemia (Figure 3B, C). One in 6 BM cells with LTHSC phenotype possessed long-term repopulation activity, whereas 1 in 80 BM LTHSC were capable of initiating leukemia (defined as development of leukocytosis) within 20 weeks (Figure 3D), suggesting heterogeneity in leukemia-initiating capacity of individual BCR-ABL+ LTHSC. The frequency of functional long-term engrafting stem cells and leukemia-inducing cells within phenotypically defined LTHSC in the spleen was similar to BM LTHSC (Figure 3E).
Figure 3. Long-term engraftment and leukemia induction by BCR-ABL expressing LTHSC.
(A) Levels of donor GFP+ cells in peripheral blood after transplantation of BCR-ABL expressing LTHSC (500 cells/mouse), MPP1 (CD150+CD48−) (2,000 cells/mouse), and MPP2 (CD150−CD48+) (2,000 cells/mouse) into irradiated recipients. Mice were followed for 16 weeks. The mean±SEM are shown. (B) Engraftment of GFP+ cells in peripheral blood, and (C) peripheral blood WBC counts in recipient mice after transplantation of BCR-ABL-expressing LTHSC in limiting dilutions. Red spots indicate mice that died of leukemia within 20 weeks. The calculated frequency of functional long-term engrafting and leukemia-initiating cells within cells with LTHSC (Lin−Sca-1+Kit+Flt3−CD150+CD48−) phenotype in the (D) BM, and (E) spleen of BCR-ABL mice is shown. The 95% confidence interval for LTHSC and LSC in the BM is 1/15-1/2 and 1/198-1/34 respectively. The 95% confidence interval for LTHSC and LSC in the spleen is 1/21-1/4 and 1/286-1/62 respectively. See also Figure S2.
BCR-ABL expression and cell cycle status of LTHSC
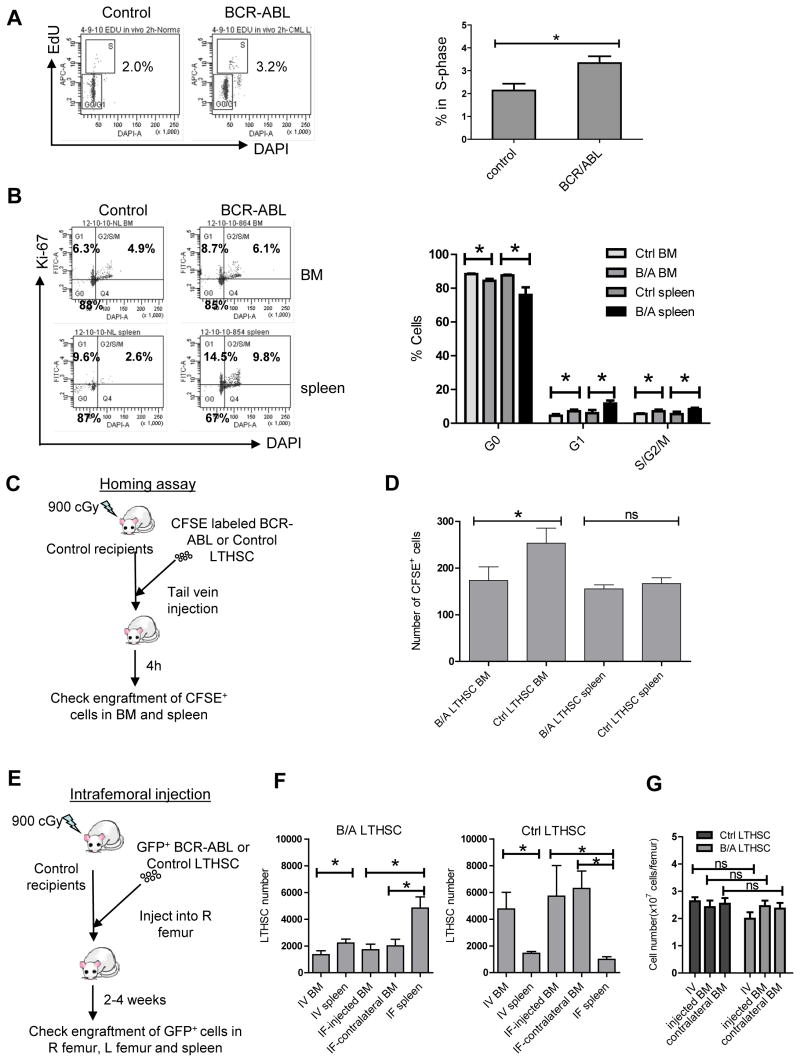
Expression of BCR-ABL mRNA levels was higher in LSK cells compared with CMP, GMP and MEP cells (Figure S3A). Within LSK cells BCR-ABL expression was higher in MPP compared with LTHSC, but was reduced in LMPP (Figure S3B). Although the BCR-ABL transgene expression through the SCL promoter in transgenic mice may differ from expression driven by the endogenous human promoter, the patterns of BCR-ABL expression in murine cells are in general consistent with previous reports for human CML subpopulations (Jamieson et al., 2004). Cell cycle status of stem and progenitor populations was evaluated by administration of 5-ethynyl-2′-deoxyuridine (EdU). EdU labeling of BM LTHSC was significantly reduced compared to total LSK cells in both normal and CML mice (Figure S3C). However, cycling of BCR-ABL+ LTHSC was increased compared to control mice both in vivo (Figure 4A) and in vitro (data not shown). Ki-67 and DAPI labeling a modest increase in the proportion of BM LTHSC from BCR-ABL mice in S/G2/M phase compared to control mice (Figure 4B). LTHSC from the spleen of BCR-ABL mice showed significantly increased S/G2/M phase cells and reduced G0 cells compared to splenic LTHSC from control mice. These results demonstrate modest increase in cycling of LTHSC in the BM and greater increase in cycling of LTHSC in the spleen of BCR-ABL mice.
Figure 4. Alterations in cell cycle, homing and trafficking of BCR-ABL expressing LTHSC.
(A) Percentage of cycling LTHSC in the BM of BCR-ABL and control mice evaluated 2 hours after in vivo administration of EdU. Representative flow cytometry plots (left) and combined results (right) are shown (n=3). (B) The proportion of LTHSC from the BM and spleen of BCR-ABL and control mice in G0, G1 and S/G2/M phase evaluated by labeling with Ki-67 and DAPI. Representative flow cytometry plots (left) and combined results (right) are shown (n=3). (C) CFSE-labeled LTHSC were transplanted by tail vein injection into irradiated congenic recipient mice and the number of CFSE expressing cells in the BM and spleen of recipient mice were evaluated 4h after injection. (D) Homing of BCR-ABL and control LTHSC to the marrow and spleen of recipient mice after IV injection is shown (n=8). (E) GFP+ BCR-ABL or control LTHSC (1,000 cells/mouse) were injected directly into the right femur of irradiated congenic mice. Donor GFP+ LTHSC numbers in the injected femur; contralateral femur and spleen were analyzed 2 weeks and 4 weeks after transplantation. (F) Mice receiving control and BCR-ABL+ LTHSC administered intrafemorally or IV were analyzed after 4 weeks (n=8 for both). The number of donor GFP+ LTHSC in the injected femur, contralateral femur and the spleen were analyzed following intra-femoral transplantation, and the number of LTHSC per femur and in the spleen were analyzed following IV transplantation. (G) The BM cellularity at 4 weeks after transplantation is shown. Results represent mean±SEM. Significance values: *, p<0.05; ns, not significant, compared with controls, or as indicated. See also Figure S3.
Reduced homing and retention of BCR-ABL expressing LTHSC in BM and increased egress to spleen
We evaluated whether altered LTHSC localization, with reduced BM LTHSC and increased splenic LTHSC, was related to impaired LTHSC homing and/or reduced retention in BM. To evaluate homing, CFSE-labeled LTHSC were injected intravenously into irradiated recipients and CFSE-labeled cells in the BM and spleen of recipient mice evaluated after 4 hours (Figure 4C). There was reduced homing of BCR-ABL+ LTHSC to the BM of recipient mice compared to controls (p=0.04), with similar levels of homing to spleen (Figure 4D). To study retention of LTHSC in the BM, BCR-ABL+ and control LTHSC were injected directly into the femur of irradiated congenic mice (Figure 4E). We observed significantly increased numbers of BCR-ABL+ LTHSC in the spleen and decreased numbers of BCR-ABL+ LTHSC in the injected and contralateral femur of recipient mice at 4 weeks post-injection (Figure S3D). In contrast, control LTHSC were present in increased numbers in the BM and lower numbers in the spleen of recipient mice, after both intrafemoral and intravenous transplantation (Figure 4F). Increased egress of BCR-ABL+ LTHSC was not related to increased BM cellularity since total BM cell counts were not increased in CML mice compared to normal mice (Figure 4G). These results indicate that both reduced homing to the BM and enhanced egress from the BM to the spleen contribute to altered LTHSC localization in BCR-ABL mice.
Altered chemokine and cytokine expression in BCR-ABL mice
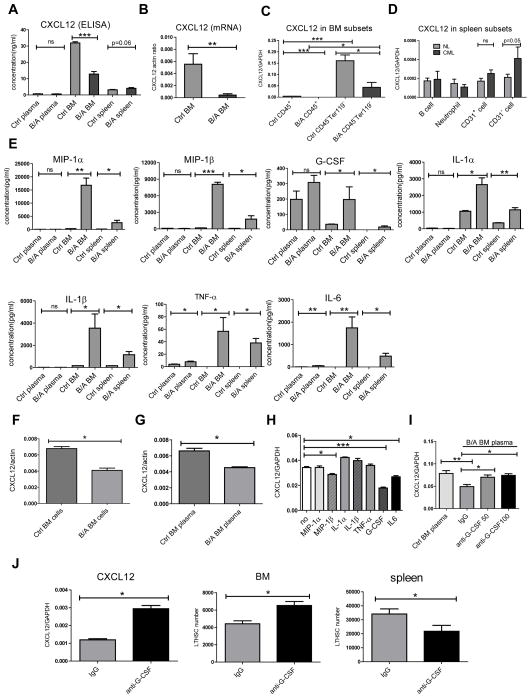
The chemokine CXCL12 mediates hematopoietic cell localization to the BM through interactions with the CXCR4 receptor on hematopoietic cells (Peled et al., 1999). ELISA analysis demonstrated significantly reduced CXCL12 levels in BM and a marginal increase of CXCL12 levels in the spleen of BCR-ABL mice compared to control mice (Figure 5A). We also observed reduced CXCL12 mRNA levels in BM cells from BCR-ABL mice compared to controls (Figure 5B). CXCL12 mRNA was expressed at higher levels in CD45− Ter119− non-hematopoietic stromal cells compared with CD45+ hematopoietic cells (Figure 5C). CXCL12 mRNA levels were significantly lower in BM stromal cells from BCR-ABL mice compared to controls. Reduced CXCL12 expression may contribute to reduced retention of LTHSC in the BM of BCR-ABL mice. In the spleen, CXCL12 was mainly expressed in CD45−Ter119−CD31− non-hematopoietic, non-endothelial stromal cells, and levels were increased in CML compared to control mice (Figure 5D).
Figure 5. Altered chemokine and cytokine expression in BCR-ABL mice.
(A) CXCL12 levels measured by ELISA in peripheral blood and BM plasma and spleen supernatants from control and BCR-ABL mice. CXCL12 mRNA levels measured by Q-RT-PCR in (B) total BM cells, (C) BM hematopoietic cells (CD45+) and BM stromal cells (CD45−Ter119−), and (D) splenic B cells (CD45+B220+), neutrophils (CD45+Gr-1+), endothelial cells (CD45−Ter119−CD31+) and non-endothelial stromal cells (CD45−Ter119−CD31−) cells, from control and BCR-ABL mice. (E) Expression of cytokines and chemokines in peripheral blood and BM plasma, and spleen supernatants, from BCR-ABL-expressing and control mice measured by ELISA using Luminex xMAP technology. CXCL12 mRNA levels in M2-10B4 murine stromal cells co-cultured for 48 hours with (F) BM cells, and (G) BM plasma from BCR-ABL and control mice. (H) CXCL12 mRNA levels in M2-10B4 murine stromal cells cultured for 24 hours in serum-free medium without addition of cytokines, or with MIP-1α (16ng/ml), MIP-1β (8ng/ml), IL-1α (2.5ng/ml), IL-1β (3.5ng/ml), TNF-α (0.05ng/ml), G-CSF (0.2ng/ml) or IL-6 (2ng/ml). (I) CXCL12 mRNA levels in M2-10B4 murine stromal cells following culture for 24 hours with BM plasma from control and BCR-ABL mice together with a function blocking anti-mouse G-CSF antibody (50ng/ml and 100ng/ml) or isotype control antibody. (J) BCR-ABL mice were induced for one week and then were treated with function blocking anti-mouse G-CSF antibody (10μg/mouse, IV, once per day) or isotype control antibody for an additional two weeks. CXCL12 mRNA levels in the BM and the numbers of LTHSC cells in the BM and spleen were measured. Results represent mean±SEM. Significance values: *, p<0.05; **, p<0.01; ***, p<0.001; ns, not significant. See also Figure S4.
We evaluated the levels of additional chemokines and cytokines in BCR-ABL and control mice using Luminex xMAP technology. In contrast to reduced CXCL12 levels, several chemokines including MIP-1α and MIP-1β; and cytokines including IL-1α, IL-1β, IL-6, G-CSF, TNF-α and LIF, showed increased expression in BM of BCR-ABL mice (Figure 5E and Figure S4A-M). Expression of mRNA for MIP-1α, MIP-1β, IL-1α, IL-1β, IL-4, MIP-2 and IL-6 was significantly higher in both hematopoietic cells and, to a lesser extent, non-hematopoietic cells from the BM of BCR-ABL compared to control mice (Figure S4N-T). Levels of several chemokines/cytokines were also increased in the spleen of BCR-ABL mice compared with control mice (Figure 5E and S4A-M). An additional subset of chemokines and cytokines, including VEGF, IL-9 and MIG, were selectively enhanced in the spleen but not BM of BCR-ABL compared to control mice. These observations indicate complex alterations of cytokine and chemokine production by leukemic and stromal cells in the BM and splenic microenvironment of BCR-ABL mice.
Co-culture with BM cells and BM plasma from BCR-ABL mice resulted in reduced CXCL12 mRNA levels in murine BM stromal cells (M2-10B4), compared to BM cells (Figure 5F) and BM plasma (Figure 5G) from control mice, indicating that reduced CXCL12 expression is related to diffusible factors produced by leukemia cells. Culture of stromal cells with MIP-1α, MIP-1β, IL-1α, IL-1β, TNF-α, G-CSF or IL6 (at concentrations similar to those measured in BCR-ABL BM plasma) indicated that CXCL12 mRNA levels are reduced in stromal cells cultured with G-CSF, and to a lesser extent MIP-1β and IL-6 (Figure 5H). Addition of a function blocking anti-G-CSF antibody increased CXCL12 levels in stromal cells exposed to CML BM plasma (Figure 5I). Finally BCR-ABL mice treated with function blocking anti-mouse G-CSF antibody or isotype control antibody demonstrated enhanced CXCL12 levels in BM cells, and enhanced numbers of BM and reduced numbers of splenic LTHSC (Figure 5J). These results support an important role for G-CSF-mediated reduction in CXCL12 levels in altered LTHSC localization in BCR-ABL mice.
Differential regulation of normal and leukemic LTHSC growth by the CML BM microenvironment
In view of the observed abnormalities of chemokine and cytokine expression, we investigated whether BCR-ABL mice demonstrated altered capacity to support LTHSC engraftment. LTHSC from control or BCR-ABL mice were transplanted into irradiated BCR-ABL mice (induced for 4 weeks) or control recipient mice by tail vein injection (Figure 6A). BCR-ABL mice showed mild to modest elevation in WBC count; most of them had not developed leukemia, and were not sick. Since it was not clear how long leukemia-induced microenvironmental alterations could be sustained after myeloablative treatment, mice were followed for up to 4 weeks after transplantation. We observed reduced engraftment of both control and BCR-ABL+ LTHSC in the BM of BCR-ABL recipients compared with control recipients 2 weeks after transplantation (Figure 6B), associated with reduced homing of control LTHSC (Figure 6C) and BCR-ABL+ LTHSC (Figure 4D) to the BM of BCR-ABL compared to control mice, possibly related to low BM CXCL12 levels (Figure 5A, B). The numbers of control LTHSC in the BM of BCR-ABL recipient mice remained low, whereas BCR-ABL+ LTHSC were increased 4 weeks after transplant and similar to the numbers seen in the BM of control recipients (Figure 6D). Similar results were seen for CD48+MPP, committed progenitors and total GFP+ leukemic cells (Figure S5A, C). There was no difference in the number of engrafted LTHSC or progenitor cells in the spleen of BCR-ABL and control mice at 2 weeks (Figure 6B, S5B), but there was a modest increase in LTHSC but not progenitor engraftment in the spleen of BCR-ABL mice at 4 weeks after transplantation (Figure 6D, S5D). These results suggest that increased splenic CXCL12 expression may not be sufficient to increase LTHSC homing, but may contribute to increased relocalization of LTHSC to the spleen at later times. Lack of increased homing of normal LTHSC to CML spleen despite increased CXCL12 levels may reflect the involvement of additional factors besides CXCL12 in the homing process.
Figure 6. Differential regulation of normal and leukemic LTHSC growth by the CML BM microenvironment.
(A) GFP+ LTHSC (1,000 cells/mouse) from control or BCR-ABL mice were transplanted into irradiated BCR-ABL or control mice by tail vein injection (n=16 for each group, with a total of 64 mice at 2 weeks and 64 mice at 4 weeks). (B) The numbers of GFP+ LTHSC in the BM and spleen of irradiated control and BCR-ABL recipient mice at 2 weeks post-transplant. (C) Homing of CFSE-labeled control LTHSC transplanted by tail vein injection into irradiated control and BCR-ABL recipient mice evaluated 4 hours after injection. (n=8). (D) The numbers of GFP+ LTHSC in the BM and spleen of irradiated control and BCR-ABL recipient mice at 4 weeks post-transplant. (E) Control LTHSC and BCR-ABL+ LTHSC were cultured with BM plasma obtained from control and BCR-ABL mice. Results shown represent fold-change in cell numbers after 2 days of culture (n=3). (F) BCR-ABL+ LTHSC (CD45.1) and control LTHSC (CD45.2) were mixed in a 1:1 ratio and cultured with CML or control BM conditioned medium (CM). The proportion of input cells was checked using flow cytometry. The ratio of CD45.1:CD45.2 cells at different times is shown (n=3). CFSE+ LTHSC from control and BCR-ABL-expressing mice were cultured with serum-free medium without cytokine or supplemented with MIP-1α (16ng/ml), MIP-1β (8ng/ml), IL-1α (2.5ng/ml), IL-1β (3.5ng/ml), TNF-α (0.05ng/ml), G-CSF (0.2ng/ml) or IL-6 (2ng/ml) for 72 hours and cell growth (G) and proliferation (H) were measured. Results represent mean±SEM. Significance values: *, p<0.05; **, p<0.01; ***, p<0.001; ns, not significant. See also Figure S5.
In vitro exposure to BM plasma from CML mice resulted in impaired growth of control LTHSC compared to BM plasma from control mice. In contrast growth of BCR-ABL+ LTHSC was similar after exposure to CML and control BM plasma (Figure 6E), suggesting that diffusible factors produced by leukemia cells contribute to reduced growth of normal compared to BCR-ABL+ LTHSC in the BM microenvironment of BCR-ABL mice, resulting in a growth advantage for the leukemic clone. To directly test the hypothesis we performed a competitive in vitro assay in which BCR-ABL+ LTHSC (CD45.1) and control LTHSC (CD45.2) were mixed in a 1:1 ratio and cultured with CML or control BM conditioned medium (CM). The proportion of the input cells was checked every 3 days using flow cytometry. We observed a markedly enhanced proportion of BCR-ABL+ compared with control cells following culture with CML CM, whereas the proportion of BCR-ABL+ and control cells was similar following culture with control CM (Figure 6F). These results indicate that factors produced by CML BM cells confer a competitive advantage to BCR-ABL+ LTHSC over control LTHSC. We further show that growth of control LTHSC was reduced compared to BCR-ABL+ LTHSC in the presence of MIP-1α, MIP-1β, IL-1α, IL-1β and TNF-α (at concentrations similar to those in BM plasma from BCR-ABL mice) (Figure 6G). Reduced growth was related to reduced proliferation of control compared to BCR-ABL+ LTHSC (Figure 6H), without significant changes in apoptosis (Figure S5E).
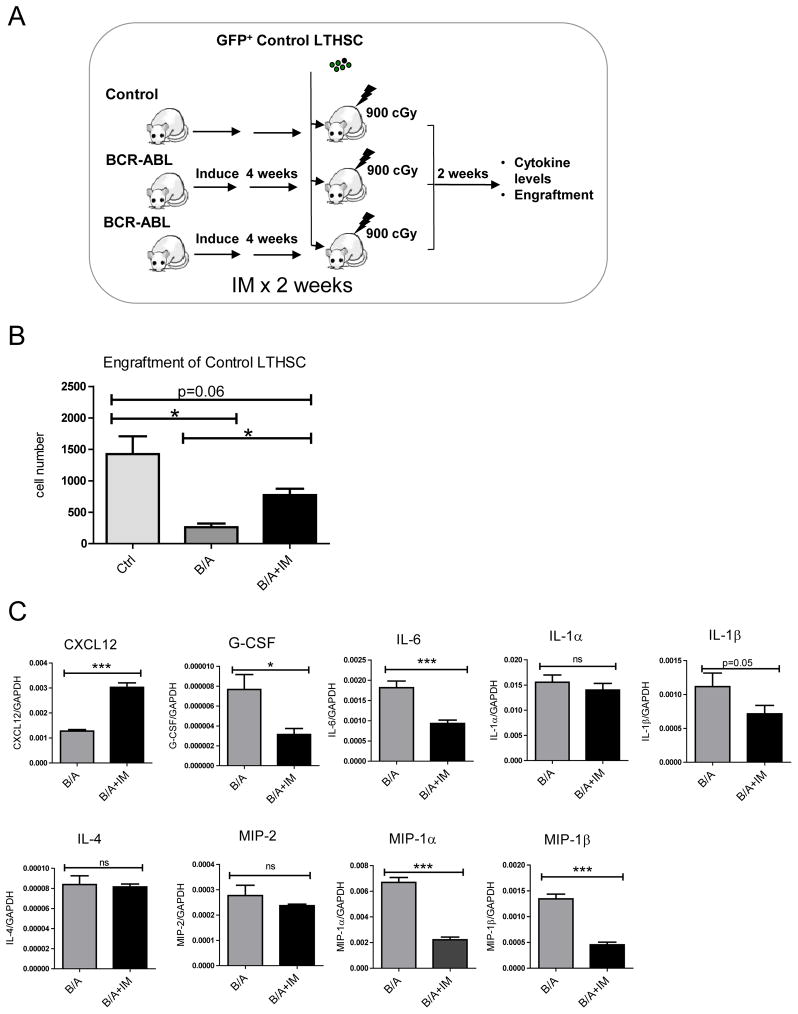
We examined the effect of IM treatment on BM microenvironmental function in BCR-ABL mice. LTHSC from control mice were transplanted into irradiated control or BCR-ABL mice that had been treated or not treated with IM for 2 weeks (Figure 7A). LTHSC engraftment was increased in IM-treated BCR-ABL mice, indicating at least partial correction of the microenvironmental defect (Figure 7B). We observed increased expression of CXCL12 and reduced expression of G-CSF, IL-6, IL-1β, MIP-1α and MIP-1β in BM cells from IM-treated compared to untreated BCR-ABL mice (Figure 7C). Levels of IL-1α, IL-4, and MIP-2 were not significantly changed. Enhanced growth of normal LTHSC in the BM of BCR-ABL mice pre-treated with IM was associated with enhanced CXCL12 and reduced cytokine levels.
Figure 7. Effect of IM treatment on cytokine levels on CML BM microenvironmental function.
(A)1,000 GFP+ control LTHSC/mouse were sorted and transplanted into irradiated control mice, BCR-ABL mice (induced 4 weeks) and BCR-ABL mice treated with IM for the last 2 weeks of the induction period (n=8 each). (B) Engraftment of control LTHSC in the BM of irradiated control, BCR-ABL and IM-treated BCR-ABL mice. (C) Cytokine and chemokine mRNA levels in the BM of BCR-ABL mice treated with or without IM were measured. Results represent mean±SEM. Significance values: *, p<0.05; **, p<0.01, n=8.
Cytokine and chemokine expression in human CML BM cells
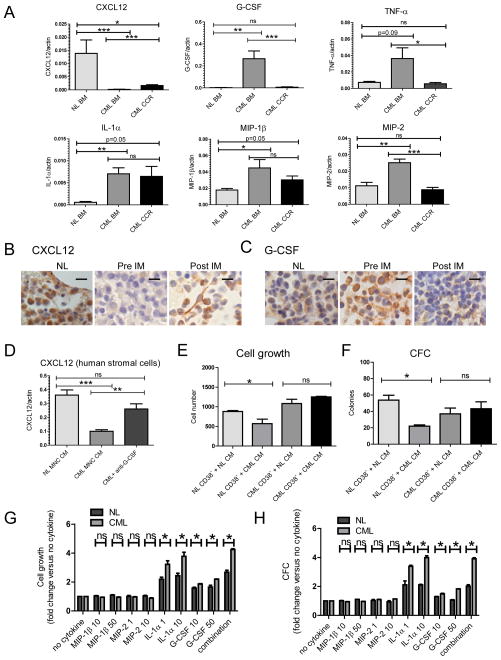
We analyzed cytokine and chemokine expression in BM cells from newly diagnosed CML CP patients, CML CP patients in complete cytogenetic remission (CCR) on IM treatment, and from healthy controls. CML BM cells demonstrated reduced expression of CXCL12, and increased expression of G-CSF, IL-1α, MIP-1β, and MIP-2, compared to normal BM cells. BM cells from patients in CCR showed increased CXCL12 and reduced G-CSF, TNF-α and MIP-2 expression, without significant change in other factors (Figure 8A, S6A). However, CXCL12 levels in CML patients in CCR remained significantly lower than in normal BM. Immunohistochemical analysis of BM sections from newly diagnosed CML CP patients showed reduced CXCL12 labeling compared to untreated lymphoma patients without BM involvement as controls. BM sections from the same CML patients after 6 months or more of IM treatment showed increased CXCL12 staining (Figure 8B, S6B). CXCL12 expression was seen in stromal cells and in a subset of mature hematopoietic cells. In contrast, G-CSF expression was increased in hematopoietic cells in BM sections from CML patients compared to controls, and was reduced after IM treatment (Figure 8C, S6B). Primary human stromal cells immortalized with hTERT (Mihara et al., 2003) were exposed to conditioned medium (CM) made with BM cells from control and CML CP patients. Exposure to CML CM resulted in reduced CXCL12 in stromal cells compared to control CM (Figure 8D). CXCL12 expression was partially restored by addition of an anti-G-CSF blocking antibody. These results support a role for increased G-CSF expression by CML hematopoietic cells in reduced CXCL12 production by human stromal cells, validating the results obtained with the murine model.
Figure 8. Cytokine and chemokine expression in human CML BM cells.
(A) Cytokine and chemokine mRNA levels in BM MNC from control, newly diagnosed CML CP patients and CML CP patients in complete cytogenetic remission (CCR) on IM treatment were measured by Q-RT-PCR, and results normalized to actin expression. (B) CXCL12 and (C) G-CSF expression in BM sections obtained from untreated lymphoma patients without BM involvement by disease (NL), newly diagnosed CML CP patients prior to starting IM treatment (pre-IM) and from the same patients after 6 months or more of IM treatment (Post-IM) were analyzed by Immunohistochemistry (n=3). Scale bars represent a size of 10μm. (D) Primary human stromal cells immortalized with hTERT expression were exposed to CM made with BM MNC from healthy controls and CML CP patients for 24 hours. The effect of addition of a blocking anti-human G-CSF (10ug/ml) antibody or isotype control antibody on CXCL12 mRNA levels was evaluated. (E) CD34+CD38− cells from control and newly diagnosed CML CP patients were cultured for 48 hours with CM made with BM MNC from healthy controls and newly diagnosed CML CP patients and cell growth measured using a luminescent cell viability assay and (F) in CFC assays. CD34+ cells from normal and newly diagnosed CML CP patients were labeled with CFSE, and CFSE+ Lin−CD34+CD38− cells were sorted and cultured for 72 hours in serum-free medium either without cytokines, or supplemented with MIP-1β (10 and 50ng/ml), MIP-2 (1 and 10ng/ml), IL-1α (1 and 10ng/ml), G-CSF (10 and 50ng/ml) or the combination (10ng/ml MIP-1β, 1ng/ml MIP2, 1ng/ml IL-1α, 50ng/ml G-CSF). Cell growth was measured using a (G) luminescent viability assay and (H) in CFC assay. Results represent mean±SEM. Significance values: *, p<0.05; **, p<0.01; ***, p<0.001; ns, not significant. See also Figure S6.
Primitive CD34+CD38− cells from newly diagnosed CML CP patients and control healthy individuals were cultured with CP CML and normal BM CM. Culture with CML CM reduced growth of normal but not CML CD34+CD38− cells in luminescent cell viability (Figure 8E) and CFC assays (Figure 8F). Human CML and normal CD34+CD38− cells were also cultured without cytokines, or with MIP-1β, MIP-2, IL-1α, G-CSF, the four cytokines significantly increased in the BM cells from CML CP patients. IL-1α and G-CSF treatment resulted in significantly less expansion of normal compared to CML CD34+CD38− cells, compared to controls cultured without cytokines in luminescent cell viability (Figure 8G) and CFC assays (Figure 8H), related to reduced proliferation of normal compared to CML CD34+CD38− cells (Figure S6C), consistent with results obtained with the mouse model.
Discussion
The transgenic BCR-ABL mouse demonstrates gradual development of a myeloproliferative disorder with neutrophilic leukocytosis, extramedullary hematopoiesis and splenomegaly, and provides a representative model of human CP CML. We used the transgenic BCR-ABL mouse model to identify LSC with a high degree of resolution and to characterize their microenvironmental regulation. Long-term engraftment and leukemia-initiating capacity was restricted to a small sub-fraction of LSK cells that had the LTHSC (Flt3−CD150+CD48−) phenotype. In contrast LSK cells with MPP or LMPP phenotypes generated only short-term engraftment. These results indicate that CML originates from transformation at the level of the LTHSC rather than more differentiated MPP or LMPP and that leukemic hematopoiesis follows a hierarchical differentiation pattern as seen in normal hematopoiesis. These results are consistent with a recent report where SCL-tTA/BCR-ABL mice in the C57BL/6 background were studied (Reynaud et al., 2011). In contrast to the FVB/N strain, leukemia developing in C57BL/6 background mice is more aggressive with a higher incidence of BM fibrosis and hypocellularity, and secondary lymphoid disorders are not seen, suggesting that strain-specific factors modulate the phenotype and course of leukemia. Our results contrast with observations made in BC CML and acute myeloid leukemia where more mature LMPP or GMP progenitors may acquire self-renewal and leukemia-initiating capacity (Jamieson et al., 2004; Sarry et al.).
LTHSC numbers were reduced in the BM of CML mice, with a concomitant increase in MPP, suggesting enhanced LTHSC differentiation and enhanced proliferation and expansion of MPP. CML MPP generated short-term engraftment with predominantly myeloid cells after transplantation, whereas CML LMPP generated expanded populations of immature pro-B cells after transplantation. Although CML LTHSC can generate progeny with both myeloid and lymphoid potential, selective expansion of the myeloid population is seen following the transplantation of purified LTHSC, and lymphoid cells are suppressed. The recent report that IL-6 produced by leukemic cells inhibits lymphoid and promotes myeloid differentiation and expansion of the leukemic clone could provide an explanation for these findings (Reynaud et al., 2011).
We observed reduced BM and increased splenic LTHSC in BCR-ABL mice related to decreased LTHSC homing to the BM, reduced retention in the BM and increased egress to the spleen, and enhanced proliferation in the spleen. The abnormal trafficking and growth of BCR-ABL+ LTHSC indicates alterations in microenvironmental interactions compared to normal LTHSC. Intrinsic defects in adhesion receptors, including β1 integrins (Verfaillie et al., 1992) and CD44 (Krause et al., 2006), may be seen in CML progenitor cells. In addition, migration of CML progenitors to CXCL12 is reported to be reduced compared to normal progenitors (Jin et al., 2008; Peled et al., 2002). We identified reduction in BM CXCL12 levels as a mechanism underlying impaired LTHSC homing and retention in CML BM. CXCL12 expression in BM stromal cells was reduced in both BCR-ABL mice and CML patients. Since CXCL12 regulates LTHSC quiescence, reduced CXCL12 levels could directly contribute to increased cycling of LTHSC in the BM of BCR-ABL mice (Cashman et al., 2002). In addition, reduced CXCL12 expression could contribute to abnormal regulation of LTHSC proliferation and differentiation indirectly by altering their niche localization.
Growth of normal LTHSC was reduced in the BM of BCR-ABL mice compared to control mice, whereas BCR-ABL+ LTHSC demonstrated similar growth in the BM of BCR-ABL and normal mice, related to diffusible factors produced by CML BM cells. These results are consistent with our previous observations that normal human progenitors demonstrate impaired in vitro growth on stromal layers derived from human CML BM, but that growth of human CML progenitors is similar on normal and CML BM derived stromal layers (Bhatia et al., 1995), and suggest that altered BM microenvironmental function contributes to suppression of normal LTHSC and provides a selective advantage to LSC in CML. Expression of several other chemokines and cytokines was significantly increased in the BM of BCR-ABL mice. These cytokines were produced mainly by leukemic hematopoietic cells. We identified the chemokines MIP-1α and β, and the cytokines IL-1α and β and TNF-α as supporting reduced proliferation and growth of normal compared to CML LTHSC. Relevant to these findings it has been previously reported that certain chemokines selectively inhibit the proliferation of normal progenitors while sparing CML progenitors (Cashman et al., 1998; Eaves et al., 1993); and that CML LTHSC have enhanced expression of proteins affecting IL-1 signaling (Jaras et al., 2010). Additional as yet unidentified inhibitory factors could also contribute to selective inhibition of normal LTHSC in the CML BM microenvironment. Growth of normal LTHSC was enhanced in the BM microenvironment of IM-treated compared to untreated BCR-ABL mice, in association with reduced levels of several of the abnormally expressed cytokines. These results support a role for specific cytokines produced by leukemic cells within the CML BM microenvironment in conferring a growth advantage to CML compared with normal LTHSC.
Cytokine alterations, in addition to direct effects on LTHSC, may also indirectly affect LTHSC by altering the function of stromal cells (Bonig et al., 2006). The cytokines G-CSF, IL-1 and TNF-α can suppress expression of CXCL12 in osteoblasts or fibroblastic cells (Peng et al., 2006; Zhang et al., 2008). We identified an important role for G-CSF produced by leukemic cells in reducing CXCL12 expression in CML BM stromal cells. Inhibition of G-CSF activity increased CXCL12 levels in BM stromal cells in vitro and in vivo, leading to increased retention of LTHSC in the BM and reduced LTHSC in the spleen of BCR-ABL mice. CXCL12 expression was increased and G-CSF expression was reduced in the BM of IM-treated BCR-ABL mice and in the BM of CML patients in remission following IM treatment. Restoration of normal niche interactions could play a role in leukemia control in a physiological setting. However CXCL12 levels in CML BM after IM treatment remained lower than in normal BM, indicating that recovery of CXCL12 expression is incomplete. Although our studies demonstrate reduced CXCL12 mRNA expression by stromal cells in CML, we recognize that additional mechanisms such as increased CXCL12 cleavage by proteolytic enzymes could also be active (Cho et al., 2010).
These studies support important new concepts regarding the contribution of leukemia-induced alterations in the BM microenvironment to a selective growth advantage to leukemic compared with normal LTHSC, and have relevance to our understanding of response and resistance to TKI at the organismal level. Although IM treatment corrects several abnormalities in cytokine and chemokine expression in CML cells, and reduces normal LTHSC inhibition by leukemic cells and facilitates their regrowth, it does not completely reverse leukemia-associated changes in the microenvironment. It will be important to determine the mechanisms underlying these persistent changes, and how leukemia-related alterations in the hematopoietic microenvironment affect LSC response to TKI treatment, regrowth of normal LTHSC, and persistence of LSC in CML patients on prolonged TKI treatment. These insights could lead to improved strategies to eliminate resistant LSC populations in TKI-treated patients.
Experimental Procedures
Mice
Inducible, transgenic SCL-tTa/BCR-ABL mice in the FVB/N background (Huettner et al., 2005) were maintained with administration of tetracycline in the drinking water (0.5 g/L) and were crossed with transgenic GFP-expressing mice (FVB.Cg-Tg [ACTB-EGFP] B5Nagy/J, Jackson Laboratories) for transplantation experiments to facilitate tracking of donor cells. If not indicated, BCR-ABL expression was induced for 3 weeks by tetracycline withdrawal. Mouse care and experimental procedures were performed in accordance with federal guidelines and protocols approved by the Institutional Animal Care and Use Committee at the City of Hope.
Samples
Aliquots of normal peripheral blood and BM mononuclear cells (MNC) were obtained from allogeneic transplant donors. CML samples were obtained from patients in CP who had not received prior IM treatment from City of Hope and the University of Glasgow. Sample acquisition was approved by the Institutional Review Boards (IRB) at City of Hope, and the North Glasgow University Hospital Division of NHS Greater Glasgow and Clyde in accordance with the Declaration of Helsinki. All donors signed informed consent forms. BM sections from NHL patients were obtained from the City of Hope Biospecimen Repository, as anonymized specimens, using an IRB-approved protocol which exempted consent requirements. MNC were isolated using Ficol separation. CD34+ cells were isolated using a positive magnetic bead selection protocol (Miltenyi).
Flow cytometry
Cells were obtained from BM (both tibias and femurs), lymph nodes or spleen. All analyses were performed on an LSRII flow cytometer (Becton Dickinson). Myeloid progenitors were identified as Lin−Sca-1−c-Kit+CD34+Fc RII/IIIlo (CMP), Lin−Sca-1−c-Kit+CD34+Fc RII/IIIhi (GMP), or Lin−Sca-1−c-Kit+CD34−Fc RII/IIIlo (MEP) (Akashi 2000). Stem and multipotent progenitor populations were identified as LSK cells (Lin−Sca-1hic-Kithi), LTHSC (LSK Flt3−CD150+CD48−), MPP (LSK Flt3−CD150−CD48−, LSK Flt3−CD150+CD48+, LSK Flt3−CD150−CD48+) and LMPP (LSK Flt3+CD150−) (Kiel et al., 2005). Human stem cells/primitive progenitors were isolated by sorting CD34+CD38− cells. For cell cycle analysis EdU (Invitrogen) was administered intraperitoneally (1mg/mouse), BM cells were obtained 2 hours post-injection and EdU incorporation into stem cell populations analyzed (Cappella et al., 2008). Cell cycle was also analyzed by Ki-67 (BD) and DAPI labeling as previously described (Jordan et al., 1996). Details are provided in the Supplemental Experimental Procedures.
Transplantation
GFP+ BM cells or purified progenitor populations from BCR-ABL/GFP transgenic mice and control mice were injected into 8 week old FVB/N or BCR-ABL mice irradiated at 900cGy. To evaluate homing, LTHSC were labeled with 1.25μM CFSE (Molecular Probes, Eugene, OR) and injected intravenously and the percentage of CFSE+ cells in the BM and spleen analyzed after 4 hours. To evaluate egress of cells from the BM to the spleen, GFP+ LTHSC cells were injected into the right femur of FVB/N mice irradiated at 900 cGy and the number of GFP+ LTHSC in the right femur, left femur and spleen of mice analyzed after 2 and 4 weeks. For some experiments mice were treated with IM for 2 weeks pre-transplantation. Details are provided in the Supplemental Experimental Procedures.
Measurement of cytokine levels
BM, peripheral blood and spleen cell suspensions were centrifuged and supernatants were aliquoted and frozen. ELISA assays for CXCL12 (R&D Systems, MCX120) and Luminex xMAP assays for a panel of murine cytokines and chemokines (MILLIPLEX MAP Mouse Cytokine/Chemokine Panel from Millipore, MPXMCYTO-70K) were performed. Expression of mRNA for different cytokines and chemokines in human and murine BM and spleen cells were analyzed using Q-RT-PCR. CXCL12 and G-CSF expression in BM sections from CML patients and controls were analyzed by immunohistochemistry. Details are provided in the Supplemental Experimental Procedures.
Statistics
Results are expressed as means ± standard error of means. Significance was estimated using Student’s t test.
Supplementary Material
Significance.
Chronic myelogenous leukemia (CML) results from stem cell transformation by the BCR-ABL oncogene. BCR-ABL tyrosine kinase inhibitors (TKI) induce remission in CML patients, but do not eliminate leukemia stem cells (LSC), which can regenerate leukemia on drug discontinuation. Improved understanding of LSC regulation is critical to understanding CML pathogenesis and developing curative therapies. We show here that leukemia-induced decrease in CXCL12 expression results in reduced retention of LSC in CML bone marrow (BM). Furthermore leukemia induced abnormalities in cytokine expression selectively suppressed normal stem cell growth and enhanced LSC growth in CML BM. These changes were partially corrected by TKI treatment. These results improve our understanding of LSC regulation in CML and could guide strategies for targeting this resistant population.
Acknowledgments
This work was supported by NIH grant R01 HL77847 and R01 CA95684 to Ravi Bhatia and CR-UK grant (C11074/A11008) to Tessa Holyoake. We acknowledge the excellent technical support of the COHNMC Analytical Cytometry core, the Clinical Immunobiology Correlative Studies laboratory, and the Animal Resources Center. Procurement of biospecimens was facilitated by the City of Hope Biospecimen Repository Protocol. This study was supported by the Glasgow Experimental Cancer Medicine Centre (ECMC), which is funded by Cancer Research UK and by the Chief Scientist’s Office (Scotland).
Footnotes
Author contributions:
Bin Zhang: Designed and performed research, analyzed data, wrote manuscript
Yin Wei Ho: Performed experiments, reviewed manuscript
Qin Huang: Performed experiments, interpreted data, reviewed manuscript
Takahiro Maeda: Designed experiments, interpreted data, reviewed manuscript
Allen Lin: Performed research, reviewed manuscript
Sung-uk Lee: Performed experiments, reviewed manuscript
Alan Hair: Performed research, reviewed manuscript
Tessa Holyoake: Provided material, interpreted data, reviewed manuscript
Claudia Huettner: Provided material, interpreted data, reviewed manuscript
Ravi Bhatia: Designed study, analyzed data, wrote manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akala OO, Park IK, Qian D, Pihalja M, Becker MW, Clarke MF. Long-term haematopoietic reconstitution by Trp53−/−p16Ink4a−/−p19Arf−/− multipotent progenitors. Nature. 2008;453:228–232. doi: 10.1038/nature06869. [DOI] [PubMed] [Google Scholar]
- Bhatia R, McGlave PB, Dewald GW, Blazar BR, Verfaillie CM. Abnormal function of the bone marrow microenvironment in chronic myelogenous leukemia: role of malignant stromal macrophages. Blood. 1995;85:3636–3645. [PubMed] [Google Scholar]
- Bonig H, Priestley GV, Papayannopoulou T. Hierarchy of molecular-pathway usage in bone marrow homing and its shift by cytokines. Blood. 2006;107:79–86. doi: 10.1182/blood-2005-05-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappella P, Gasparri F, Pulici M, Moll J. Cell proliferation method: click chemistry based on BrdU coupling for multiplex antibody staining. In: Paul Robinson J, editor. Current protocols in cytometry/editorial board. Unit7. Chapter 7. 2008. p. 34. [DOI] [PubMed] [Google Scholar]
- Cashman J, Clark-Lewis I, Eaves A, Eaves C. Stromal-derived factor 1 inhibits the cycling of very primitive human hematopoietic cells in vitro and in NOD/SCID mice. Blood. 2002;99:792–799. doi: 10.1182/blood.v99.3.792. [DOI] [PubMed] [Google Scholar]
- Cashman JD, Eaves CJ, Sarris AH, Eaves AC. MCP-1, not MIP-1alpha, is the endogenous chemokine that cooperates with TGF-beta to inhibit the cycling of primitive normal but not leukemic (CML) progenitors in long-term human marrow cultures. Blood. 1998;92:2338–2344. [PubMed] [Google Scholar]
- Cho SY, Xu M, Roboz J, Lu M, Mascarenhas J, Hoffman R. The effect of CXCL12 processing on CD34+ cell migration in myeloproliferative neoplasms. Cancer Res. 2010;70:3402–3410. doi: 10.1158/0008-5472.CAN-09-3977. [DOI] [PubMed] [Google Scholar]
- Chomel JC, Bonnet ML, Sorel N, Bertrand A, Meunier MC, Fichelson S, Melkus M, Bennaceur-Griscelli A, Guilhot F, Turhan AG. Leukemic stem cell persistence in chronic myeloid leukemia patients with sustained undetectable molecular residual disease. Blood. 2011;118:3657–3660. doi: 10.1182/blood-2011-02-335497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin AS, Agarwal A, Loriaux M, Cortes J, Deininger MW, Druker BJ. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J Clin Invest. 2011;121:396–409. doi: 10.1172/JCI35721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes J, Hochhaus A, Hughes T, Kantarjian H. Front-Line and Salvage Therapies With Tyrosine Kinase Inhibitors and Other Treatments in Chronic Myeloid Leukemia. J Clin Oncol. 2011 doi: 10.1200/JCO.2010.31.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N, Deininger MW, Silver RT, Goldman JM, Stone RM, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. The New England journal of medicine. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- Eaves CJ, Cashman JD, Wolpe SD, Eaves AC. Unresponsiveness of primitive chronic myeloid leukemia cells to macrophage inflammatory protein 1 alpha, an inhibitor of primitive normal hematopoietic cells. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:12015–12019. doi: 10.1073/pnas.90.24.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fialkow PJ, Jacobson RJ, Papayannopoulou T. Chronic myelocytic leukemia: clonal origin in a stem cell common to the granulocyte, erythrocyte, platelet and monocyte/macrophage. Am J Med. 1977;63:125–130. doi: 10.1016/0002-9343(77)90124-3. [DOI] [PubMed] [Google Scholar]
- Graham SM, Jorgensen HG, Allan E, Pearson C, Alcorn MJ, Richmond L, Holyoake TL. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood. 2002;99:319–325. doi: 10.1182/blood.v99.1.319. [DOI] [PubMed] [Google Scholar]
- Holtz MS, Slovak ML, Zhang F, Sawyers CL, Forman SJ, Bhatia R. Imatinib mesylate (STI571) inhibits growth of primitive malignant progenitors in chronic myelogenous leukemia through reversal of abnormally increased proliferation. Blood. 2002;99:3792–3800. doi: 10.1182/blood.v99.10.3792. [DOI] [PubMed] [Google Scholar]
- Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, Gotlib J, Li K, Manz MG, Keating A, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. The New England journal of medicine. 2004;351:657–667. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- Jaras M, Johnels P, Hansen N, Agerstam H, Tsapogas P, Rissler M, Lassen C, Olofsson T, Bjerrum OW, Richter J, Fioretos T. Isolation and killing of candidate chronic myeloid leukemia stem cells by antibody targeting of IL-1 receptor accessory protein. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:16280–16285. doi: 10.1073/pnas.1004408107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Tabe Y, Konoplev S, Xu Y, Leysath CE, Lu H, Kimura S, Ohsaka A, Rios MB, Calvert L, et al. CXCR4 up-regulation by imatinib induces chronic myelogenous leukemia (CML) cell migration to bone marrow stroma and promotes survival of quiescent CML cells. Molecular cancer therapeutics. 2008;7:48–58. doi: 10.1158/1535-7163.MCT-07-0042. [DOI] [PubMed] [Google Scholar]
- Jordan CT, Yamasaki G, Minamoto D. High-resolution cell cycle analysis of defined phenotypic subsets within primitive human hematopoietic cell populations. Exp Hematol. 1996;24:1347–1355. [PubMed] [Google Scholar]
- Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M, Moiraghi B, Shen Z, Mayer J, Pasquini R, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. The New England journal of medicine. 2010;362:2260–2270. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- Koschmieder S, Gottgens B, Zhang P, Iwasaki-Arai J, Akashi K, Kutok JL, Dayaram T, Geary K, Green AR, Tenen DG, Huettner CS. Inducible chronic phase of myeloid leukemia with expansion of hematopoietic stem cells in a transgenic model of BCR-ABL leukemogenesis. Blood. 2005;105:324–334. doi: 10.1182/blood-2003-12-4369. [DOI] [PubMed] [Google Scholar]
- Krause DS, Lazarides K, von Andrian UH, Van Etten RA. Requirement for CD44 in homing and engraftment of BCR-ABL-expressing leukemic stem cells. Nature medicine. 2006;12:1175–1180. doi: 10.1038/nm1489. [DOI] [PubMed] [Google Scholar]
- Li S, Ilaria RL, Jr, Million RP, Daley GQ, Van Etten RA. The P190, P210, and P230 forms of the BCR/ABL oncogene induce a similar chronic myeloid leukemia-like syndrome in mice but have different lymphoid leukemogenic activity. The Journal of experimental medicine. 1999;189:1399–1412. doi: 10.1084/jem.189.9.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon FX, Rea D, Guilhot J, Guilhot F, Huguet F, Nicolini F, Legros L, Charbonnier A, Guerci A, Varet B, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. The lancet oncology. 2010;11:1029–1035. doi: 10.1016/S1470-2045(10)70233-3. [DOI] [PubMed] [Google Scholar]
- Mansson R, Hultquist A, Luc S, Yang L, Anderson K, Kharazi S, Al-Hashmi S, Liuba K, Thoren L, Adolfsson J, et al. Molecular evidence for hierarchical transcriptional lineage priming in fetal and adult stem cells and multipotent progenitors. Immunity. 2007;26:407–419. doi: 10.1016/j.immuni.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Michor F, Hughes TP, Iwasa Y, Branford S, Shah NP, Sawyers CL, Nowak MA. Dynamics of chronic myeloid leukaemia. Nature. 2005;435:1267–1270. doi: 10.1038/nature03669. [DOI] [PubMed] [Google Scholar]
- Mihara K, Imai C, Coustan-Smith E, Dome JS, Dominici M, Vanin E, Campana D. Development and functional characterization of human bone marrow mesenchymal cells immortalized by enforced expression of telomerase. Br J Haematol. 2003;120:846–849. doi: 10.1046/j.1365-2141.2003.04217.x. [DOI] [PubMed] [Google Scholar]
- Pear WS, Miller JP, Xu L, Pui JC, Soffer B, Quackenbush RC, Pendergast AM, Bronson R, Aster JC, Scott ML, Baltimore D. Efficient and rapid induction of a chronic myelogenous leukemia-like myeloproliferative disease in mice receiving P210 bcr/abl-transduced bone marrow. Blood. 1998;92:3780–3792. [PubMed] [Google Scholar]
- Peled A, Hardan I, Trakhtenbrot L, Gur E, Magid M, Darash-Yahana M, Cohen N, Grabovsky V, Franitza S, Kollet O, et al. Immature leukemic CD34+CXCR4+ cells from CML patients have lower integrin-dependent migration and adhesion in response to the chemokine SDF-1. Stem cells (Dayton, Ohio) 2002;20:259–266. doi: 10.1634/stemcells.20-3-259. [DOI] [PubMed] [Google Scholar]
- Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, Nagler A, Ben-Hur H, Many A, Shultz L, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science (New York, NY. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- Peng H, Erdmann N, Whitney N, Dou H, Gorantla S, Gendelman HE, Ghorpade A, Zheng J. HIV-1-infected and/or immune activated macrophages regulate astrocyte SDF-1 production through IL-1beta. Glia. 2006;54:619–629. doi: 10.1002/glia.20409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotti D, Jamieson C, Goldman J, Skorski T. Chronic myeloid leukemia: mechanisms of blastic transformation. J Clin Invest. 2010;120:2254–2264. doi: 10.1172/JCI41246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzer AL, Eaves CJ, Lansdorp PM, Ponchio L, Barnett MJ, Eaves AC. Characterization of primitive subpopulations of normal and leukemic cells present in the blood of patients with newly diagnosed as well as established chronic myeloid leukemia. Blood. 1996;88:2162–2171. [PubMed] [Google Scholar]
- Reynaud D, Pietras E, Barry-Holson K, Mir A, Binnewies M, Jeanne M, Sala-Torra O, Radich JP, Passegue E. IL-6 controls leukemic multipotent progenitor cell fate and contributes to chronic myelogenous leukemia development. Cancer cell. 2011;20:661–673. doi: 10.1016/j.ccr.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C, Pasquini R, Clark RE, Hochhaus A, Hughes TP, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. The New England journal of medicine. 2010;362:2251–2259. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- Sarry JE, Murphy K, Perry R, Sanchez PV, Secreto A, Keefer C, Swider CR, Strzelecki AC, Cavelier C, Recher C, et al. Human acute myelogenous leukemia stem cells are rare and heterogeneous when assayed in NOD/SCID/IL2Rgammac-deficient mice. J Clin Invest. 2011;121:384–395. doi: 10.1172/JCI41495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyers CL. Chronic myeloid leukemia. The New England journal of medicine. 1999;340:1330–1340. doi: 10.1056/NEJM199904293401706. [DOI] [PubMed] [Google Scholar]
- Schemionek M, Elling C, Steidl U, Baumer N, Hamilton A, Spieker T, Gothert JR, Stehling M, Wagers A, Huettner CS, et al. BCR-ABL enhances differentiation of long-term repopulating hematopoietic stem cells. Blood. 2010;115:3185–3195. doi: 10.1182/blood-2009-04-215376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verfaillie CM, McCarthy JB, McGlave PB. Mechanisms underlying abnormal trafficking of malignant progenitors in chronic myelogenous leukemia. Decreased adhesion to stroma and fibronectin but increased adhesion to the basement membrane components laminin and collagen type IV. J Clin Invest. 1992;90:1232–1241. doi: 10.1172/JCI115985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Guo R, Schwarz EM, Boyce BF, Xing L. TNF inhibits production of stromal cell-derived factor 1 by bone stromal cells and increases osteoclast precursor mobilization from bone marrow to peripheral blood. Arthritis research & therapy. 2008;10:R37. doi: 10.1186/ar2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.