Abstract
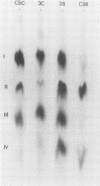
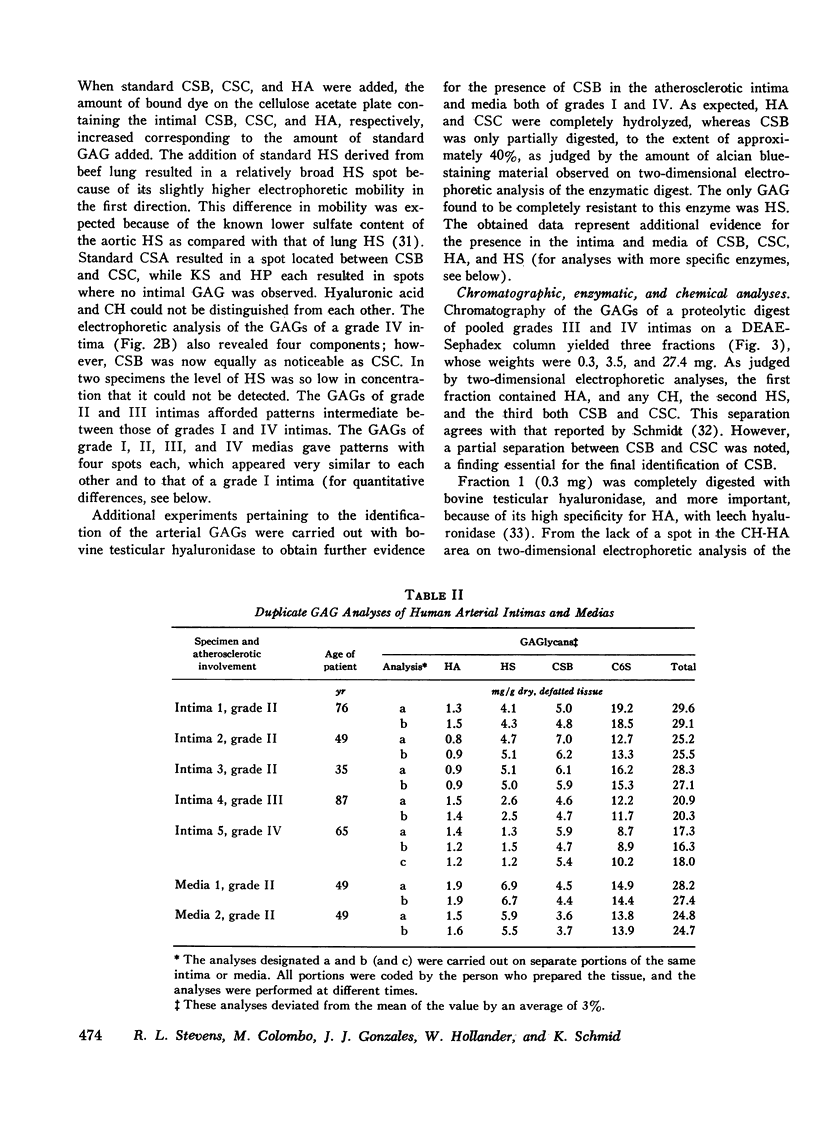
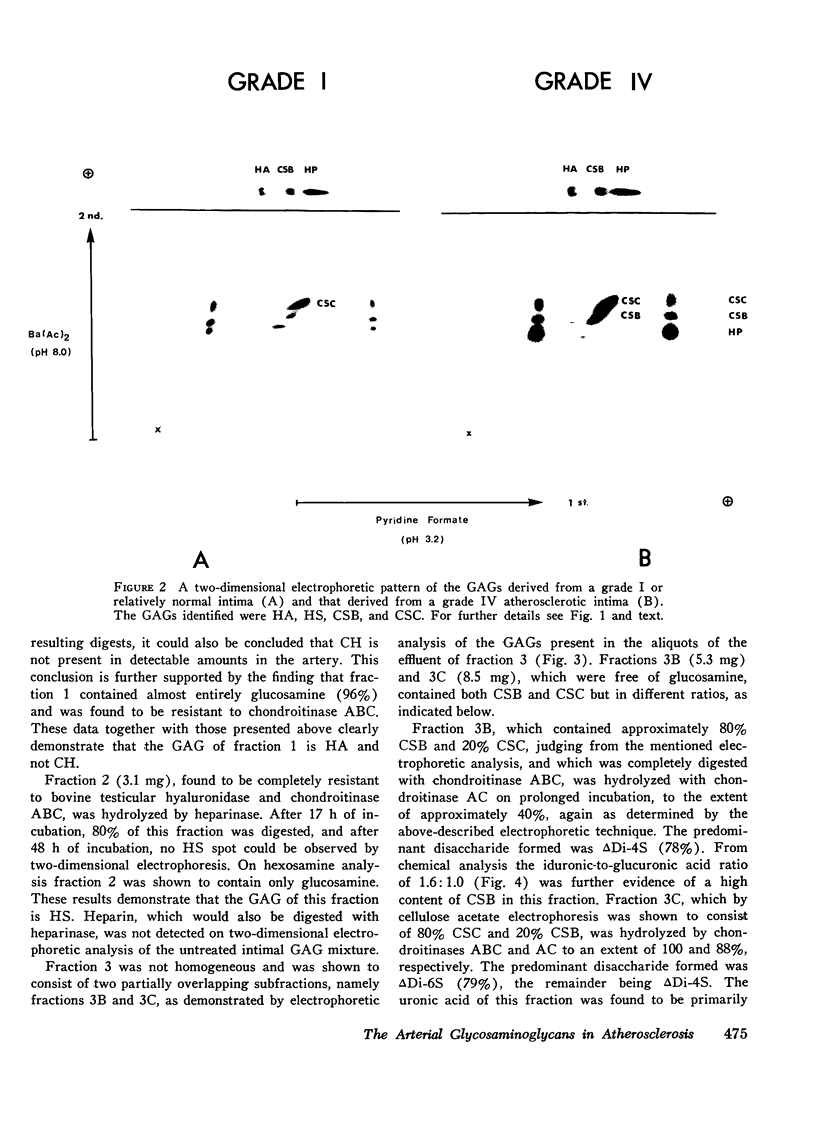
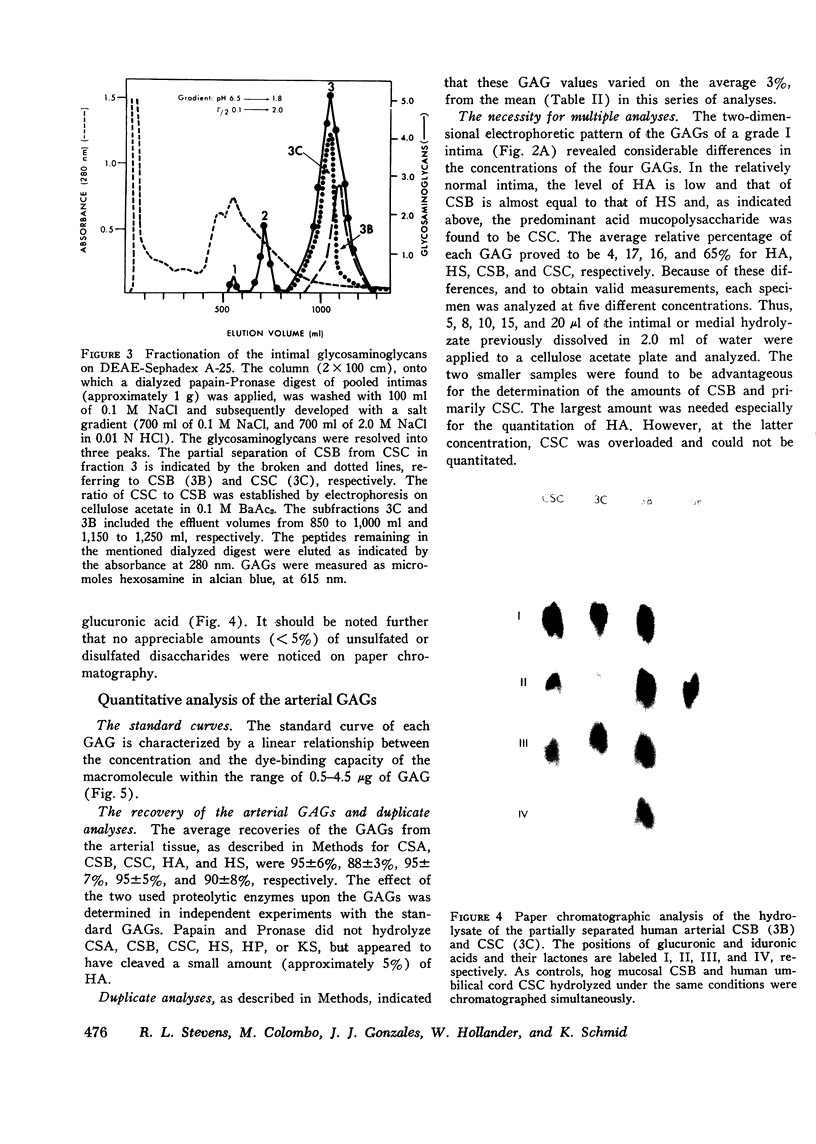
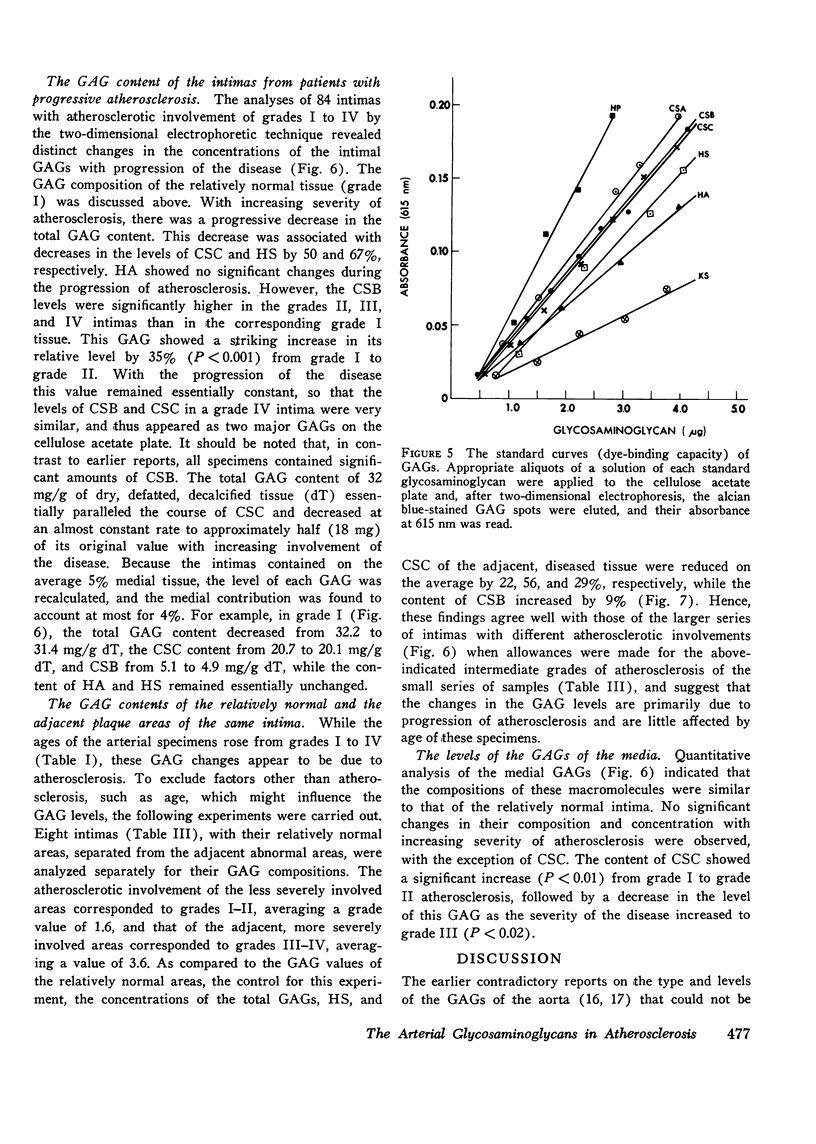
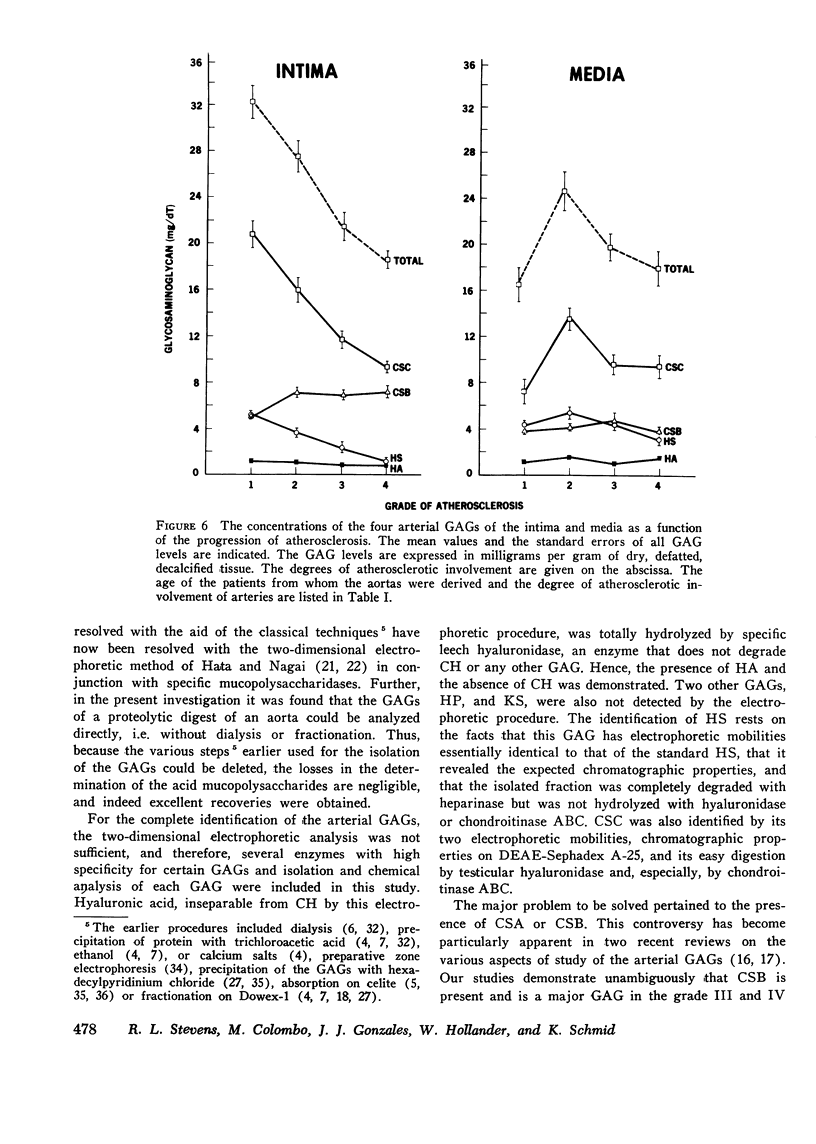
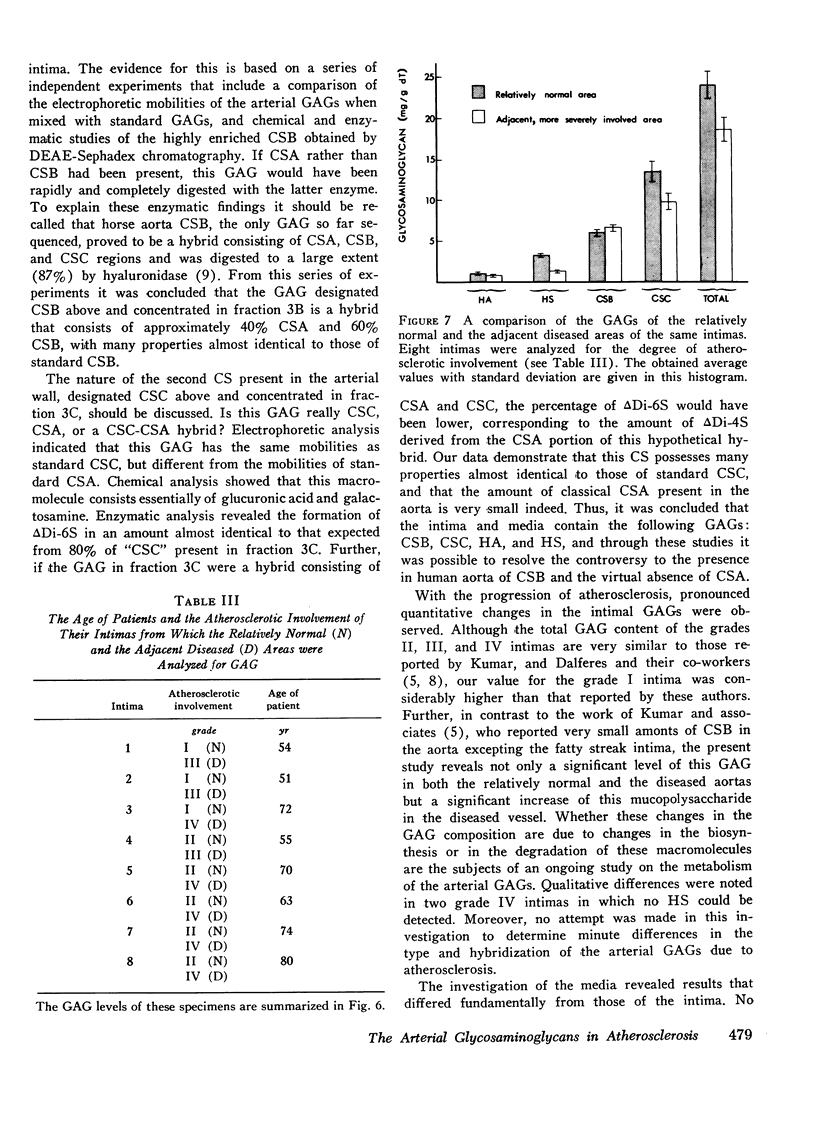
The changes in levels of glycosaminoglycans (GAGs) of the intima and media of the human artery in atherosclerosis were determined by a recently introduced two-dimensional electrophoresis technique that permits direct measurments of each of these macromolecules. To identify the arterial GAGs, they were fractionated by chromatography on a DEAE-Sephadex A-25 column, and the resulting three fractions (hyaluronic acid [HA], heparan sulfate [HS], and the partially separated chondroitin sulfates B [CSB] and C [CSC]) were analyzed for their electrophoretic mobilities by this electrophoretic method, for their digestability by highly specific hydrolases (leech hyaluronidase, heparinase, and chondroitinases ABC and AC) and for their iduronic acid content. From these studies we concluded that normal and atherosclerotic human aortas contain CSB, CSC, HA, and HS. Further, we demonstrated that CSB is a hybrid consisting of approximately 40% CSA and 60% CSB and that CSC appears to be a polymer consisting essentially of glucuronic acid and N-acetylgalactosamine-6-sulfate. Classical CSA as well as chondroitin (CH) were not present in detectable amounts. In the relatively normal intima, the mean concentrations of the GAGs were found to be 4.7, 20.9, 1.3, and 5.1 mg/g of dry, defatted, decalcified tissue for CSB, CSC, HA, and HS, respectively. With the progression of atherosclerosis, there was a pronounced decrease in the total GAG content (from 32 to 18 mg) associated with a decrease in the CSC and HS levels but without a change in the HA concentrations. Of particular interest, however, was the increase in the CSB level. In the media whose total GAG content averaged approximately 20 mg, no significant changes in these GAG levels were noted with the progression of the disease except for that of CSC. These findings may be important in explaining the increased lipoprotein and collagen deposition in the diseased aorta.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anseth A., Fransson L. A. Studies on corneal polysaccharides. VI. Isolation of dermatan sulfate from corneal scar tissue. Exp Eye Res. 1969 Jul;8(3):302–309. doi: 10.1016/s0014-4835(69)80043-6. [DOI] [PubMed] [Google Scholar]
- BUDDECKE E., DRZENIEK R. [Stability constants of the calcium complexes of acid mucopolysaccharides]. Hoppe Seylers Z Physiol Chem. 1962 May 4;327:49–64. doi: 10.1515/bchm2.1962.327.1.49. [DOI] [PubMed] [Google Scholar]
- Berenson G. S., Dalferes E. R., Jr Urinary excretion of mucopolysaccharides in normal individuals and in the Marfan syndrome. Biochim Biophys Acta. 1965 Jul 1;101(2):183–192. doi: 10.1016/0926-6534(65)90049-7. [DOI] [PubMed] [Google Scholar]
- Brandt K. D., Hardingham T. E., Tsiganos C. P., Muir H. Arterial mesenchyme and arteriosclerosis. Some aspects of the organization and interaction of connective tissue proteoglycans. Adv Exp Med Biol. 1974;43(0):161–172. doi: 10.1007/978-1-4684-3243-5_8. [DOI] [PubMed] [Google Scholar]
- CORNWELL D. G., KRUGER F. A. Molecular complexes in the isolation and characterization of plasma lipoproteins. J Lipid Res. 1961 Apr;2:110–134. [PubMed] [Google Scholar]
- Dalferes E. R., Jr, Ruiz H., Kumar V., Radhakrishnamurthy B., Berenson G. S. Acid mucopolysaccharides of fatty streaks in young, human male aortas. Atherosclerosis. 1971 Jan-Feb;13(1):121–131. doi: 10.1016/0021-9150(71)90013-x. [DOI] [PubMed] [Google Scholar]
- Fransson L. A., Havsmark B. Structure of dermatan sulfate. VII. The copolymeric structure of dermatan sulfate from horse aorta. J Biol Chem. 1970 Sep 25;245(18):4770–4783. [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. Hyaluronic acid in cartilage and proteoglycan aggregation. Biochem J. 1974 Jun;139(3):565–581. doi: 10.1042/bj1390565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata R., Nagai Y. A micro colorimetric determination of acidic glycosaminoglycans by two dimensional electrophoresis on a cellulose acetate strip. Anal Biochem. 1973 Apr;52(2):652–656. doi: 10.1016/0003-2697(73)90075-4. [DOI] [PubMed] [Google Scholar]
- Hata R., Nagai Y. A rapid and micro method for separation of acidic glycosaminoglycans by two-dimensional electrophoresis. Anal Biochem. 1972 Feb;45(2):462–468. doi: 10.1016/0003-2697(72)90208-4. [DOI] [PubMed] [Google Scholar]
- Hollander W., Kramsch D. M., Farmelant M., Madoff I. M. Arterial wall metabolism in experimental hypertension of coarctation of the aorta of short duration. J Clin Invest. 1968 May;47(5):1221–1229. doi: 10.1172/JCI105811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander W. Recent advances in experimental and molecular pathology; influx, synthesis, and transport of arterial lipoproteins in atherosclerosis. Exp Mol Pathol. 1967 Oct;7(2):248–258. doi: 10.1016/0014-4800(67)90033-0. [DOI] [PubMed] [Google Scholar]
- Iverius P. H. The interaction between human plasma lipoproteins and connective tissue glycosaminoglycans. J Biol Chem. 1972 Apr 25;247(8):2607–2613. [PubMed] [Google Scholar]
- Iverius P. H. The interaction between human plasma lipoproteins and connective tissue glycosaminoglycans. J Biol Chem. 1972 Apr 25;247(8):2607–2613. [PubMed] [Google Scholar]
- Izuka K., Murata K. Occurrence of heparin or its related acidic glucosaminoglycan in human aortic tissue. Experientia. 1973 Jun 15;29(6):655–657. doi: 10.1007/BF01944754. [DOI] [PubMed] [Google Scholar]
- JEANLOZ R. W. The nomenclature of mucopolysaccharides. Arthritis Rheum. 1960 Jun;3:233–237. doi: 10.1002/art.1780030306. [DOI] [PubMed] [Google Scholar]
- KAPLAN D., MEYER K. Mucopolysaccharides of aorta at various ages. Proc Soc Exp Biol Med. 1960 Oct;105:78–81. doi: 10.3181/00379727-105-26015. [DOI] [PubMed] [Google Scholar]
- Keeley F. W., Partridge S. M. Amino acid composition and calcification of human aortic elastin. Atherosclerosis. 1974 Mar-Apr;19(2):287–296. doi: 10.1016/0021-9150(74)90063-x. [DOI] [PubMed] [Google Scholar]
- Klynstra F. B., Böttcher C. J., van Melsen J. A., van der Laan E. J. Distribution and composition of acid mucopolysaccharides in normal and atherosclerotic human aortas. J Atheroscler Res. 1967 May-Jun;7(3):301–309. doi: 10.1016/s0368-1319(67)80057-7. [DOI] [PubMed] [Google Scholar]
- Kumar V., Berenson G. S., Ruiz H., Dalferes E. R., Jr, Strong J. P. Acid mucopolysaccharides of human aorta. 2. Variations with atherosclerotic involvement. J Atheroscler Res. 1967 Sep-Oct;7(5):583–590. doi: 10.1016/s0368-1319(67)80036-x. [DOI] [PubMed] [Google Scholar]
- Lazarus G. S., Goggins J. F. Mechanisms of connective tissue degradation. Science. 1974 Nov 15;186(4164):653–654. doi: 10.1126/science.186.4164.653. [DOI] [PubMed] [Google Scholar]
- Linker A., Hovingh P. The heparitin sulfates (heparan sulfates). Carbohydr Res. 1973 Jul;29(1):41–62. doi: 10.1016/s0008-6215(00)82069-8. [DOI] [PubMed] [Google Scholar]
- McGill H. C., Jr Introduction to the geographic pathology of atherosclerosis. Lab Invest. 1968 May;18(5):465–467. [PubMed] [Google Scholar]
- Murata K., Harada T., Okubo K. Enzymatic studies of chondroitin sulphates in human arterial tissue. J Atheroscler Res. 1968 Nov-Dec;8(6):951–958. doi: 10.1016/s0368-1319(68)80009-2. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Tokita K., Tateno S., Kotoku T., Ohba T. Human aortic acid mucopolysaccharides and glycoproteins. Changes during ageing and in atherosclerosis. J Atheroscler Res. 1968 Nov-Dec;8(6):891–902. doi: 10.1016/s0368-1319(68)80003-1. [DOI] [PubMed] [Google Scholar]
- SCHILLER S., SLOVER G. A., DORFMAN A. A method for the separation of acid mucopolysaccharides: its application to the isolation of heparin from the skin of rats. J Biol Chem. 1961 Apr;236:983–987. [PubMed] [Google Scholar]
- SCHMIDT M. Fractionation of acid mucopolysaccharides on DEAE-Sephadex anion exchanger. Biochim Biophys Acta. 1962 Sep 24;63:346–348. doi: 10.1016/0006-3002(62)90692-3. [DOI] [PubMed] [Google Scholar]
- Saito H., Yamagata T., Suzuki S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J Biol Chem. 1968 Apr 10;243(7):1536–1542. [PubMed] [Google Scholar]
- Smith E. B. Acid glycosaminoglycan, collagen and elastin content of normal artery, fatty streaks and plaques. Adv Exp Med Biol. 1974;43(0):125–139. doi: 10.1007/978-1-4684-3243-5_6. [DOI] [PubMed] [Google Scholar]
- Srinivasan S. R., Dolan P., Radhakrishnamurthy B., Berenson G. S. Isolation of lipoprotein-acid mucopolysaccharide complexes from fatty streaks of human aortas. Atherosclerosis. 1972 Jul-Aug;16(1):95–104. doi: 10.1016/0021-9150(72)90012-3. [DOI] [PubMed] [Google Scholar]
- Toole B. P., Lowther D. A. Dermatan sulfate-protein: isolation from and interaction with collagen. Arch Biochem Biophys. 1968 Dec;128(3):567–578. doi: 10.1016/0003-9861(68)90064-7. [DOI] [PubMed] [Google Scholar]
- Toole B. P., Lowther D. A. The organisation of hexosamine-containing compounds in bovine skin. Biochim Biophys Acta. 1966 Jun 29;121(2):315–325. doi: 10.1016/0304-4165(66)90120-6. [DOI] [PubMed] [Google Scholar]
- YUKI H., FISHMAN W. H. Purification and characterization of leech hyaluronic acid-endo-beta-glucuronidase. J Biol Chem. 1963 May;238:1877–1879. [PubMed] [Google Scholar]
- Yu S. Y. Calcification processes in atherosclerosis. Adv Exp Med Biol. 1974;43(0):403–425. doi: 10.1007/978-1-4684-3243-5_20. [DOI] [PubMed] [Google Scholar]