Abstract
pVHL, product of von Hippel-Lindau (VHL) tumor suppressor gene, functions as the substrate recognition component of an E3-ubiquitin ligase that targets proteins for ubiquitination and proteasomal degradation. Hypoxia-inducible factor α (HIFα) is the well-known substrate of pVHL. Besides HIFα, pVHL also binds to many other proteins and has multiple functions. In this manuscript, we report that the nuclear clusterin (nCLU) is a target of pVHL. We found that pVHL had a direct interaction with nCLU. nCLU bound to pVHL at pVHL's β domain, the site for recognition of substrate, indicating that nCLU might be a substrate of pVHL. Interestingly, pVHL bound to nCLU but did not lead to nCLU destruction. Further studies indicated that pVHL mediated K63-linked ubiquitination of nCLU and promoted nCLU nuclear translocation. In summary, our results disclose a novel function of pVHL that mediates K63-linked ubiquitination and identify nCLU as a new target of pVHL.
Introduction
pVHL, product of von Hippel-Lindau (VHL) tumor suppressor gene, acts as a multipurpose adaptor protein that controls a diverse array of gene expression programs, as well as extracellular matrix assembly and microtubule-based processes by linking various target proteins to appropriate enzymatic activities [1]. Germline mutations in VHL can be found in patients with VHL disease, a familial tumor syndrome characterized by the development of highly vascularized tumors in multiple organs [2], [3]. Major clinical manifestation of VHL disease include hemangioblastomas of the retina and central nervous system, renal cysts and clear cell renal cell carcinoma, pancreatic cysts and tumors, as well as pheochromocytomas [2], [3]. VHL is also mutated in the majority of sporadic clear cell renal cell carcinoma. pVHL forms a multi-protein complex that contained elongin B, elongin C, Cul2, and Rbx1 and functions as the substrate recognition component of an E3-ubiquitin ligase complex that targets proteins for degradation [4]–[6]. The best-understood function of pVHL relates to its role that targets the alpha subunit of hypoxia-inducible factor (HIFα) for destruction [3], [7]. pVHL binds to hydroxylated HIFα and mediates HIFα ubiquitination and proteasomal degradation [8], [9]. The pVHL-mediated degradation of HIFα is involved in tumor progression as well as oxygen sensing [10]. Besides HIFα, pVHL has been found to bind to a dozen different proteins and mediates other biological processes [1], [3], [11]. pVHL has a direct interaction with atypical protein kinase C and inhibits its activities [12]. pVHL binds to hydroxylated α-chain of collagen IV [13] and reacts with fibronectin [14]. pVHL is also involved in regulation of NF-κB [15] and Wnt signaling [16].
Clusterin (CLU) is an enigmatic glycoprotein with a nearly ubiquitous tissue distribution, involved in many biological processes [17]. CLU is also believed to be involved in many pathological states including cancer [18]. It may promote cell survival or induce cell apoptosis, depending on its different isoforms. [18], [19]. The secreted form of CLU (sCLU) is present in almost all physiological fluids [20] and is considered to be a survival factor [21]. In addition to sCLU, a nuclear form of CLU (nCLU) exists. And nCLU has been found to trigger cell death in a few systems [22].
Previous study demonstrated that expression of sCLU was attenuated in cells lacking wild-type pVHL [23]. However, the effect of pVHL on nCLU is unknown. We found in our work that pVHL bound to nCLU but did not target it for destruction. Interestingly, we found that pVHL mediated K63-linked ubiquitination of nCLU and promoted its nuclear translocation. Our results reveal a novel function of pVHL that mediates K63-linked ubiquitination and identify nCLU as a new target of pVHL.
Materials and Methods
Cell culture and reagents
Human embryonic kidney 293T cells and human cervix cancer Hela cells were cultured in DMEM with 10% serum, penicillin (100 U/mL), and streptoMycin (100 µg/mL). Human colon cancer HCT116 cells were cultured in McCoy's 5A media (M5A) with 10% serum, penicillin (100 U/mL), and streptoMycin (100 µg/mL). pVHL antibody was from BD Biosciences (Bedford, MA). nCLU (B5), HA, Myc and His antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Antibody against Ub(K63) (BML-PW0600-0025) was from Enzo Life Sciences (Farmingdale, NY). Beta-actin and α-tubulin antibodies, cycloheximide and MG132 were from Sigma (St. Louis, MO).
Construction of vectors
nCLU was constructed by polymerase chain reaction (PCR) and cloned into pcDNA3.0 vector (with HA tag) or pcDNA3.1 vector (with Myc tag). Deletion forms of VHL were cloned into pCDNA3.1 vector or into pET-28a Vector (with His epitope). R-VHL was constructed through inserting VHL into pDsRed-Monomer-N1 vector between Xho I and Hind III. Glutathione S-transferase (GST)-nCLU fusion proteins were constructed by inserting PCR-generated DNA fragments encoding nCLU into pGEX4T1. E. coli BL21-Gold(DE3)pLysS cells were transformed with pGEX4T1 or pET28a expression vectors and treated with 0.1 mmol/L of isopropyl-D-thiogalactoside for 4 h. Ubiquitin was constructed by PCR and various mutated ubiquitin constructs were generated by site-directed mutagenesis. The diagram of ubiquitin mutants is as follows.
6 11 27 29 33 48 63
Ub ----K-----K----K----K------K------K-----K---
Ub(K48) ----R-----R----R----R-----R------K-----R---
Ub(K63) ----R-----R----R----R-----R------R-----K---
Ub(K63R) ----K-----K----K----K------K------K-----R---
Ub(K48R) ----K-----K----K----K------K------R-----K---
Immuno-staining of cells
The cells, grown on glass coverslip were washed and fixed with 4% formaldehyde for 20 minutes, followed by permeabilization with 0.1% Triton X-100. After wash, the coverslips were blocked in 3% BSA for 1 h, followed by the incubation with primary antibody at 4°C overnight. After wash, the coverslips were incubated with fluorescence dye-conjugated secondary antibody for 30 min. Finally, the cells were stained with DAPI in the dark for 3 min. The stained cells were mounted and observed in A Zeiss LSM 510 META (Axiovert 200) microscope.
Nuclear protein extraction
Cells were washed and suspended in 100 µL of buffer A (20 mM HEPES, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 1 mM dithiothreitol, 0.1 mM EGTA, 0.1 mM EDTA, 0.5 mM phenylmethylsulfonyl fluoride, and 0.2 mM phenylmethylsulfonyl fluoride, 0.5% Nonidet P-40). The cells were lysed, followed by centrifugation at 1700× g for 10 min. After separation of the cytoplasmic fraction, nuclei were washed once with Buffer A and resuspended in ice-cold buffer B (10 mM HEPES, pH 7.9, 25% glycerol, 1.5 mM MgCl2 and 0.4 mM NaCl, 1 mM dithiothreitol, and 0.2 mM phenylmethylsulfonyl fluoride). After 1 h incubation on ice and a centrifuge to clear the cellular debris, the nuclear extracts were collected and used for immunoblotting.
Transient transfection
Transient transfection of cells was performed using LipofectAMINE-2000 or LipofectAMINE-PLUS (Invitrogene) as per the manufacturer's instructions.
Immunoprecipitation and immunoblotting
Immunoblotting and immunoprecipitation was performed as described [24]. Briefly, 500 µg of cell lysates were incubated with 1 µg of primary antibody at 4°C for 3 h. Then, 20 µL of protein A/G PLUS agarose beads was added and the incubation continued overnight. The beads were washed and boiled in SDS-PAGE loading buffer for 5 minutes before electrophoresis.
GST pull-down assay
GST pull-down assay was performed as described [24]. Bacterial cells were lysed using the following buffer: 20 mmol/L Tris-Cl, 150 mmol/L NaCl, 2 mmol/L EDTA, 0.5% NP40, pH 7.5. To determine the interaction between nCLU and pVHL, bacterial lysates containing GST-nCLU were incubated with glutathione-Sepharose 4B beads at 4°C for 1 h. The beads were washed and incubated with bacterial cell lysates containing His-pVHL, allowing the interaction between GST-nCLU and pVHL. After washing, GST-nCLU and the bound pVHL were eluted from the beads and subjected to electrophoresis.
In vitro ubiquitination assay
In vitro ubiquitination assay was performed as described [25] and the Reaction II reticulocyte conjugation kit (Bostonbiochem, MA) was employed in this assay. To isolate the pVHL complex, Myc-VHL was transiently expressed in 293T cells and four hundred micrograms of the cellular proteins were immunoprecipitated with 1 µg of Myc antibody at 4°C for 3 h. Twenty microliters of protein A/G PLUS agarose beads were added and the incubation continued at 4°C overnight. The beads were washed for four times with RIPA buffer, followed by two washes with buffer containing 50 mM Tris-HCl and 3 mM DTT, pH 8.0. The agarose beads that contained purified pVHL complex were suspended in a final reaction volume of 50 µL containing ATP-regenerating buffer, 5 µg/µL ubiquitin, 1 µg/µL GST-nCLU, 100 ng/µL ubiquitin aldehyde, MG132 (5 µM), and 16 µL of purified rabbit reticulocyte fraction II, and incubated at 37°C for 2 h. After reaction, the total proteins generated in the ubiquitination reaction were analyzed by western-blot.
Statistical analysis
The data represent the mean ± SD from three independent experiments except where indicated. Statistical analysis was performed using the unpaired two-tailed Student's t-test. The difference is considered significant when a P value is less than 0.05.
Results
pVHL interacts with nCLU
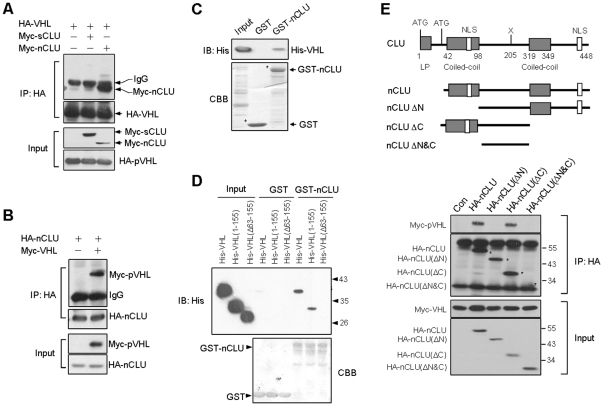
pVHL is an adaptor protein and binds to many proteins. We determined whether pVHL had an interaction with sCLU and nCLU. 293T cells were transiently transfected with HA-VHL plus Myc-sCLU or HA-VHL plus Myc-nCLU plasmids. Immunoprecipitation was performed and the results indicated that HA-pVHL co-immunoprecipitated with Myc-nCLU (Fig. 1A, Fig. S1A) but not Myc-sCLU (Fig. 1A), indicating that pVHL had an interaction with nCLU. We repeated the experiment in 293T cells to confirm the interaction between pVHL and nCLU. The cells were transfected with Myc-VHL and HA-nCLU vectors. The results showed that Myc-pVHL and HA-nCLU also co-immunoprecipitated (Fig. 1B, Fig. S1B). A direct interaction between pVHL and nCLU was ascertained by GST-pull down assays (Fig. 1C). These results suggest that pVHL binds to nCLU directly.
Figure 1. pVHL binds to nCLU.
(A) 293T cells were transfected as indicated. In 24 h, the cells were harvested and cellular proteins were prepared. Immunoprecipitation was performed using HA antibody. (B) 293T cells were transfected with Myc-VHL and HA-nCLU. In 24 h, the cells were harvested and immunoprecipitation was performed using HA antibody. (C) Direct interaction between nCLU and pVHL. Equal amount of bacterial lysates containing His-pVHL were incubated with the glutathione-sepharose beads that already captured GST or GST-nCLU. The beads were washed and His-pVHL retained on beads was determined by immunoblotting. CBB, Coomassie Blue Staining. (D) nCLU bound to pVHL at β domain. Equal amount of bacterial lysates containing His-pVHL, His-pVHL(1-155) or His-pVHL(Δ63-155) were incubated with the glutathione-sepharose beads that already captured GST or GST-nCLU. The beads were washed and His-pVHL or the truncated His-pVHL retained on beads was determined by immunoblotting. (E) A various constructs encoding different nCLUs were designed. 293T cells were transfected with Myc-VHL and nCLU construct as indicated. 24 h post-transfection, the cells were harvested for immunoprecipitation using HA antibody.
We determined the domain of pVHL that nCLU might bind to. pVHL contains two functional sub-domains termed α and β, and the β domain (residues 63–155) functions for recognition of substrate. GST-pull down assay was performed using pVHL fragments covering different regions. VHL encodes wild-type pVHL, VHL(1-155) encodes pVHL without α domain, and VHL(Δ63-155) encodes pVHL without β domain. The results showed that pVHL and pVHL(1-155) bound to nCLU (Fig. 1D). However, pVHL(Δ63-155) had no interaction with nCLU. The results suggest that nCLU binds to the β domain of pVHL and nCLU might be a substrate of pVHL.
We next determined the site of nCLU that pVHL bound to. Our results showed pVHL had no interaction with nCLU if the N-terminal coil-coil domain of nCLU was deleted (Fig. 1E), suggesting that this domain is required for nCLU-pVHL interaction. sCLU also has the coil-coil domain, however, it does not bind to pVHL (Fig. 1A). sCLU was translated from the first ATG and nCLU was translated from the second ATG (Fig. 1E). So, the N-terminal of sCLU is different from that of nCLU. This might be the reason why nCLU binds to pVHL but sCLU does not. This needs further investigation.
pVHL mediates ubiquitination of nCLU
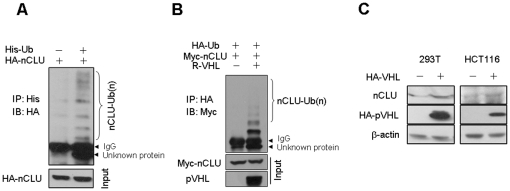
pVHL functions as the substrate recognition component of an E3-ubiquitin ligase that targets proteins for degradation. pVHL interacted with nCLU (Fig. 1). Previous study demonstrated that nCLU undergoes proteasomal degradation [26]. We therefore tested whether pVHL mediated ubiquitination of nCLU. We found that nCLU had polyubiquitination (Fig. 2A) and overexpression of VHL increased polyubiquitination of nCLU (Fig. 2B). The identity of the band below IgG is unknown (Fig. 2A and B). We determined whether pVHL promoted degradation of nCLU. Unexpectedly, ectopic expression of VHL didn't inhibit expression of nCLU (Fig. 2C).
Figure 2. pVHL mediates ubiquitination of nCLU.
(A) Modification of nCLU by ubiquitin. 293T cells were transfected with indicated plasmids and incubated for 24 h before harvest for immunoprecipitation and western-blot. (B) pVHL enhanced the ubiquitination of nCLU. 293T cells were transfected with HA-Ub, Myc-nCLU and R-VHL plasmids. In 24 h, the cells were harvested and cellular proteins were prepared for immunoprecipitation using HA antibody. (C) HCT116 and 293T cells were transfected with Myc-VHL. In 48 h, the cells were harvested and lysed. Expression of nCLU was determined by western-blot.
pVHL mediates K63-linked polyubiquitination of nCLU
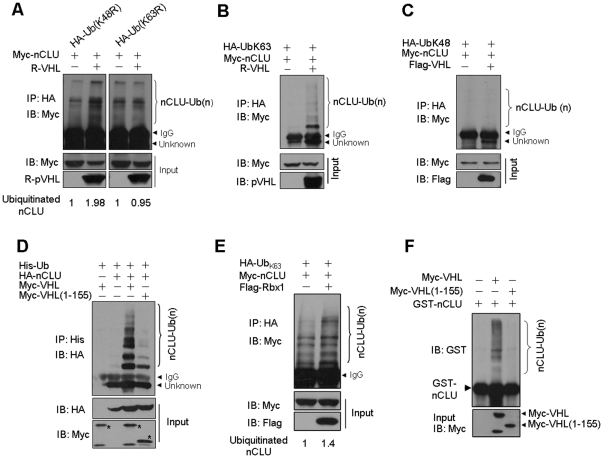
It is known that, the K48-linked polyubiquitination leads to protein proteasomal degradation and the K63-linked polyubiquitination plays a role in protein trafficking, kinase and phosphatase activation [27]. One of the examples is the non-degradative K63-polyubiquitination of NEMO that leads to NF-κB activation [28]. This drove us to determine the kind of ubiquitination of nCLU that pVHL mediated. We employed in our work two ubiquitin mutants Ub(K48R) and Ub(K63R) where K48 or K63 was mutated to arginine (R). 293T cells were transfected with nCLU, VHL and Ub(K48R) or Ub(K63R) constructs. Overexpression of VHL enhanced polyubiquitination of nCLU in the presence of Ub(K48R) (Fig. 3A). However, overexpression of VHL had little effect on the polyubiquitination of nCLU in the presence of Ub(K63R) (Fig. 3A), implying that pVHL mediates K63-linked ubiquitination of nCLU. Next, we employed a construct encoding Ub(K63) in our experiments. Ub(K63) is the ubiquitin that the lysine residues are mutated to R except K63. We found that overexpression of VHL enhanced polyubiquitination of nCLU in the presence of Ub(K63) (Fig. 3B). We also used a construct encoding Ub(K48). All lysine residues of it are mutated to R except K48. We found that overexpression of VHL did not enhance polyubiquitination of nCLU in the presence of Ub(K48) (Fig. 3C). Taken together, these results suggest that pVHL mediates K63-linked ubiquitination of nCLU.
Figure 3. pVHL mediates K63-linked ubiquitination of nCLU.
(A) pVHL enhanced the K63-linked ubiquitination of nCLU. 293T cells were transfected with Myc-nCLU, R-VHL and HA-Ub(K48R) (or Ub(K63R)). In 24 h, the cells were lysed and immunoprecipitation was done using HA antibody. The relative amount of ubiquitinated nCLU was determined by densitometry assay. (B) pVHL enhanced the ubiquitination of nCLU in the presence of Ub(K63). 293T cells were transfected with Myc-nCLU, R-VHL and HA-Ub(K63) plasmids. In 24 h, the cells were harvested and then lysed for immunoprecipitation using HA antibody. (C) pVHL did not enhance nCLU ubiquitination in the presence of Ub(K48). 293T cells were transfected with Myc-nCLU, R-VHL and HA-Ub(K48) plasmids. In 24 h, the cells were harvested and then lysed for immunoprecipitation using HA antibody. (D) pVHL(1-155) did not mediate ubiquitination of nCLU. 293T cells were transfected with various constructs as indicated. In 24 h, the cell lysates were prepared for immunoprecipitation and western-blot. (E) Overexpression of Rbx1 enhanced K63-linked ubiquitination of nCLU. 293T cells were transfected as indicated. In 24 h, the cells were harvested and cell lysates were prepared for immunoprecipitation. The relative amount of ubiquitinated Myc-nCLU was determined by densitometry assay. (F) pVHL mediated K63-linked ubiquitination of nCLU in vitro. In vitro ubiquitination assay was performed as described under Methods.
The α domain of pVHL associates with the E3 ubiquitin ligase complex [2]. We found that the ubiquitination of nCLU was reduced significantly when VHL(1-155) was tested (Fig. 3D, Fig. S2). Because pVHL(1-155) did not have α domain, the results imply that the α domain of pVHL is required for pVHL to mediate ubiquitination of nCLU. Moreover, expression of VHL(Δ63-155) had little effect on ubiquitination of nCLU (Fig. S2).
Next, we determined the effects of Rbx1, a subunit of the pVHL E3 ubiquitin ligase complex, on K63-linked ubiquitination of nCLU. We found that ectopic expression of Rbx1 enhanced K63-linked ubiquitination of nCLU (Fig. 3E). We also determined whether pVHL could mediate K63-linked polyubiquitination of nCLU in vitro. The pVHL complex was isolated by immunoprecipitation and incubated with purified nCLU in Reaction II Reticulocyte Conjugation Kit as described previously [25]. We found that the isolated pVHL complex mediated K63-linked ubiquitination of nCLU in vitro (Fig. 3F). However, the isolated pVHL(1-155) could not mediate K63-linked ubiqitination of nCLU in vitro. A control experiment was done. The results showed that the ubiquitin chains were attached to GST-nCLU and not to pVHL itself and Ub(K63R) was not linked to nCLU (Fig. S3). Taken together, these results suggest that pVHL E3 ubiquitin ligase complex mediates K63-linked ubiquitination of nCLU.
pVHL promotes nucleus localization of nCLU
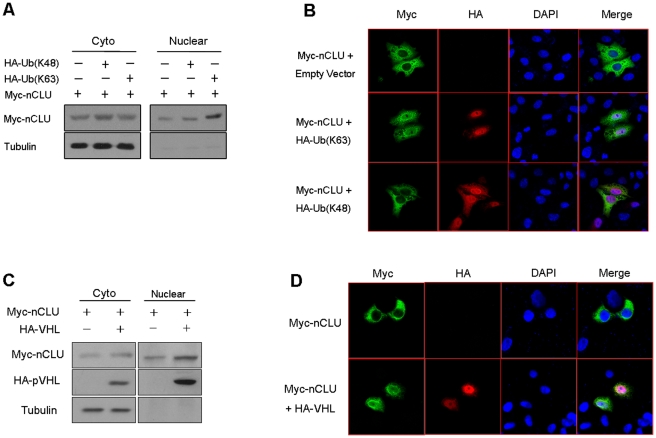
It is believed that nCLU functions in nucleus. We therefore asked whether the K63-linked ubiquitination promoted nuclear translocation of nCLU. To know this, we determined the effect of expression of Ub(K63) on cellular distribution of nCLU. We found that ectopic expression of Ub(K63) enhanced nuclear localization of nCLU (Fig. 4A). However, overexpression of Ub(K48) had little effect on nCLU nuclear translocation. Immuno-staining of the cells also indicated that ectopic expression of Ub(K63) enhanced nuclear localization of nCLU (Fig. 4B). It was noted that overexpression of Ub(K63)+nCLU, but not Ub(K48)+nCLU, led to alteration of cell morphology (Fig. 4B). Taken together, these results suggest that the K63-linked ubiquitination of nCLU promotes nCLU nuclear translocation.
Figure 4. pVHL enhances nuclear translocation of nCLU.
(A) Overexpression of Ub(K63) promoted nCLU nucleus translocation. 293T cells were transfected with the constructs as indicated. In 24 h, the nuclear and cytoplasmic proteins were prepared for immunoblotting. (B) Overexpression of Ub(K63) promoted nCLU nucleus translocation. Hela cells were transfected with the constructs as indicated. In 24 h, the cells were fixed and immunostained as described under methods. The pictures were obtained under a confocal microscope. (C) Overexpression of pVHL promoted nCLU nuclear translocation. 293T cells were transfected as indicated. In 24 h, the cells were harvested and nuclear and cytoplasmic proteins were prepared for immunoblotting. (D) pVHL promoted nCLU nuclear translocation. Hela cells were transfected as indicated. In 24 h, the cells were fixed and immuno-stained as described in Methods.
Next, we determined the effect of pVHL on nCLU nuclear translocation. The nuclear and cytoplasmic proteins were isolated and western-blot was performed. We found that overexpression of pVHL enhanced protein levels of nCLU in nucleus (Fig. 4C). Immuno-staining of the cells was also performed to indicate nCLU distribution in cells. We found that Myc-nCLU proteins were mainly cytoplasmic when the cells were transfected with Myc-nCLU alone (Fig. 4D). If Myc-nCLU were co-transfected with HA-VHL, the Myc-nCLU proteins in nucleus were increased. These results suggest that pVHL promotes nuclear translocation of nCLU. We noted that expression of exogenous VHL+nCLU altered cell morphology (Fig. 4D). This is similar to the results of Fig. 4B. The altered cell morphology might be due to increased nCLU in nucleus.
Discussion
It is known that pVHL targets HIFα for proteasomal degradation. As an adaptor protein, pVHL not only binds to HIFα but also binds to many other proteins and has multiple functions [1]. We found in our work that pVHL bound to nCLU and mediated nCLU K63-linked ubiquitination, which may lead to nuclear translocation of nCLU. To our knowledge, this is the first report to demonstrate that pVHL mediates K63-linked ubiquitination of a protein.
The addition of ubiquitin to proteins occurs through an enzymatic cascade. Firstly, an activating enzyme, E1, is charged with ubiquitin, then the ubiquitin is transferred to an E2-conjugating enzyme and, finally, it is attached to a substrate by an E3 ligase [29]. There are a few types of polyubiquitin chains [27] and in many conditions the type of polyubiquitin chain formed appears to be directed primarily by the cooperating E2 enzyme. E2 dictates the type of inter-ubiquitin linkages and thereby determines the fate of the substrate [30]. We found that pVHL mediated K63-linked ubiquitination of nCLU (Fig. 3) and promoted nCLU nuclear translocation (Fig. 4). Our results suggest that, for different substrates, pVHL may mediate different types of ubiquitination, probably using different E2 enzymes.
Although protein ubiquitination has been best characterized as a mechanism for targeting proteins to the proteasome for degradation, recently it has become clear that this modification can also serve many other functions. For example, K48-linked ubiquitination tags the target protein to be degraded by the proteasome, and K29-linked ubiquitination is considered as an indicator of subsequent lysosomal degradation [31]. During the last few years the K63-ubiquitination has emerged as a novel post-translational modification of remarkable functional interest for fine-tuning signal transduction pathways [27], [32]. We found that ectopic expression of pVHL promoted nuclear translocation of nCLU (Fig. 4). This might be one function of pVHL that mediates K63-linked ubiquitination of nCLU. There are currently a few examples of ubiquitin serving as a nuclear import signal. For example, TRAF6, an E3 ubiquitin ligase, was shown to mediate K63-linked ubiquitination of p75 cytoplasmic interactor (NRIF) and this modification is necessary for NRIF nuclear translocation [33].
nCLU has been found to trigger cell death when in nucleus in a few systems [22]. However, we did not find that overexpression of nCLU led to cell apoptosis in our work. The difference might be due to different cells used in these works. It should be noted that in our work the increased nucleus nCLU do altered the morphology of these cell (Fig. 4B and D). The physiological function of pVHL that mediates nCLU ubiquitination needs further investigation. For example, it will be interesting to determine whether pVHL-mediated ubiquitination and nucleus translocation of nCLU plays an important role in regulation of cell proliferation and apoptosis. Another important question is whether pVHL is required for nCLU nucleus translocation.
In this manuscript, we have disclosed a novel function of pVHL that mediates K63-linked ubiquitination and identified nCLU as a new target of pVHL. These results indicate that pVHL mediates not only K48- but also K63-linked ubiquitination of proteins. It is known that K48-linked ubiquitination usually leads to protein degradation and K63-linked ubiquitination functions as a post-translational modification for protein trafficking and fine-tuning signal transduction pathways. Our results may provide a new sight of pVHL's function.
Supporting Information
(A) 293T cells were transfected with Myc-nCLU plus empty vector or Myc-nCLU plus HA-VHL. In 24 h, the cells were harvested and cellular proteins were prepared. Immunoprecipitation was performed using HA antibody. The results of negative control experiment showed that HA antibody did not interact with Myc-tagged nCLU. (B) 293T cells were transfected as indicated. In 24 h, the cells were harvested and immunoprecipitation was performed using HA antibody. The results of negative control experiment indicated that the HA antibody did not interact with Myc-tagged VHL.
(TIF)
pVHL(1-155) and pVHL(Δ63-155) had little effect on ubiquitination of nCLU. 293T cells were transfected with HA-nCLU, His-Ub and various Myc-VHL constructs as indicated. In 24 h, the cells were harvested and cellular proteins were prepared for immunoprecipitation and western-blot.
(TIF)
pVHL mediated K63-linked ubiquitination of nCLU in vitro . In vitro ubiquitination of nCLU by pVHL was performed as described under Methods. Ubiquitin or Ub(K63R) at 5 µg/µL was added to the reaction system.
(TIF)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The work was supported by National Natural Science Foundation of China (30970586)(http://www.nsfc.gov.cn); Chinese Academy of Sciences (KSCX2-EW-R-08). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Frew IJ, Krek W. pVHL: a multipurpose adaptor protein. Sci Signal. 2008;1:e30. doi: 10.1126/scisignal.124pe30. [DOI] [PubMed] [Google Scholar]
- 2.Kim WY, Kaelin WG. Role of VHL gene mutation in human cancer. J Clin Oncol. 2004;22:4991–5004. doi: 10.1200/JCO.2004.05.061. [DOI] [PubMed] [Google Scholar]
- 3.Kaelin WG. Von Hippel-Lindau disease. Annu Rev Pathol. 2007;2:145–173. doi: 10.1146/annurev.pathol.2.010506.092049. [DOI] [PubMed] [Google Scholar]
- 4.Kamura T, Koepp DM, Conrad MN, Skowyra D, Moreland RJ, et al. Rbx1, a component of the VHL tumor suppressor complex and SCF ubiquitin ligase. Science. 1999;284(5414):657–61. doi: 10.1126/science.284.5414.657. [DOI] [PubMed] [Google Scholar]
- 5.Iwai K, Yamanaka K, Kamura T, Minato N, Conaway RC, et al. Identification of the von Hippel-lindau tumor-suppressor protein as part of an active E3 ubiquitin ligase complex. Proc Natl Acad Sci USA. 1999;96:12436–12441. doi: 10.1073/pnas.96.22.12436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lisztwan J, Imbert G, Wirbelauer C, Gstaiger M, Krek W. The von Hippel-Lindau tumor suppressor protein is a component of an E3 ubiquitin-protein ligase activity. Genes Dev. 1999;13:1822–1833. doi: 10.1101/gad.13.14.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5:343–354. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- 8.Ivan M, Kondo K, Yang H, Kim W, Valiando J, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 9.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 10.Kaelin WG., Jr The von Hippel–Lindau tumour suppressor protein: O2 sensing and cancer. Nat Rev Cancer. 2008;8:865–873. doi: 10.1038/nrc2502. [DOI] [PubMed] [Google Scholar]
- 11.Czyzyk-Krzeska MF, Meller J. von Hippel-Lindau tumor suppressor: not only HIF's executioner. Trends Mol Med. 2004;10:146–149. doi: 10.1016/j.molmed.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Okuda H, Saitoh K, Hirai S, Iwai K, Takaki Y, et al. The von Hippel-Lindau tumor suppressor protein mediates ubiquitination of activated atypical protein kinase C. J Biol Chem. 2001;276:43611–43617. doi: 10.1074/jbc.M107880200. [DOI] [PubMed] [Google Scholar]
- 13.Grosfeld A, Stolze IP, Cockman ME, Pugh CW, Edelmann M, Kessler B, Bullock AN, Ratcliffe PJ, Masson N. Interaction of Hydroxylated Collagen IV with the von Hippel-Lindau Tumor Suppressor. J Biol Chem. 2007;282:13264–13269. doi: 10.1074/jbc.M611648200. [DOI] [PubMed] [Google Scholar]
- 14.Ohh M, Yauch RL, Lonergan KM, Whaley JM, Stemmer-Rachamimov AO, et al. The von Hippel-Lindau tumor suppressor protein is required for proper assembly of an extracellular fibronectin matrix. Mol Cell. 1998;1:959–968. doi: 10.1016/s1097-2765(00)80096-9. [DOI] [PubMed] [Google Scholar]
- 15.Yang H, Minamishima YA, Yan Q, Schlisio S, Ebert BL, et al. pVHL acts as an adaptor to promote the inhibitory phosphorylation of the NF-kappaB agonist Card9 by CK2. Mol Cell. 2007;28:15–27. doi: 10.1016/j.molcel.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chitalia VC, Foy RL, Bachschmid MM, Zeng LL, Panchenko MV, et al. Jade-1 inhibits Wnt signaling by ubiquitilating β-catenin and mediates Wnt pathway inhibition by pVHL. Nat Cell Biol. 2008;10:1208–1216. doi: 10.1038/ncb1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pucci S, Bonanno E, Pichiorri F, Angeloni C, Spagnoli LG. Modulation of different clusterin isoforms in human colon tumorigenesis, Oncogene. 2004;23:2298–2304. doi: 10.1038/sj.onc.1207404. [DOI] [PubMed] [Google Scholar]
- 18.Shannan B, Seifert M, Leskov K, Willis J, Boothman D, et al. Challenge and promise: roles for clusterin in pathogenesis, progression and therapy of cancer. Cell Death and Diff. 2006;13:12–19. doi: 10.1038/sj.cdd.4401779. [DOI] [PubMed] [Google Scholar]
- 19.Leskov KS, Klokov DY, Li J, Kinsella TJ, Boothman DA. Synthesis and functional analyses of nuclear clusterin, a cell death protein. J Biol Chem. 2003;274:11590–11600. doi: 10.1074/jbc.M209233200. [DOI] [PubMed] [Google Scholar]
- 20.de Silva HV, Harmony JA, Stuart WD, Gil CM, Robbins J. A Apolipoprotein J: structure and tissue distribution. Biochemistry. 1990;29:5380–5389. doi: 10.1021/bi00474a025. [DOI] [PubMed] [Google Scholar]
- 21.Klock G, Baiersdörfer M, Koch-Brandt C. Chapter 7: Cell protective functions of secretory Clusterin (sCLU). Adv Cancer Res. 2009;104:115–138. doi: 10.1016/S0065-230X(09)04007-X. [DOI] [PubMed] [Google Scholar]
- 22.Bettuzzi S, Rizzi F. Chapter 5: Nuclear CLU (nCLU) and the fate of the cell. Adv Cancer Res. 2009;104:59–88. doi: 10.1016/S0065-230X(09)04005-6. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura E, Abreu-e-Lima P, Awakura Y, Inoue T, Kamoto T, et al. Clusterin Is a Secreted Marker for a Hypoxia-Inducible Factor-Independent Function of the von Hippel-Lindau Tumor Suppressor Protein. Am J Pathol. 2006;168:574–584. doi: 10.2353/ajpath.2006.050867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xue J, Li X, Jiao S, Wei Y, Wu G, Fang J. Prolyl hydroxylase-3 is down-regulated in colorectal cancer cells and inhibits IKKbeta independent of hydroxylase activity. Gastroenterology. 2010;138:606–615. doi: 10.1053/j.gastro.2009.09.049. [DOI] [PubMed] [Google Scholar]
- 25.Kuznetsova AV, Meller J, Schnell PO, Nash JA, Ignacak ML, et al. von Hippel–Lindau protein binds hyperphosphorylated large subunit of RNA polymerase II through a proline hydroxylation motif and targets it for ubiquitination. Proc Natl Acad Sci USA. 2003;100:2706–2711. doi: 10.1073/pnas.0436037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rizzi F, Caccamo AE, Belloni L, Bettuzzi S. Clusterin is a short half-life, poly-ubiquitinated protein, which controls the fate of prostate cancer cells. J Cell Physiol. 2009;219:314–323. doi: 10.1002/jcp.21671. [DOI] [PubMed] [Google Scholar]
- 27.Yang W-L, Zhang X, Lin H-K. Emerging role of Lys-63 ubiquitination in protein kinase and phosphatase activation and cancer development. Oncogene. 2010;29:4493–4503. doi: 10.1038/onc.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu CJ, Conze DB, Li T, Srinivasula SM, Ashwell JD. Sensing of Lys 63-linked polyubiquitination by NEMO is a key event in NF-κB activation. Nature Cell Biol. 2006;8:398–406. doi: 10.1038/ncb1384. [DOI] [PubMed] [Google Scholar]
- 29.Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem. 2001;70:503–533. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- 30.van Wijk SJ, de Vries SJ, Kemmeren P, Huang A, Boelens R, et al. A comprehensive framework of E2–RING E3 interactions of the human ubiquitin–proteasome system. Mol Syst Biol. 2009;5:295. doi: 10.1038/msb.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nath D, Shadan S. The ubiquitin system. Nature. 2009;458:421. doi: 10.1038/458421a. [DOI] [PubMed] [Google Scholar]
- 32.Bhoj VG, Chen ZJ. Ubiquitylation in innate and adaptive immunity. Nature. 2009;458:430–437. doi: 10.1038/nature07959. [DOI] [PubMed] [Google Scholar]
- 33.Geetha T, Kenchappa RS, Wooten MW, Carter BD. TRAF6-mediated ubiquitination regulates nuclear translocation of NRIF, the p75 receptor interactor. EMBO J. 2005;24:3859–3868. doi: 10.1038/sj.emboj.7600845. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) 293T cells were transfected with Myc-nCLU plus empty vector or Myc-nCLU plus HA-VHL. In 24 h, the cells were harvested and cellular proteins were prepared. Immunoprecipitation was performed using HA antibody. The results of negative control experiment showed that HA antibody did not interact with Myc-tagged nCLU. (B) 293T cells were transfected as indicated. In 24 h, the cells were harvested and immunoprecipitation was performed using HA antibody. The results of negative control experiment indicated that the HA antibody did not interact with Myc-tagged VHL.
(TIF)
pVHL(1-155) and pVHL(Δ63-155) had little effect on ubiquitination of nCLU. 293T cells were transfected with HA-nCLU, His-Ub and various Myc-VHL constructs as indicated. In 24 h, the cells were harvested and cellular proteins were prepared for immunoprecipitation and western-blot.
(TIF)
pVHL mediated K63-linked ubiquitination of nCLU in vitro . In vitro ubiquitination of nCLU by pVHL was performed as described under Methods. Ubiquitin or Ub(K63R) at 5 µg/µL was added to the reaction system.
(TIF)