Abstract
It has long been observed that many cancer cells exhibit increased aerobic glycolysis and rely more on this pathway to generate ATP and metabolic intermediates for cell proliferation. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is a key enzyme in glycolysis and has been known as a housekeeping molecule. In the present study, we found that GAPDH expression was significantly up-regulated in human colorectal carcinoma tissues compared to the adjacent normal tissues, and also increased in colon cancer cell lines compared to the non-tumor colon mucosa cells in culture. The expression of GAPDH was further elevated in the liver meta-static tissues compared to the original colon cancer tissue of the same patients, suggesting that high expression of GAPDH might play an important role in colon cancer development and metastasis. Importantly, we found that 3-bromopyruvate propyl ester (3-BrOP) preferentially inhibited GAPDH and exhibited potent activity in inducing colon cancer cell death by causing severe depletion of ATP. 3-BrOP at low concentrations (1–10 μM) inhibited GAPDH and a much higher concentration (300 μM) was required to inhibit hexokinase-2. The cytotoxic effect of 3-BrOP was associated with its inhibition of GAPDH, and colon cancer cells with loss of p53 were more sensitive to this compound. Our study suggests that GAPDH may be a potential target for colon cancer therapy.
Keywords: Warburg effect, GAPDH, 3-Bromopyruvate, Colon cancer, Metastasis
Introduction
One of the major metabolic alterations in cancer cells is that they exhibit an increase in aerobic glycolysis and seem to largely rely on this non-oxidative glucose metabolism for generation of ATP and production of certain metabolic intermediates as the building blocks for the synthesis of macromolecules for cell proliferation. This phenomenon, known as the Warburg effect (Warburg 1956a; Warburg 1956b), has been observed in various cancer types including solid tumors and leukemia. Whether such alteration in energy metabolism is a cause malignant transformation or a symptom of cancer cells still remains as a matter of debate. Accumulating evidence suggests that increase in aerobic glycolysis is associated with tumorigenesis and seems necessary to maintain the malignant behaviors of cancer cells. Therefore, the increased dependence of cancer cells on the glycolytic pathway may serve as a biochemical basis to selectively kill cancer cells (Chen et al. 2007).
GAPDH is a key enzyme that catalyzes the redox reaction in the glycolytic pathway by converting glyceraldehyde-3-phosphate to 1,3-bisphosphoglycerate, coupled with the reduction of NAD+ to NADH. Although GAPDH is expressed in most cell types as a housekeeping enzyme and is often used as a control molecule for the expression of other genes in various experimental settings, recent studies have shown that, in addition to its well-known function as a glycolytic enzyme, GAPDH may play important roles in a diverse range of cellular processes and may affect the functions of multiple molecules that interact with GAPDH (Nicholls et al. 2011). Elevation of GAPDH mRNA and protein expression has been observed in pancreatic cancer and lung cancer (Tokunaga et al. 1987; Schek et al. 1988; Mikuriya et al. 2007), indicating that increased GAPDH expression may be associated with cell proliferation and tumorigenesis. Consistently, it has been found that GAPDH gene expression is up-regulated in oncogene-transformed cells and in human prostate cancer of late pathological stage (Persons et al. 1989; Rondinelli et al. 1997), suggesting that increased GAPDH expression may be related to tumor progression.
Active glycolysis in cancer cells suggests a possibility to preferentially inhibit cancer cell metabolism by targeting the glycolytic enzymes. Various glycolytic inhibitors with potential anticancer activity have been identified. 2-Deoxyglucose, lonidamine, and 3-bromopyruvate (3BP) are among the small molecular weight compounds that exhibit inhibitory effect on glycolysis (Pelicano et al. 2006). Previous studies have shown that 3BP, in addition to its ability to inhibit the enzyme activity of hexokinase (HK), is able to cause a disassociation of hexokinase-2 (HK-2) from the mitochondria and induce activation of Bad, leading to mitochondrial membrane permeability transition and apoptosis (Xu et al. 2005a,b; Chen et al. 2009). Recent studies showed that 3-BrOP, an ester derivative of 3BP, exhibited a more potent anticancer activity in vitro and caused rapid depletion of cellular ATP in several types of human cancer cells including leukemia, lymphoma, and neuroblastoma (Xu et al. 2005b; Levy et al. 2010; Akers et al. 2011). Although HK and GAPDH have been considered as potential targets of 3-BrOP, the exact mechanisms by which this compound inhibits glycolysis and exerts its potent anti-cancer activity largely remain unclear.
In the current study, we demonstrated that 3-BrOP exhibited much more potent inhibitory effect on GAPDH than on HK, and that this compound exerted significant cytotoxicity against cancer cells at the low concentrations that inhibited GAPDH but not HK. Importantly, we found that the expression of GAPDH was elevated in colorectal carcinoma and further increased in their liver metastatic tissues. Our study suggests that GAPDH may be a preferred target of 3-BrOP and may serve as a molecular target for cancer therapy.
Materials and methods
Chemicals
Purified GAPDH from rabbit muscle and purified hexokinase-2 from Saccharomyces cerevisiae were purchased from Sigma. 3BP and 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) were also from Sigma. 3-BrOP was synthesized as described previously (Xu et al. 2005b). GAPDH mouse monoclonal antibody was purchased from Chemicon (Temecula, CA). α–Tublin mouse monoclonal antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Biotinylated goat anti-mouse IgG and streptavidin-peroxidase conjugate 3,5-diaminobenzidine (DAB) Substrate Kit were from Zhongshanjinqiao Biotechnology Co., Ltd (Beijing, China). Chemiluminescence kit and apoptosis detection kit (annexin-V-FITC, propidiumiodide, and binding buffer,) were purchased from Keygen Biotech. Co., Ltd (Nanjing, China).
Tumor specimens and immunohistochemistry
Tumor specimens from patients with primary colorectal carcinoma who had undergone initial surgery between 1999 and 2009 at Sun Yat-sen University Cancer Center were used in this study with proper informed consents. Paraffin specimens were collected from 195 patients with colorectal carcinoma, including 28 patients with liver metastasis. None of these patients received preoperative chemotherapy or radiotherapy. The patients aged from 19 to 83 years (median, 54 years). Hematoxylin-eosin-stained tissue slides were reviewed by a pathologist to identify proper tissue sections for staining by immunohistochemistry (IHC). Specimens embedded in paraffin slides (4 μm sections) were first treated with 3% H2O2 for 30 min to quench the endogenous peroxidase activity. Tissue slides were immersed in citrate buffer (pH 6.0) and heated for 5 min, and then blocked with 10% goat serum. A 1:8000 dilution of mouse monoclonal antibody against human GAPDH, biotinylated goat anti-mouse IgG and streptavidin-peroxidase conjugate were then added sequentially. After incubation and washing, the tissue slides were incubated with the DAB Substrate Kit and counterstained with hematoxylin before examination by light microscopy. The expression levels of GAPDH were scored according to the relative intensity of the immunostaining.
Cell culture
Human colon cancer cells HCT116 and HT29 were cultured in McCoy’s 5A medium supplemented with 10% fetal bovine serum (FBS). THC8307 and DLD1 cells were cultured in RPMI 1640 medium supplemented with 10% FBS. Normal human colon mucosal epithelial cells (NCM460) were cultured in DMEM medium supplemented with 10% FBS. The cells were maintained at 37 °C in a humidified chamber containing 5% CO2.
Western blot
Cellular proteins (50 μg) from cell lysates were separated by standard SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was then probed with primary mouse anti-GAPDH (dilution, 1:10,000) over night at 4 °C. The membrane was then incubated with horseradish peroxides-conjugated goat anti-mouse secondary antibody (dilution, 1:10,000) for 1 h at room temperature, and the protein band was revealed by chemiluminescent detection. α–tublin was also probed as a loading control.
Assay of GAPDH and HK enzyme activities
Purified GAPDH from rabbit muscle (Sigma-Aldrich) was used in the in vitro GAPDH assays with a GAPDH Assay Kit (ScienCell Research Labotatories) according to the manufacturer’s instructions. Purified GAPDH was incubated in vitro with various concentrations of 3-BrOP for 30 min and then added to a mixture of 6.67 mM 3-phosphoglyceric acid, 3.33 mM L-cysteine, 117μM β-NADH, 1.13 mM ATP, 3.33 U/ml 3-phosphoglycerate kinase, and 100 mM triethanolamine buffer (pH 7.6). The change in NADH fluorescence was then monitored using a Fluoroskan spectrometer every minute for up to 20 min. Purified hexokinase-2 from Saccharomyces cerevisiae (Sigma-Aldrich) was incubated with various concentrations of 3-BrOP for 30 min and then added to a mixture of 6.5 mM MgCl2, 220 mM glucose, 2.7 mM ATP, 0.83 mM β-NAD, 0.24 U/ml glucose-6-phosphate dehydrogenase (Sigma-Aldrich), and 100 mM triethanolamine buffer (pH 7.6). The change in NADH fluorescence was measured using a Fluoroskan fluorescence scanner every 1 min for up to 20 min.
Cell proliferation assay
Cells were plated in 96-well microplates at a density of 2000 cells/well and incubated with 3-BrOP for 72 h. 20 μL of 5 mg/mL MTT solution was added to each well and incubated at 37 °C for an additional 4 h. Medium was removed and 200 μL of dimethyl sulfoxide (DMSO) were added to each well. Absorbance was measured using a microtiter plate reader at 570 nm.
Flow cytometry analysis
Cells were seeded in 6-well plates at 2×105 cells/well and incubated with 3-BrOP for 24–48 h. The cells were collected, washed twice with PBS and re-suspended in the binding buffer. The samples were then stained with annexin-V-FITC and PI for 15 min in dark. Analysis was performed by a FACS Calibur flow cytometer (Becton Dickinson).
Statistical analysis
The expression levels of GAPDH (IHC scores) in colorectal carcinoma tissues, the adjacent normal tissues, and the corresponding liver metastatic tissues were compared using the Wilcoxon signed rank test. The SPSS software (version 16.0) was used for the data analysis. The statistical difference between colorectal adenomas and normal mucosa tissues in their GAPDH mRNA expression was calculated by two-tailed t test. A p value of less than 0.05 was considered statistically significant.
Results
Elevated expression of GAPDH in colon cancer and liver metastatic tissues
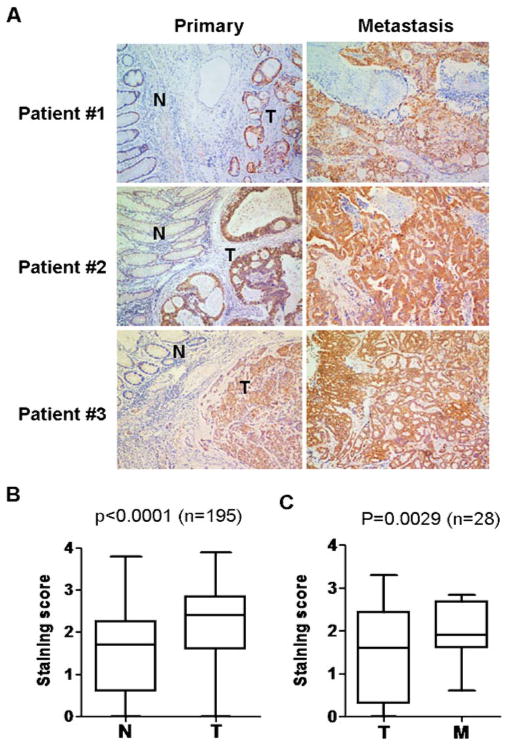
Since GAPDH is an essential enzyme in glycolysis which is highly active in many cancer cells, we examined the expression of this enzyme by immunohistochemistry using 195 paraffin-embedded, archived colon cancer tissues, including 28 matched liver metastatic tissues. Figure 1a shows the immunostaining of GAPDH in 3 pairs of representative slides of the primary colon cancer tissues and the matched liver metastatic lesions. Each primary tumor slide contained both tumor tissue (T) and the adjacent noncancerous tissue (N). The immunostaining results showed that the expression of GAPDH was substantially higher in the tumor tissues compared to the adjacent noncancerous tissues. The increase in GAPDH expression seemed specific to the cancer cells, since the stromal cells within the tumor tissue showed a weak staining. Comparison of GAPDH expression in the tumor tissues and non-tumor tissues in all 195 cases showed that the increase in GAPDH expression in cancer cells is highly significant (Fig. 1b, p<0.0001, Wilcoxon signed rank test). Interestingly, when we compared the expression of GAPDH in the primary cancer tissues with the matched liver metastatic tissues, there was a further increase of GAPDH in the metastatic lesions (Fig. 1a). Statistical analysis of 28 pairs of primary colorectal cancer tissues with their respective live metastatic lesions showed that this increase was significant (Fig. 1c, p = 0.0029, Wilcoxon signed rank test). These data together suggest that GAPDH may potentially play a role in colon cancer metastasis.
Fig. 1.
GAPDH protein expression in colorectal carcinoma, the adjacent non-cancerous tissues, and the liver metastasis tissues. a Immunostaining of GAPDH in three pairs of representative colorectal tumor tissues (T) with adjacent non-cancerous tissues (N) and in liver meta-static leisions (M). b Quantitative analysis of GAPDH expression in primary colorectal tumor tissues and adjacent noncancerous tissues. (Wilcoxon signed rank test, n = 195, p<0.0001). c Comparison of GAPDH expression in primary colorectal tumor tissues and the matched liver metastatic tissue (Wilcoxon signed rank test, n = 28, P = 0.0029). Data in B and C are shown as mean±S.D.
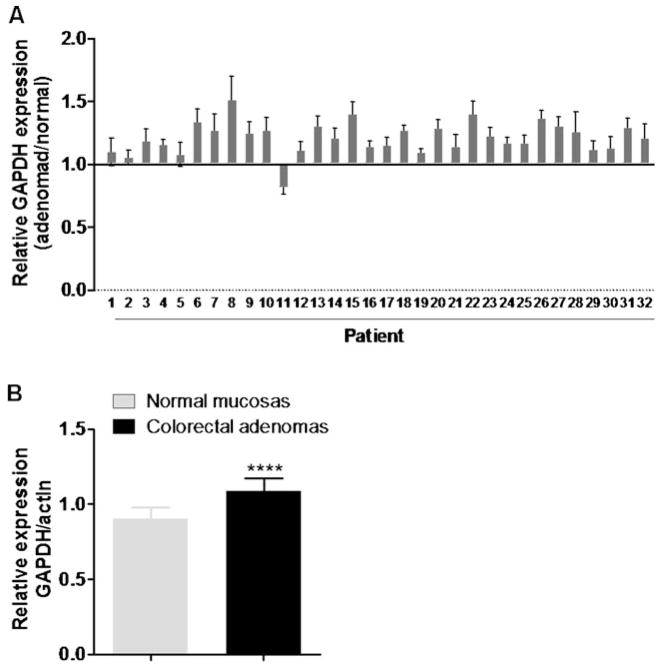
Interestingly, through the analysis of gene expression profiles obtained from the NCBI GEO database (accession GDS2947, Sabates-Bellver et al. 2007), the expression of GAPDH mRNA was found to be also increased in colorectal adenoma samples. As shown in Fig. 2a, we used the expression of β-actin gene (ACTB) as an internal control to calculate the relative GAPDH expression in 32 adenoma patient samples in comparison with their respective normal mucosa control samples, and found that almost all adenoma specimens (except patient #11) showed certain degrees of increase in GAPDH expression. The normalized GAPDH expression values in adenomas were significantly higher than that of normal mucosas (Fig. 2b, p<0.0001). Since colorectal adenomas (polyps) are considered pre-cancerous lesions and may develop into adenocarcinoma, the increased expression of GAPDH in colorectal adenomas suggests a potential involvement of GAPDH in colon cancer development.
Fig. 2.
Increased expression of GAPDH mRNA in colorectal adenoma. a The relative GAPDH expression were calculated using the average of GAPDH expression values from the gene expression profiles in the NCBI GEO database generated from the Affymetrix Human Genome U133 Plus 2.0 Array (Sabates-Bellver J. et al. 2007, accession GDS2947) of the paired colorectal adenoma and normal mucosa sample from each patient (n = 32), each normalized by its β-actin (ACTB) expression. b Comparison of relative GAPDH expression in colorectal adenomas and normal colon mucosa. The relative expression values were calculated using the averages of GAPDH expression values normalized by ACTB expression values of 32 pairs of colorectal adenomas and normal mucosas. The statistical significance was calculated by two-tailed t test (p<0.0001)
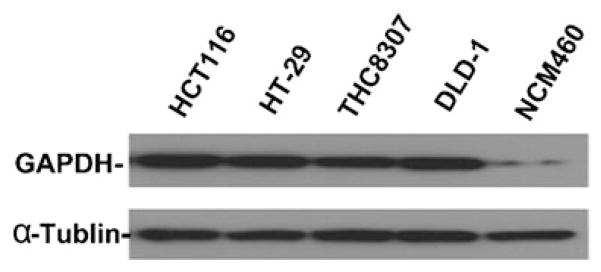
We then used western blot analysis to compare GAPDH protein expression levels in colon cancer cells and normal colon epithelial cells in culture. As shown in Fig. 3, the protein extracts from 4 colon cancer cell lines (HCT116, HT29, THC8307, DLD-1) all showed a substantial higher GAPDH expression than the normal colon mucosal epithelial cells (NCM460). It seemed that the expression of GAPDH remained high when colon cancer cells were cultured in vitro as established cell lines, further suggesting the important role of this enzyme in colon cancer.
Fig. 3.
Western blot analysis of GAPDH expression in colorectal cancer cell lines in comparison with normal colon mucosa epithelial cells. Protein lysates (50 μg) from colorectal cancer cell lines (HCT116p53+/+, HT-29, THC8307, DLD-1) and normal colon mucosa epithelial cells (NCM460) were separated by SDS-PAGE. After the proteins were transferred to a membrane, GAPDH signal was detected using a specific antibody as described in Methods. α-tublin was used as a protein loading control
Preferential inhibition of GAPDH enzyme activity by 3-BrOP
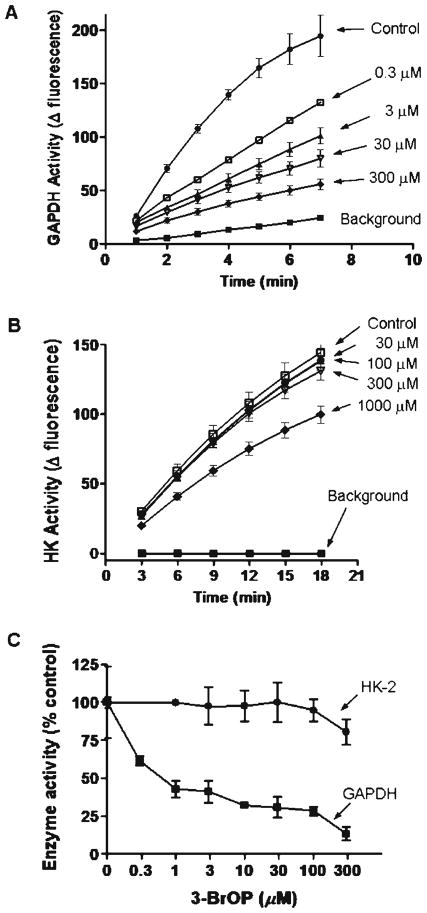
Based on the observations that GAPDH expression was elevated in colon cancer tissues and in metastatic tumor tissues, we speculated that this enzyme might be essential for the survival and proliferation of colon cancer cells and thus could be a potential therapeutic target. Considering that 3-BrOP is a potent glycolytic inhibitor and that its parental compound 3BP has inhibitory effect on HK and GAPDH, we tested the potential inhibitory effect of 3-BrOP on HK and GAPDH, using in vitro assays with the purified enzymes. As shown in Fig. 4a, the purified GAPDH from rabbit muscle showed high enzyme activity under the in vitro assay conditions. Incubation of this enzyme with 3-BrOP resulted in a concentration-dependent inhibition of the enzyme activity. This inhibitory effect was observed when the concentration of 3-BrOP was as low as 0.3 μM. In contrast, incubation of purified HK-2 led to only a modest inhibition of the enzyme activity at high concentrations (300–100 μM) of 3-BrOP (Fig. 4b), suggesting that this compound had limited ability to inhibit HK-2. Quantitative comparison of the effect of 3-BrOP on GAPDH and HK-2 clearly showed that 3-BrOP preferentially inhibited GAPDH (Fig. 4c).
Fig. 4.
Preferential inhibition of GAPDH enzyme activity by 3-BrOP. a GAPDH enzyme activity assay was performed as described in Methods, using purified GAPDH from rabbit muscle in vitro with the indicated concentrations of 3-BrOP. b HK-2 enzyme activity assay was performed as described in Methods, using purified HK-2 from yeast in vitro with the indicated concentrations of 3-BrOP. c Quantitative comparison of the inhibitory effect of 3-BrOP on purified GAPDH and purified HK-2
3-BrOP induced cancer cell death at the concentrations that inhibited GAPDH
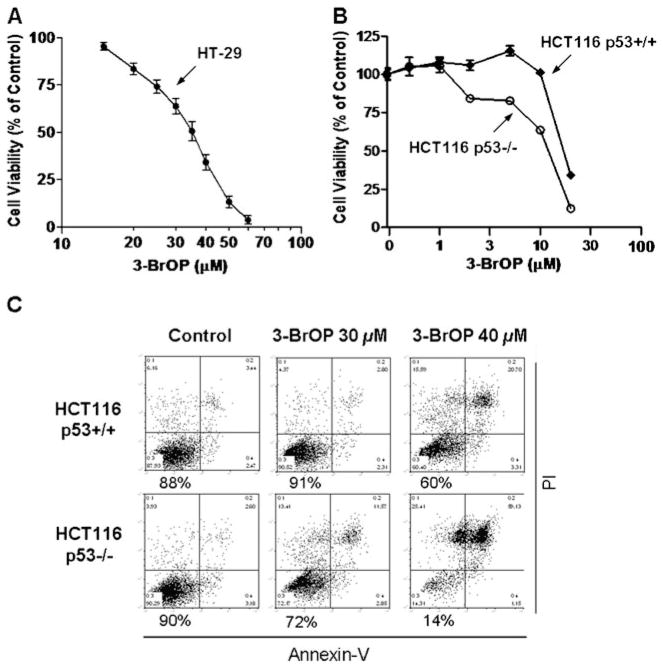
To evaluate the cytotoxic activity of 3-BrOP against cancer cells in relation to its inhibitory effect on GAPDH and HK, we tested the cytotoxicity of various concentrations of 3-BrOP in several colon cancer cell lines including HT29, HCT116p53+/+ and HCT116p53−/− cells. MTT assay revealed that 3-BrOP inhibited the colon cancer cell proliferation in a concentration-dependent manner, with the IC50 values of 30 μM and 13 μM for HT-29 cells and HCT116 (p53+/+) cells, respectively, in a 72-h drug incubation period (Fig. 5a–b). Of note, these concentrations of 3-BrOP (10–30 μM) were sufficient to inhibit GAPDH, but had little effect on HK-2 activity in vitro (Fig. 4), suggesting that the cytotoxic effect of 3-BrOP was likely due to inhibition of GAPDH.
Fig. 5.
Effect of 3-BrOP on colon cancer cell growth and viability. a Concentration-dependent growth inhibition of HT-29 colon cancer cells by 3-BrOP. HT-29 cells were incubated with the indicated concentration of 3-BrOP for 72 h and then subjected to MTT assay. Error bars represent SD from 3 independent experiments. b Growth inhibition of HCT116p53+/+ and HCT116p53−/− cells by 3-BrOP. Cells were incubated with the indicated concentration of 3-BrOP for 72 h, and then subjected to MTT assay. Error bars represent SD from 3 independent experiments c Comparison of the cytotoxic effect of 3-BrOP in HCT116p53+/+ and HCT116p53−/− cells. The colon cancer cells were incubated with the indicated concentrations of 3-BrOP for 24 h, and cells viability was detected by annexin-PI staining followed by flow cytometry analysis. The number below each panel shows the % of viable cells after drug treatment
Interestingly, the MTT assay also showed that HCT116 cells with p53 double knockout genotype (p53−/−) seemed to be more sensitive to 3-BrOP compared to the isogenic HCT116 cells with wild-type p53 (Fig. 5b). We then used annexin-V and PI double staining and flow cytometry analysis to further test the acute cytotoxic effect of 3-BrOP in the isogenic HCT116 p53+/+ and p53−/− cell pair. As shown in Fig. 5c, incubation of HCT116 p53−/− cells with 40 μM 3-BrOP for 24 h caused a massive cell death, with only 14% of viable cells remained after a one-day incubation. Under the identical drug exposure conditions (40 μM, 24 h), the HCT116p53+/+ cells were significantly less sensitive, with 60% of the cells remained viable after the drug incubation. Thus, it appeared that cancer cells with a loss of p53 function might be more dependent on glycolysis and were more sensitive to glycolytic inhibition.
3-BrOP caused rapid depletion of cellular ATP before cancer cell death
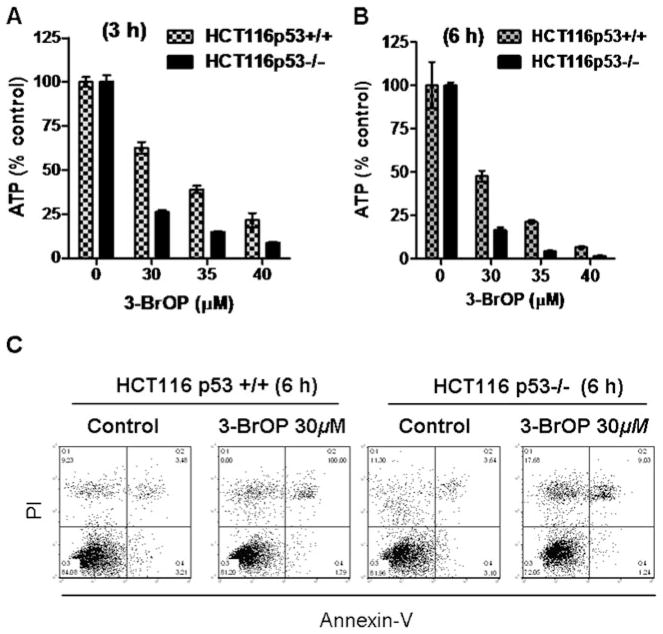
Considering that colon cancer cells had elevated expression of GAPDH and appeared more dependent on glycolysis for ATP generation, we reasoned that if inhibition of GAPDH were the key mechanism responsible for the cytotoxic action of 3-BrOP, incubation of colon cancer cells should cause severe depletion of cellular ATP. To test this possibility, we treated HCT116 cells with various concentrations of 3-BrOP and then measured cellular ATP after various periods of the drug incubation. As shown in Fig. 6a–b, severe ATP depletion was observed as early as 3 h after incubation with 30–40 μM 3-BrOP, and over 90% of ATP was depleted at 6 h when the cells were incubated with 40 μM 3-BrOP. Interestingly, compared with HCT116 cells with wild-type p53 (+/+), the colon cancer cells without p53 (−/−) were more vulnerable to ATP depletion by 3-BrOP at all concentrations and time points tested (Fig. 6a–b), again suggesting that a loss of p53 would render cancer cells more glycolytic and sensitive to inhibition of glycolysis.
Fig. 6.
Comparison of cellular ATP depletion by 3-BrOP in HCT116p53+/+ and HCT116p53−/− cells. a–b HCT116 cells were incubated with the indicated concentrations of 3-BrOP for 3–6 h as indicated, and cellular ATP was measured using a luminescence ATP assay kit. c Effect of a short-term treatment of 3-BrOP on cell viability in HCT116p53 +/+ and HCT116p53−/− cells. Cells were incubated with 30 μM 3-BrOP for 6 h, and cell viability was measured by annexin-PI staining followed by flow cytometry analysis
Flow cytometry analysis of HCT116 cells after staining with annexin-V and PI showed that there was minimum cell death detectable at 6 h after incubation with 3-BrOP (Fig. 6c), suggesting that the severe decrease in cellular ATP observed in the drug-treated cells occurred before cell death, and thus was an important primary biochemical event rather than a later event subsequent to cell death (non-specific loss of ATP).
Discussion
Increased aerobic glycolysis in cancer cells has been recognized for decades. Otto Warburg was a pioneer in cancer metabolism, and he suggested that a switch from oxidative phosphorylation to glycolysis for ATP generation due to impairment of respiration might be the “origin of cancer” (Warburg 1956a,b ). In addition to its role in the production of ATP, the glycolytic pathway also provides important metabolic intermediates needed for cell growth and proliferation (Vander Heiden et al. 2009). Because of the highly active glycolysis in cancer cells, it is reasonable to speculate that the expression of glycolytic enzymes may be up-regulated in tumor cells. In the current study, we analyzed the expression of GAPDH in 195 cases of colorectal carcinoma (CRC) and the paired adjacent non-cancerous tissues. Twenty-eight matched liver metastatic tissues were also analyzed. We discovered that GAPDH expression was highly elevated in CRC tissues compared to the adjacent non-cancerous tissues. The expression of GAPDH was further increased in metastatic tissues. Western blot analysis confirmed that colon cancer cells exhibited substantially higher expression of GAPDH protein than the normal colon mucosa epithelial cells in culture. Furthermore, GAPDH expression was found to be further increased in matched metastatic lesions compared to their original colon cancer tissues. Interestingly, analysis of the NCBI GEO database also revealed that GAPDH mRNA expression was elevated in colorectal adenoma, a clinical condition considered to be pre-cancerous. These data together suggest that increased expression of GAPDH may be associated with colon cancer development, and may play a role in metastasis. Indeed, early studies have suggested an association between GAPDH expression and cell motility and meta-static potential in prostate cancer (Epner et al. 1993; Epner and Coffey 1996), although the underlying mechanisms still remain unclear. Since tumorigenesis and metastasis are complex and multi-step processes, the exact role of GAPDH in these pathological processes requires further investigations.
The important role of GAPDH in colon cancer cell survival was further suggested by the observations that 3-BrOP potently inhibited this enzyme activity (Fig. 4) and caused a severe ATP depletion (Fig. 6) and subsequent cell death (Fig. 5). The mechanism by which 3-BrOP preferentially inhibits GAPDH is unclear at the present time. 3BP, the parental compound of 3-BrOP, is known to inhibit glycolysis and appears to be an active-site-specific alkylating agent under certain conditions or a non-specific alkylating agent under other conditions (Cini et al. 1978; Lowe and Perham 1984; Honda and Tokushige 1985; Kirley and Day 1985; Banas et al. 1988). The alkylating property of 3BP seems to confer this compound the ability to interact with various proteins and inhibit their functions, including modification of tryptophanase (Yoshida and Wood 1978; Borthwick et al. 1995) and inhibition of carbonic anhydrase (Yoshida and Wood 1978; Borthwick et al. 1995), pyruvate dehydrogenase (Yoshida and Wood 1978; Borthwick et al. 1995), GAPDH (Ganapathy-Kanniappan et al. 2009), and hexokinase (Ko et al. 2001; Pedersen 2007). Of note, HK-2 seems to be largely bound to mitochondria, plays an important role in cancer metabolism, and is considered a pivotal target for cancer therapy (Mathupala et al. 2009). It is possible that hexokinase-2 may be a key target of 3BP, whereas 3-PrOP preferentially inhibits GAPDH. Thus, it would be of interest to compare the relative potency of 3BP in inhibiting HK-2 and GAPDH, and to evaluate the relative contributions of each enzyme inhibition to the cytotoxic action of this compound. In the case of 3-BrOP, it seems that inhibition of GAPDH was the main event leading to ATP depletion and cancer cell death, since 3-BrOP at the concentrations of 10–40 μM showed significant cytotoxicity in colon cancer cells and inhibited purified GAPDH, but did not inhibit HK-2 at these drug concentrations.
It is of great interest to note that colon cancer cells with loss of p53 were more sensitive to 3-BrOP compared to the p53+/+ cells. Previous study showed that p53 plays important roles in guarding mitochondrial genomic stability through its interaction with mitochondrial pol γ (Achanta et al. 2005), and in promoting mitochondrial respiratory function through its downstream effector SCO2 (Matoba et al. 2006). Thus, it is possible that a loss of p53 function would cause mitochondrial dysfunction and compromise the ability to generate ATP through oxidative phosphorylation, leading to increased glycolysis and more dependence on this metabolic pathway. As such, the p53−/− cells would be expected to be more sensitive to glycolytic inhibition by 3-BrOP. Importantly, loss of p53 often leads to a decrease in sensitivity to many chemotherapeutic agents due to a loss of the pro-apoptotic function of p53. The observation that 3-BrOP was more effective in killing p53−/− cells suggests that this compound might be useful in eliminating p53-null cells that are resistant to standard therapeutic drugs.
Although the results from experimental models have shown that inhibition of glycolysis by targeting its key enzymes such as HK-2 and GAPDH may provide a new approach to cancer treatment, cautions should be exercised in considering the potential toxic side effects of this therapeutic strategy. Glycolysis is a conserved metabolic pathway used by various normal cell types. It is also a main pathway that generates pyruvate as a critical metabolic intermediate for entering mitochondria to fuel the TCA cycle and energy generation. A potent inhibition of glycolysis might significantly affect these metabolism in normal cells and thus have potential toxic side effect, especially in the tissues such as brain, the heart, and testis that are highly active in glucose metabolism. It would be of pivotal importance to compare the relative cytotoxicity of 3-BrOP, 3BP, and other glycolytic inhibitors in cancer and normal cells. Further investigations are required to evaluate the therapeutic activity and selectivity of glycolytic inhibition, and to develop strategies to minimize/overcome the potential toxic side effects.
In summary, our study revealed a significant increase of GAPDH expression in colon cancer and liver metastatic tissues, and suggested that the increased expression of GAPDH might be associated with cancer development and disease progression. GAPDH appeared to be a preferred target of 3-BrOP, which induced severe ATP depletion and significant cell death in colon cancer cells. Our findings identified GAPDH as a potential therapeutic target for colon cancer treatment. This promising anticancer strategy warrants further investigations.
Abbreviations
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- 3BP
3-Bromopyruvate
- 3-BrOP
3-Bromopyruvate propyl ester
- HK
Hexokinase
- MTT
3- (4, 5-Dimethyl-thiazol-2-yl)-2, 5-diphenyl tetrazolium bromide
- DAB
3, 5-Diaminobenzidine
- CRC
Colorectal carcinoma
- IHC
Immunohistochemistry
- PI
Propidiumiodide
Contributor Information
Zhenjie Tang, State Key Laboratory of Oncology in Southern China, Sun Yat-Sen University Cancer Center, 651 Dong Feng Road East, Guangzhou 510060, China.
Shuqiang Yuan, State Key Laboratory of Oncology in Southern China, Sun Yat-Sen University Cancer Center, 651 Dong Feng Road East, Guangzhou 510060, China. Department of Molecular Pathology, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030, USA.
Yumin Hu, State Key Laboratory of Oncology in Southern China, Sun Yat-Sen University Cancer Center, 651 Dong Feng Road East, Guangzhou 510060, China.
Hui Zhang, Department of Molecular Pathology, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030, USA.
Wenjing Wu, State Key Laboratory of Oncology in Southern China, Sun Yat-Sen University Cancer Center, 651 Dong Feng Road East, Guangzhou 510060, China.
Zhaolei Zeng, State Key Laboratory of Oncology in Southern China, Sun Yat-Sen University Cancer Center, 651 Dong Feng Road East, Guangzhou 510060, China.
Jing Yang, State Key Laboratory of Oncology in Southern China, Sun Yat-Sen University Cancer Center, 651 Dong Feng Road East, Guangzhou 510060, China.
Jingping Yun, State Key Laboratory of Oncology in Southern China, Sun Yat-Sen University Cancer Center, 651 Dong Feng Road East, Guangzhou 510060, China.
Ruihua Xu, Email: xurh@sysucc.org.cn, State Key Laboratory of Oncology in Southern China, Sun Yat-Sen University Cancer Center, 651 Dong Feng Road East, Guangzhou 510060, China.
Peng Huang, Email: phuang@mdanderson.org, State Key Laboratory of Oncology in Southern China, Sun Yat-Sen University Cancer Center, 651 Dong Feng Road East, Guangzhou 510060, China. Department of Molecular Pathology, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030, USA.
References
- Achanta G, Sasaki R, Feng L, Carew JS, Lu W, Pelicano H, Keating MJ, Huang P. Novel role of p53 in maintaining mitochondrial genetic stability through interaction with DNA Pol gamma. EMBO J. 2005;24:3482–3492. doi: 10.1038/sj.emboj.7600819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akers LJ, Fang W, Levy AG, Franklin AR, Huang P, Zweidler-McKay PA. Targeting glycolysis in leukemia: a novel inhibitor 3-BrOP in combination with rapamycin. Leuk Res. 2011;35:814–820. doi: 10.1016/j.leukres.2010.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banas T, Gontero B, Drews VL, Johnson SL, Marcus F, Kemp RG. Reactivity of the thiol groups of Escherichia coli phosphofructo-1-kinase. Biochim Biophys Acta. 1988;957:178–184. doi: 10.1016/0167-4838(88)90270-1. [DOI] [PubMed] [Google Scholar]
- Borthwick EB, Connell SJ, Tudor DW, Robins DJ, Shneier A, Abell C, Coggins JR. Escherichia coli dihydrodipicolinate synthase: characterization of the imine intermediate and the product of bromopyruvate treatment by electrospray mass spectrometry. Biochem J. 1995;305(Pt 2):521–524. doi: 10.1042/bj3050521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Lu W, Garcia-Prieto C, Huang P. The Warburg effect and its cancer therapeutic implications. J Bioenerg Biomembr. 2007;39:267–274. doi: 10.1007/s10863-007-9086-x. [DOI] [PubMed] [Google Scholar]
- Chen Z, Zhang H, Lu W, Huang P. Role of mitochondria-associated hexokinase II in cancer cell death induced by 3-bromopyruvate. Biochim Biophys Acta. 2009;1787:553–560. doi: 10.1016/j.bbabio.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cini C, Foppoli C, De Marco C. On the product of the reaction between cysteamine and 3-bromopyruvate. Ital J Biochem. 1978;27:233–246. [PubMed] [Google Scholar]
- Epner DE, Coffey DS. There are multiple forms of glyceraldehyde-3-phosphate dehydrogenase in prostate cancer cells and normal prostate tissue. Prostate. 1996;28:372–378. doi: 10.1002/(SICI)1097-0045(199606)28:6<372::AID-PROS6>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Epner DE, Partin AW, Schalken JA, Isaacs JT, Coffey DS. Association of glyceraldehyde-3-phosphate dehydrogenase expression with cell motility and metastatic potential of rat prostatic adenocarcinoma. Cancer Res. 1993;53:1995–1997. [PubMed] [Google Scholar]
- Ganapathy-Kanniappan S, Geschwind JF, Kunjithapatham R, Buijs M, Vossen JA, Tchernyshyov I, Cole RN, Syed LH, Rao PP, Ota S, Vali M. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is pyruvylated during 3-bromopyruvate mediated cancer cell death. Anticancer Res. 2009;29:4909–4918. [PMC free article] [PubMed] [Google Scholar]
- Honda T, Tokushige M. Active site-directed modification of tryptophanase by 3-bromopyruvate. J Biochem. 1985;97:851–857. doi: 10.1093/oxfordjournals.jbchem.a135126. [DOI] [PubMed] [Google Scholar]
- Kirley JW, Day RA. Irreversible inhibition of carbonic anhydrase by the carbon dioxide analog cyanogen. Biochem Biophys Res Commun. 1985;126:457–463. doi: 10.1016/0006-291x(85)90627-8. [DOI] [PubMed] [Google Scholar]
- Ko YH, Pedersen PL, Geschwind JF. Glucose catabolism in the rabbit VX2 tumor model for liver cancer: characterization and targeting hexokinase. Cancer Lett. 2001;173:83–91. doi: 10.1016/s0304-3835(01)00667-x. [DOI] [PubMed] [Google Scholar]
- Levy AG, Zage PE, Akers LJ, Ghisoli ML, Chen Z, Fang W, Kannan S, Graham T, Zeng L, Franklin AR, Huang P, Zweidler-McKay PA. The combination of the novel glycolysis inhibitor 3-BrOP and rapamycin is effective against neuroblastoma. Invest New Drugs. 2010 Oct 5; doi: 10.1007/s10637-010-9551-y. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe PN, Perham RN. Bromopyruvate as an active-site-directed inhibitor of the pyruvate dehydrogenase multienzyme complex from Escherichia coli. Biochemistry. 1984;23:91–97. doi: 10.1021/bi00296a015. [DOI] [PubMed] [Google Scholar]
- Mathupala SP, Ko YH, Pedersen PL. Hexokinase-2 bound to mitochondria: cancer’s stygian link to the “Warburg Effect” and a pivotal target for effective therapy. Semin Cancer Biol. 2009;19:17–24. doi: 10.1016/j.semcancer.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoba S, Kang JG, Patino WD, Wragg A, Boehm M, Gavrilova O, Hurley PJ, Bunz F, Hwang PM. p53 regulates mitochondrial respiration. Science. 2006;312:1650–1653. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- Mikuriya K, Kuramitsu Y, Ryozawa S, Fujimoto M, Mori S, Oka M, Hamano K, Okita K, Sakaida I, Nakamura K. Expression of glycolytic enzymes is increased in pancreatic cancerous tissues as evidenced by proteomic profiling by two-dimensional electrophoresis and liquid chromatography-mass spectrometry/mass spectrometry. Int J Oncol. 2007;30:849–855. [PubMed] [Google Scholar]
- Nicholls C, Li H, Liu JP. GAPDH: a common enzyme with uncommon functions. Clin Exp Pharmacol Physiol. 2011 doi: 10.1111/j.1440-1681.2011.05599.x. [DOI] [PubMed] [Google Scholar]
- Pedersen PL. Warburg, me and Hexokinase 2: multiple discoveries of key molecular events underlying one of cancers’ most common phenotypes, the “Warburg Effect”, i.e., elevated glycolysis in the presence of oxygen. J Bioenerg Biomembr. 2007;39:211–222. doi: 10.1007/s10863-007-9094-x. [DOI] [PubMed] [Google Scholar]
- Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25:4633–4646. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
- Persons DA, Schek N, Hall BL, Finn OJ. Increased expression of glycolysis-associated genes in oncogene-transformed and growth-accelerated states. Mol Carcinog. 1989;2:88–94. doi: 10.1002/mc.2940020207. [DOI] [PubMed] [Google Scholar]
- Rondinelli RH, Epner DE, Tricoli JV. Increased glyceraldehyde-3-phosphate dehydrogenase gene expression in late pathological stage human prostate cancer. Prostate Cancer Prostatic Dis. 1997;1:66–72. doi: 10.1038/sj.pcan.4500208. [DOI] [PubMed] [Google Scholar]
- Sabates-Bellver J, Van der Flier LG, de Palo M, Cattaneo E, Maake C, Rehrauer H, Laczko E, Kurowski MA, Bujnicki JM, Menigatti M, Luz J, Ranalli TV, Gomes V, Pastorelli A, Faggiani R, Anti M, Jiricny J, Clevers H, Marra G. Transcriptome profile of human colorectal adenomas. Mol Cancer Res. 2007;5:1263–1275. doi: 10.1158/1541-7786.MCR-07-0267. [DOI] [PubMed] [Google Scholar]
- Schek N, Hall BL, Finn OJ. Increased glyceraldehyde-3-phosphate dehydrogenase gene expression in human pancreatic adenocarcinoma. Cancer Res. 1988;48:6354–6359. [PubMed] [Google Scholar]
- Tokunaga K, Nakamura Y, Sakata K, Fujimori K, Ohkubo M, Sawada K, Sakiyama S. Enhanced expression of a glyceraldehyde-3-phosphate dehydrogenase gene in human lung cancers. Cancer Res. 1987;47:5616–5619. [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O. On respiratory impairment in cancer cells. Science. 1956a;124:269–270. [PubMed] [Google Scholar]
- Warburg O. On the origin of cancer cells. Science. 1956b;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Xu RH, Pelicano H, Zhou Y, Carew JS, Feng L, Bhalla KN, Keating MJ, Huang P. Inhibition of glycolysis in cancer cells: a novel strategy to overcome drug resistance associated with mitochondrial respiratory defect and hypoxia. Cancer Res. 2005a;65:613–621. [PubMed] [Google Scholar]
- Xu RH, Pelicano H, Zhang H, Giles FJ, Keating MJ, Huang P. Synergistic effect of targeting mTOR by rapamycin and depleting ATP by inhibition of glycolysis in lymphoma and leukemia cells. Leukemia. 2005b;19:2153–2158. doi: 10.1038/sj.leu.2403968. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Wood HG. Crystalline pyruvate, phosphate dikinase from Bacteroides symbiosus. Modification of essential histidyl residues and bromopyruvate inactivation. J Biol Chem. 1978;253:7650–7655. [PubMed] [Google Scholar]