Abstract
Objective
Hyperthermia uses exogenous heat induction as a cancer therapy. This work addresses the acute and long-term effects of hyperthermia in the highly metastatic melanoma cell line B16-F10.
Materials and Methods
Melanoma cells were submitted to one heat treatment, 45°C for 30 min, and thereafter were kept at 37°C for an additional period of 14 days. Cultures maintained at 37°C were used as control. Cultures were assessed for the heat shock reaction.
Results
Immediately after the heat shock, cells began a process of fast degradation, and, in the first 24 h, cultures showed decreased viability, alterations in cell morphology and F-actin cytoskeleton organization, significant reduction in the number of adherent cells, most of them in a process of late apoptosis, and an altered gene expression profile. A follow-up of two weeks after heat exposure showed that viability and number of adherent cells remained very low, with a high percentage of early apoptotic cells. Still, heat-treated cultures maintained a low but relatively constant population of cells in S and G2/M phases for a long period after heat exposure, evidencing the presence of metabolically active cells.
Conclusion
The melanoma cell line B16-F10 is susceptible to one hyperthermia treatment at 45°C, with significant induced acute and long-term effects. However, a low but apparently stable percentage of metabolically active cells survived long after heat exposure.
Introduction
Hyperthermia, a cancer therapy, uses exogenous heat induction as therapeutic agent [1]. It can be used alone or as adjuvant to other therapies, especially chemotherapy and radiotherapy [2]. Hyperthermia localized techniques apply heat only to the tumour using electromagnetic energy ultrasounds [3]. The use of hyperthermia is controversial and presents significant technical challenges. Scientific reports show contradictory results relatively to hyperthermia therapeutic potential, some attesting its utility and others showing no effect. This is probably due to the lack of knowledge correlating the complex hyperthermic effects at cellular and tissue level [1].
In vivo animal studies have been performed in a variety of tumour models, as melanoma [4], glioma [5], mammary carcinoma [6], osteosarcoma [7], prostate cancer [8], most showing some benefit with the use of hyperthermia. These studies have shown that with hyperthermia exposure involving lower temperatures, as 43°C, the tumour regression can only be observed with more than one treatment session [5], [7], [9], [10]. In this situation, it has been reported that the hyperthermia treatment gives rise to an immune reaction against the tumour cells, leading to a second deleterious effect to the tumour, which appears to be important to a good outcome [11], [12]. On the other hand, higher temperatures, as 45–46°C, lead to tumour regression with fewer applications; in this case, the heat shock reaction takes place immediately, and tumour treatment is faster, apparently without side effects [8], [11].
In vitro studies have demonstrated a variety of effects, for heating temperatures ranging from 41–46°C and different heating times. At cellular level, the heat shock effect involves almost all known structural and functional systems, namely alterations in gene expression and ion homeostasis, protein degradation and denaturation, which can be reversible or lethal [1], [2], [13], [14], [15], [16], [17], [18]. Depending on the treatment protocol, hyperthermia outcome alternates from cells that resist to damage and are able to recover, to cells that can not compensate and undergoes predominantly apoptosis, to cells that immediately suffer necrosis [17]. A variety of results have been reported, depending on the cell line, temperature and exposure time [17], [19], [20], [21], [22]. Regarding melanoma cells, significant differences in the experimental protocols have been reported, namely involving human melanoma cells heated at temperatures ranging from 41–43°C for 3–6 h [14], 41.8–45°C for 15–150 min [15] and 43°C for 120 min [19] and, also, B16-F10 murine melanoma cells heated at 43 and 46°C for 30 min [23]. Differences were also observed in the characterization of the heat shock reaction, but these studies have focused in the acute effects of the thermal treatment, i.e. evaluated immediately after the heat shock. However, independently of the treatment protocol (temperature and exposure time), there are always the possibility of surviving cells, whose long-term behaviour has received little attention.
In this context, the aim of this work was to study the acute and long-term effects of hyperthermia, 45°C for 30 min, in the highly metastatic murine melanoma cell line B16-F10. The heat shock reaction was characterized by testing viability/proliferation, F-actin cytoskeleton organization, mechanism of cell death, cell cycle analysis and expression of functional melanoma genes and genes associated with the heat shock reaction.
Materials and Methods
Cell culture experiments
B16-F10 cell line from C57BL/6J mice was purchased from ATCC. Cells were cultured in 90 mm culture plates and were maintained in standard culture conditions, i.e., α-minimal essential medium (α-MEM) containing 10% foetal bovine serum (FBS), 2.5 µg/ml fungizone, penicillin-steptomycin (100 IU/ml and 10 mg/ml, respectively), and incubated in a humidified atmosphere of 5% CO2 in air at 37°C. Culture medium was changed twice a week. At 70 to 80% cell confluence, adherent cells were enzymatically released with a solution of 0.04% trypsin in 0.25% EDTA.
B16-F10 cells, seeded at 5×103 cell/cm2, were cultured in the experimental conditions described above. At day 5, 50–60% confluence, cell cultures were submitted to a hyperthermia treatment. In this procedure, culture plates, sealed with parafilm sealing film, were totally submerged in a water bath and incubated at 45°C (±0.1°C) for 30 minutes. Following, the heat-treated cultures were kept for a further 14 days at 37°C, and the medium was changed twice a week. Control cultures, which were kept at 37°C, were evaluated simultaneously.
Control and heat-treated cultures were characterized immediately after the treatment (0 h) and throughout the further 14 d after the heat shock, as follows.
Cell viability/proliferation
B16-F10 melanoma cells were cultured (5×103 cell/cm2) in 96-well culture plates in standard culture conditions. Cell viability/proliferation of control and heat-treated cultures was analysed by the MTT and LDH assays.
The MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium) assay is a method based in the capability of mitochondrial dehydrogenase to transform the MTT in a dark blue formazan product [24], [25]. MTT (0.5 mg/ml) was added to each well, and the culture plates were incubated for 2 h at 37°C. Following, the formazan salts were dissolved with 100 µl of dimethylsulphoxide (DMSO) and the absorbance (Abs) was determined at λ = 600 nm on an Elisa reader (Synergy HT, Biotek).
The lactate dehydrogenase (LDH) assay is based on the reduction of NAD by the action of LDH released to the medium [26]. The resulting reduced NAD (NADH) is utilized in the stoichiometric conversion of a tetrazolium dye. Determination of the total LDH was performed in control and heated culture plates using the In vitro toxicology assay kit lactate dehydrogenase based (Sigma-Aldrich; St. Louis, MO). To determine the total LDH in the culture, LDH assay lysis solution (1/10 of culture medium volume) was added to the wells and the plates were incubated at 37°C for 45 minutes. Medium was centrifuged at 250 g for 4 minutes to pellet debris. Samples aliquots (25 µl) were mixed with 50 µl lactate dehydrogenase assay mixture (LDH assay substrate, cofactor and dye solution), and incubated for 20 minutes at room temperature in the dark. Absorbance was determined at λ = 492 nm on a Elisa reader (Synergy HT, Biotek). The amount of LDH leakage to the medium was normalized by total LDH, and calculated as follows: LDH leakage % = LDH medium/total LDH ×100.
Cell morphology and F-actin cytoskeleton labelling
B16-F10 melanoma cells were cultured in 30 mm culture plates (5×103 cell/cm2) in standard culture conditions. Control and heat-treated cultures were fixed at defined time points with formaldehyde 3.7% (methanol free). After, cells were labelled for F-actin cytoskeleton and nucleus and observed by confocal laser scanning microscopy (CLSM).
For labelling, cells were permeabilized with 0.1% triton in PBS for 5 min. After, cultures were incubated with 10 mg/mL of BSA with 1 µg/mL RNAse in PBS, for 1 h. Following, they were treated with 5 U/mL Alexa Fluor® 488-Phalloidin (Invitrogen, Barcelona, Spain), for 20 min in the dark. Cells were washed with PBS and a solution of propidium iodide (PI,10 µg/mL in PBS; Sigma-Aldrich, St. Louis, MO) was added (10 min in the dark, at room temperature). Cells were maintained in mounting medium (20 mM Tris pH 8.0, 0.5% N-propyl gallate, 90% Glycerol) before CLSM observation. CLSM was performed in a Laser Scanning Confocal Microscope Leica SP2 AOBS SE (Leica Microsystems, Germany).
Mechanism of cell death and cell cycle analysis
B16-F10 melanoma cells were cultured (5×103 cell/cm2) in 75 cm2 culture flasks, in the conditions described above. At defined time-points, control and heat-treated cell layers were washed twice in PBS and cells were enzymatically released (0.04% trypsin in 0.25% EDTA). The resulting cell suspension, reflecting the number of adherent cells in control and heat-treated cultures, was used to evaluate the mechanism of cell death and cell cycle phase.
Mechanism of cell death
Alterations in the apoptotic cell index, namely the relative percentage of cells undergoing early and late apoptosis, were assessed with TACSTM Annexin V-FITC Apoptosis Detection Kit (R & D Systems) that includes Annexin V conjugated to FITC and PI. Early in the apoptotic process, caspases and other proteases cleave the cytoskeleton and the plasma membrane loses its rigidity, due to the deleterious effects in the underlying net of actin. This process disrupt the phospholipid asymmetry, leading to an increase of exposure of phosphatidylserine (PS) on the outer leaflet of plasma membrane [27]. Annexin V binds to PS; still, at this stage, damaged plasma membrane is ion selective for a long time excluding PI (Annexin V-FITC+/PI−; early apoptotic cells). In later stages of apoptosis, cells enter a blebbing process, leading to a shrunken morphology, and loose the membrane integrity and the ability to exclude PI [27], [28] (Annexin V-FITC+/PI+; late apoptotic and/or necrotic cells). Normal, viable cells are unstained. In the experimental protocol used, the cell suspension obtained as described above was centrifuged at 500 g for 7 min. Cells were re-suspended in PBS with 1% of BSA, and an aliquot of 106 cell/ml was used for apoptotic cell index determination. After a centrifugation at 500 g for 7 min, cell pellets were incubated with 50 µL Annexin V incubation reagent (5 µL 10× binding buffer, 5 µL PI, 0.5 µL Annexin V-FITC, 39.5 µL dH2O), for 20 min in the dark, at room temperature. After this period, 500 µL of 1× Binding buffer was added, and the samples were assessed by flow cytometry analysis.
Cell cycle analysis
Alterations in the cell cycle were assessed with PI. An aliquot of 106 cell/mL, from the cell suspension obtained as described above, was fixed with formaldehyde 1.9% (methanol free) in PBS and kept at 4°C. Fixed cells were washed twice in PBS, and centrifuged at 500 g for 7 min. Cells were stained in 1 mL PBS, 50 µg/mL PI and 50 µg/mL RNAse (1 h, in the dark at room temperature), and assessed for cell cycle analysis, by flow cytometry.
Total RNA extraction and RT-PCR analysis
Control and heat-treated cultures of B16-F10 melanoma cells were analysed by RT-PCR, during the first 24 h following the heat treatment, to access the expression of the housekeeping gene β-actin, TYR (Tyrosinase), TRP-1 (Tyrosinase related protein 1), TRP-2 (Tyrosinase related protein 2), pMel17 (Premelanosome protein), MITF (Microphthalmia-associated transcription factor) and MART-1 (Melanoma associated antigen recognized by T-cells 1), Bcl-2, BAX, p53 and Hsp70 (Heat shock protein 70). Total RNA was extracted using the Nucleospin RNA II kit (Machery-Nagel), according to the manufacturer's instructions. RNA was analysed for its concentration and purity in each sample by UV spectrophotometry at 260 nm and by calculating the A260nm/A280nm ratio, respectively. RT-PCR was done using the Titan One Tube RT-PCR System (Roche Applied Science), according to the manufacturer's instructions. Half microgram of total RNA from each sample was reverse transcribed and amplified (25 cycles) with Titan One Tube RT-PCR System (Roche), with an annealing temperature of 50°C. The amplification conditions were chosen accordingly to preliminary calibration experiments (data not shown), in order to become possible to use the RT-PCR analysis as a quantitative tool. The primers used (Sigma Genosys) are listed on Table 1. RT-PCR products were separated on a 1% (w/V) agarose gel and gel band intensities were analysed with ImageJ 1.41 software. Values were considered as a percentage of the corresponding β-actin value of each experimental condition.
Table 1. Primers used on RT-PCR analysis.
| Gene | 5′ Primer | 3′ Primer |
| β-actin | AAGAGCTATGAGCTGCCTGA | CAGGAGGAGCAATGATCTTG |
| TYR | GGCCAGCTTTCAGGCAGAGGT | TGGTGCTTCATGGGCAAAATC |
| TRP-1 | GCTGCAGGAGCCTTCTTTCTC | AAGACGCTGCACTGCTGGTCT |
| TRP-2 | GGATGACCGTGAGCAATGGCC | CGGTTGTGACCAATGGGTGCC |
| pMel17 | CGGATGGTCAGGTTATCTGG | ATGGTGAAGGTTGAACTGGC |
| MITF | GTATGAACACGCACTCTCTCGA | CTTCTGCGCTCATACTGCTC |
| MART-1 | TGCCCCAAGAAGACATTCAC | GTGAATAAGGTGGCGGTGAA |
| Bcl-2 | CTCGTCGCTACCGTCGTGACTTCG | CAGATGCCGGTTCAGGTACTCAGTC |
| BAX | AAGCTGAGCGAGTGTCTCCGGCG | GCCACAAAGATGGTCACTGTCTGCC |
| p53 | ATGACTGCCATGGAGGAGT | CTCGGGTGGCTCATAAGGTA |
| Hsp70 | CCATCCAGAGACAAGCGAAG | CGTTTAGACCGCCGATCACA |
Statistical analysis
Four independent experiments were performed. In the cell viability/proliferation assays (MTT and LDH assays) four replicas were performed in each experiment. Quantitative data are presented as mean ±SEM. Data were analysed with Mann Whitney U test. A p value of ≤0.05 was considered statistically significant.
Results
Cell viability/proliferation
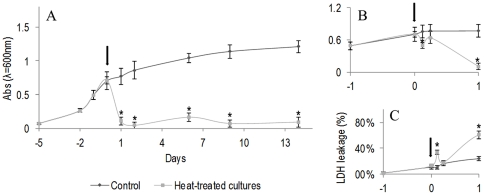
Figure 1A shows the results of the MTT assay. In control cultures, kept at 37°C, cell viability/proliferation increased gradually with the culture time, especially during the first week; afterwards, cultures reached confluence, and the MTT reduction values increased slowly. The hyperthermia treatment, performed at day 5 of the culture, resulted in a significant decrease in the viability/proliferation during the first 24 hours after the heat shock, Figure 1A. Within this period, the cell response was not uniform, i.e. there was a slight decrease in the viability during the first 3 h, followed by a partial recovery (found at 6 h), but, afterwards, cell viability decreased significantly, Figure 1B; 24 h after the heat treatment, values were in average 80% lower than those observed in control cultures, Figure 1B. Following, 2 d to 14 d after the heat treatment, cell viability/proliferation remained very low, compared to the control cultures, Figure 1A.
Figure 1. Cell viability/proliferation of B16-F10 melanoma cells submitted to a heat treatment (HT), 45°C for 30 min.
Cultures were heated at day 5 (Arrow) and further maintained at 37°C for 2 weeks. A: MTT assay, throughout the culture period; B: Detail of the MTT assay during the first 24 h after the heat treatment; C: LDH assay during the first 24 h after the heat treatment. *Significantly different from control (cultures kept at 37°C).
Results from the LDH assay were in line with those found in MTT assay. Figure 1C shows the results observed in the first 24 h following the heat treatment. In control cultures, LDH leakage remained low, but heat treated cultures showed an increase in the amount of LDH released to the medium 3 h after the treatment, followed by a decrease after 6 h, and, again, an increase in the next hours.
Cell morphology and F-actin cytoskeleton organization
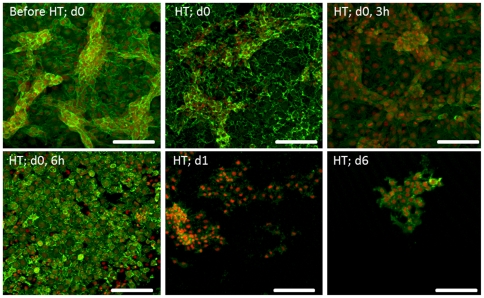
Control and heat-treated cultures were stained for the F-actin cytoskeleton and nucleus and representative CLSM images are shown in Figure 2.
Figure 2. CLSM observation of B16-F10 melanoma cells submitted to a heat treatment (HT), 45°C for 30 min, stained for F-actin cytoskeleton (green) and nucleus (red).
Representative images (from four independent experiments) showing the effect of heat exposure on cell morphology and cell layer organization, at defined time-points (d 0, 0 h–d 6, immediately to 6 days after heat exposure). Bar represents 400 µm.
Control cultures presented an organized cell layer with extensive cell-to-cell contact and areas of higher cell density. In addition, cells displayed a spread F-actin cytoskeleton with well-defined peripheral limits and a prominent nucleus. The heat treatment induced significant alterations on this behaviour. Deleterious effects were observed immediately after the heat shock (time point, 0 h) and were well evident 3 h after the treatment, reflected mainly by a lower definition of the dense peripheral F-actin ring. After 6 h, some recovery in the organization of the cytoskeleton occurred, as cells displayed a better definition of the peripheral limits; still, cultures presented alterations in the organization of the cell layer and the presence of some rounded and shrunken cells. However, 1 d after the heat shock, cultures appeared greatly affected, with evident alterations in the F-actin cytoskeleton, loss of the cell limits, rounded shape, cytoplasm shrinkage and condensation of the nuclear material. Other effects included loss of the organization of the cell layer and cell-to-cell contact and a significant decrease in the number of attached cells. These deleterious effects were observed throughout the culture time.
Mechanism of cell death and cell cycle analysis
At defined time-points, the adherent cells present in control and heat-treated cultures were suspended and counted, and the results showed significantly lower values in the cultures submitted to hyperthermia, Table 2. Aliquots of these cell suspensions were analysed by flow cytometry for the presence of apoptosis and cell cycle phase, Figures 3, 4, 5.
Table 2. Number of adherent cells/cm2 in control and heat-treated cultures throughout the culture period, at defined time-points.
| Days | 0 | 0.125 (3h) | 0.250 (6h) | 1 | 2 | 6 | 9 | 14 |
| Control | 50.0×104 | 49.3×104 | 53.3×104 | 56.8×104 | 48.0×104 | 48.0×104 | 43.2×104 | 38.7×104 |
| (±4.6×104) | (±4.4×104) | (±4.8×104) | (±5.1×104) | (±4.3×104) | (±4.4×104) | (±3.8×104) | (±3.4×104) | |
| Heat-treated Cultures | 39.2×104 | 5.2×104 | 2.8×104 | 2.4×104 | 3.5×104 | 1.9×104 | 0.9×104 | 1.1×104 |
| (±3.3×104) | (±0.4×104) | (±0.2×104) | (±0.3×104) | (±0.2×104) | (±0.2×104) | (±0.1×104) | (±0.1×104) |
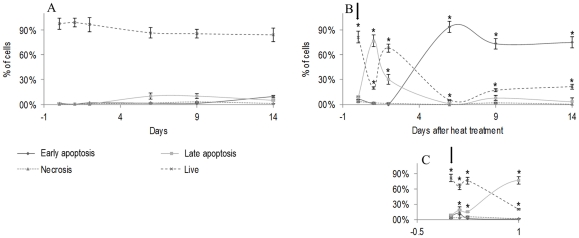
Figure 3. Apoptosis assessment of B16-F10 melanoma cells submitted to a heat treatment (HT), 45°C for 30 min.
Flow cytometry analysis of Annexin-FITC/PI uptake to evaluate the percentage of cells in early and late apoptosis. Following the heat treatment, cultures were maintained at 37°C for 2 weeks. A: control cultures (kept at 37°C during the culture time); B: heat-treated cultures; C: detail of the heat-treated cultures during the first 24 h after heat exposure. *Significantly different from control (cultures kept at 37°C).
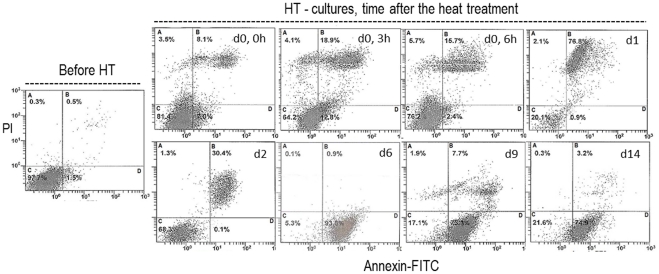
Figure 4. Apoptosis assessment of B16-F10 melanoma cells submitted to a heat treatment (HT), 45°C for 30 min.
Representative histograms (from four independent experiments), before the heat treatment (d 0) and after, at defined time-points (d 0, 0 h–d 14, immediately to 14 days after heat exposure). Flow cytometry analysis of Annexin-FITC/PI uptake (A, necrosis; B, late apoptosis; C, viable cells; D, early apoptosis).
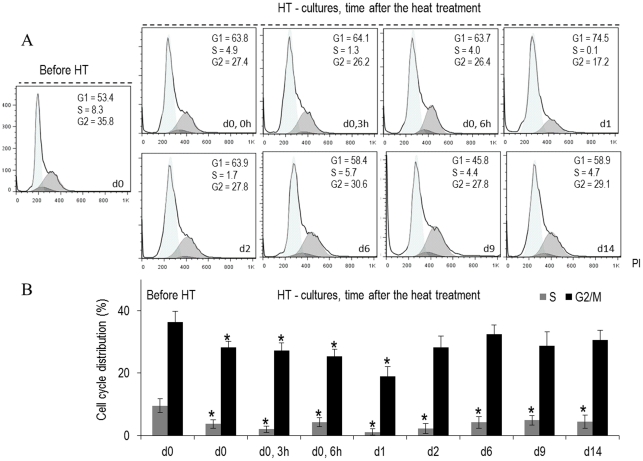
Figure 5. Cell cycle analysis of B16-F10 melanoma cells submitted to a heat treatment (HT), 45°C for 30 min, evaluated by flow cytometry.
Representative histograms (A) (from four independent experiments) and quantitative data (B) of the control and the heat-treated cultures, at defined time-points throughout the culture period (d 0, 0 h–d 14, immediately to 14 days after heat exposure). *Significantly different from control (cultures kept at 37°C).
Control cultures exhibited a high percentage of live cells throughout the culture time, Figure 3A. In the heat-treated cultures, Figure 3B, significant changes occurred during the first 24 h following the heat shock. The percentage of live cells decreased immediately after the treatment (∼64%, at 3 h), increased slightly in the next hours (∼75%, at 6 h), but at 24 h, the number of live cells was low (∼20%), Figures 3B, C. During this phase, cultures presented a high percentage of cells in late apoptosis. Following, there was a slight recovery, and 2 d after the heat treatment, the percentage of live cells was around 68%. At later times, the surviving cells were engaged in early apoptosis, i.e. 6 d after the heat shock, cultures presented ∼5% of live cells and ∼94% of early apoptotic cells, Figure 3B. In the following days, the percentage of live cells increased again, an average of 18–20% at 9 d and 14 d after the heat treatment. Figure 4 shows representative histograms of the cultures before and following the heat treatment.
Regarding cell cycle analyses, Figure 5 shows that at the culture time selected for the heat treatment, control cultures presented most of the cell population in G1 and G2/M phases, with a small percentage of cells in S phase. After the heat exposure, the percentage of S cells decreased significantly and was almost negligible 1 d and 2 d after the treatment; at this stage, the cell population remained mostly in G1 phase. Following, 6 d–14 d after heat exposure, there was a small increase in the percentage of S phase cells (∼5%) accompanied by a slight decrease in the percentage of G1 phase cells, and the culture remained stable during this period.
However, it is worth mentioning that the number of cells in heat-treated cultures was significantly lower than that in control cultures, as shown in Table 2.
Gene expression profile
Control cultures, at day 5 (the day of the heat treatment), expressed genes that are involved in melanin production, cell proliferation and differentiation events, namely TYR, TRP-1, TRP-2, pMel17, MITF and MART-1. Hyperthermia caused a significant decrease in the expression of TYR immediately after the heat treatment, a recovery after 3 h and a high decrease at 6 and 24 h. Expression of TRP-2 also decreased immediately after the heat shock, recovered at 3 and 6 h and decreased significantly at 24 h. Expression levels of pMel17 and MITF were similar to control, and MART-1 expression increased progressively after the heat treatment, attaining significantly higher values at 3, 6 and 24 h. Results are shown in Figure 6A.
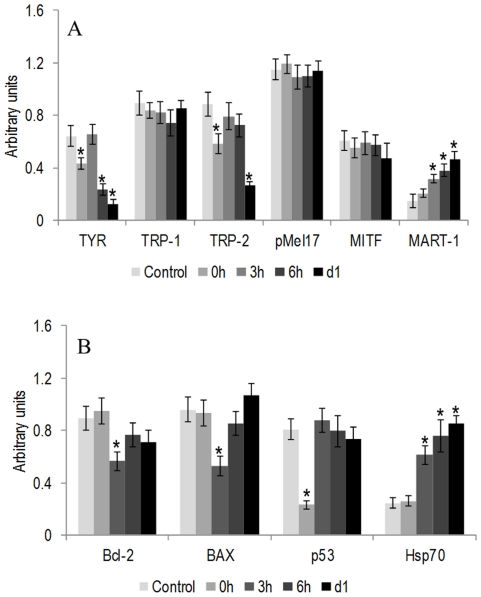
Figure 6. RT-PCR gene expression of B16-F10 melanoma cells submitted to a heat treatment (HT), 45°C for 30 min.
Expression of TYR, TRP-1, TRP-2, pMel17, MITF and MART-1(A) and Bcl-2, BAX, p53 and Hsp70 (B) during the first 24 h following the heat treatment. RT-PCR products were subjected to a densitometric analysis and were normalized to the corresponding β-actin value. *Significantly different from control (cultures kept at 37°C).
At the same stage of the culture (day 5), control melanoma cells expressed genes associated with cell response to injury, Bcl-2, BAX, p53 and Hsp70. Hyperthermia induced some significant changes. Expression of Bcl-2 and BAX decreased 3 h after the heat treatment, but recovered afterwards, especially in the case of BAX. Expression levels of p53 suffered a significant decrease immediately after the heat treatment, recovered to normal values at 3 h, but, afterwards, decreased slowly (although without statistical significance). Expression of Hsp70 was similar to control immediately after the treatment, but increased significantly afterwards. Results are presented in Figure 6B.
Discussion
Hyperthermia is a cancer therapy based on the knowledge that heat can kill cells after a temperature breakpoint, above fever temperature [1]. Although it's already being used as cancer therapy, there still is a lot to uncover regarding the effects that are critical to the destruction of cancer cells and cancerous tissues. An important feature of hyperthermia is the period of the heat shock reaction. Most studies focused in the immediate effects, and the long-term effects on the surviving cells are less known. In this work, the melanoma cell line B16-F10 was submitted to hyperthermia, at 45°C for 30 min, and cell response was characterized regarding the acute and long-term effects of the heat treatment.
Control cultures (maintained at 37°C) attained confluence around day 7, explaining the slow increase in the viability/proliferation observed afterwards. Accordingly, cell cycle analysis revealed that most of the cell population was in G1 phase. Towards later incubation times, cultures showed already a low percentage of late apoptotic cells, which is probably related with the aging of the confluent cell layer. The hyperthermia treatment induced significant acute and long-term effects in this behaviour.
Detailed characterization of the acute cell response to the heat treatment showed significant cell damage during the first 24 h, with a time-dependent cell response, which resulted in low number of adherent viable cells (Figure 1; Table 2). Cultures exhibited a disrupted cellular organization, an altered actin cytoskeleton and, at day 1, the remaining cells showed typical features of late apoptotic/necrotic cells (Figure 2). This was confirmed by the flow cytometry results (Figures 3 and 4). Also, cell cycle analysis revealed a negligible percentage of S phase cells (Figure 5). These observations suggest that in the acute heat shock reaction, cell death occurred mainly by a process of late apoptosis/necrosis, which would be expected from exposure to a severe trauma, such a high temperature [28]. In this drastic mechanism of cell death, the membrane-permeabilized cells release a variety of intracellular molecules that may damage neighbouring cells leading to later manifestations of cytotoxicity [28]. Following, assessment of the small remaining population throughout a period of 14 d after the heat exposure showed several discrete attempts of cell recovery from the inflicted damage. However, the number of adherent cells remained low until the end of the culture period, and, in this later stage, a high percentage of cells were in early apoptosis (Figures 3 and 4). During this early stage of programed cell death, dying cells are characterized as having an intact plasma membrane but expose PS on the cell surface, a process that, in physiological conditions, mediate their recognition by phagocytes and subsequent clearance [27], [28]. In this study, the presence of early apoptotic cells long after the heat shock might result from more subtle effects of the acute cell response which take longer to be expressed, and/or from indirect cytotoxicity induced by the release of toxic molecules from the high number of late apoptotic/necrotic cells generated during the acute phase reaction. However, nevertheless the significant cell injury elicited by the heat exposure, a small percentage of cells was able to survive long after the treatment. In line with this, cell-cycle analysis revealed that most of the remaining cells were in G1 phase, but cultures still maintained a low but relatively constant percentage of cells engaged in S and G2/M phases (Figure 5), suggesting the presence of a metabolically active cell population.
The in vitro effects of hyperthermia have been studied in several cell lines. Temperatures in the range of 45°C have induced alterations in a variety of cellular parameters [22]. Some cells, like RIF-1 line, appear to be resistant to this treatment and cellular viability was not affected [22]. Other cells, such as melanoma cells (Bowes) and lung adenocarcinoma cells, have demonstrated to be tolerant to hyperthermia for a short period, but afterwards cell viability decreased [19]. A great number of cell lines appears to present a low tolerance to hyperthermia at 45°C, showing a variety of deleterious effects including alterations in cell viability and increase of cellular death by apoptosis or necrosis, as observed in human erythroleukaemia cells [21], murine melanoma cells [23] and prostatic stromal cells [29]. The effect of hyperthermia in melanoma cells have been reported in a few studies, although with a great variability of experimental protocols, which difficult the comparison with the present study. In human melanoma cells heated from 41–43°C for 3 and 6 h, an increase on the shedding of soluble intercellular adhesion molecule-1 (sICAM-1) was found, with maximum values at 3 h [14]. Also, human melanoma cells were evaluated for cell survival and gene expression as a function of temperature (41.8–45°C) and time of exposure (15–150 min) for a period up to three days after the heat shock [15]. Another study showed that human melanoma cells incubated at 43°C for 120 min showed poor heat resistance and a higher fibrinolytic potential, measured immediately after the heat shock [19]. B16-F10 murine melanoma cells were also addressed in a study involving the treatment at 43 and 46°C in a PBS-EDTA solution for 30 min, and showed a significant decrease in cell viability immediately after hyperthermia [23]. Some of the deleterious substances that are expected to be generated as a result of the heat exposure are free radicals and reactive oxygen species (ROS). Flanagan and co-workers have described that treatment at 45°C can increase the flux of free radicals and ROS [30]. These are highly reactive species that can interact with key molecules, leading to cellular damage [31], [32]. Grasso et al, though at a lower temperature (42°C), also reported that the formation of ROS after hyperthermia contributes to growth arrest [33].
As described above, the heat treatment caused acute and long-term effects on the melanoma cell line B16-F10. However, the most significant effects were noted during the first 24 h after the heat shock, and the present results suggested that this acute response had a determinant effect in the long-term behaviour of melanoma cells, as cultures never recovered from the drastic reduction in the number of adherent/live cells that occurred during this period. Considering this, cultures were also assessed for the expression of melanoma functional genes and genes involved in response to stress, during the first 24 h of the heat shock reaction.
Control cultures, assessed at day 5 of the culture time (at the moment of the heat treatment), was in a pre-confluent stage, as referred above, and expressed several typical melanoma genes, namely TYR, TRP-1, TRP-2 and pMel17, genes that are involved in melanin synthesis. Melanin pigment protects the skin against UV-induced skin damage through its optical and chemical filtering properties, being essential for skin homeostasis. It is synthesized in the melanosomes, being the end-product of multistep transformations of L-tyrosine, under complex regulatory mechanisms [34], [35], [36]. Expression of MITF was also observed; this gene encodes a transcription factor that has a key role in melanoma cells by regulating cell cycle progression, survival, differentiation and invasion, and also modulates the expression of several pigmentation genes [36], [37]. MART-1, which function is not entirely understood although it appears to be involved in melanosome biogenesis [36], [38], was also expressed. The heat treatment decreased significantly the expression of TYR (and to a lesser extent, TRP-2), suggesting a deleterious effect in the expression of genes associated with the synthesis of melanin. Cultures were heated at day 5, at a pre-confluent stage, being greatly engaged in melanin synthesis, with might explain this deleterious effect. These results are in accordance with previous studies in human melanoma cells reporting a decrease in the expression of TYR in the first hours after heat-treatment at 45°C for 22 min [15]. The heat treatment had little effect in the expression of MITF, most probably because, as referred above, cultures were relatively stabilized regarding cell proliferation and cell cycle progression, which might explain the similar expression in control and heat-treated cultures. MITF appears to have a role in the expression of pMel17 [36], [37], which probably contributes for the lack of effect of the heat shock in the expression of this gene. On the other hand, expression of MART-1 was not affected in the first hours following the heat treatment, but an increase was observed afterwards, compared to control, probably reflecting an attempt to restore melanosome biogenesis. In a previous report using human melanoma cells MART-1 expression was not affected [15]. Although the temperature used was the same, 45°C, the heating time was only 22 min, which could explain the different results. An exposition to the heat for a longer period has been reported to increase cell damage [15], [17].
Control cultures also expressed genes associated with apoptotic events and cell response to stress, namely Bcl-2, BAX, p53 and Hsp70. Control cultures showed a similar expression profile of the competing genes of the same family Bcl-2 and BAX, respectively an anti-apoptotic gene and a pro-apoptotic gene [39], expression of p53, encoding a transcription factor that is activated as part of the cell response to stress that regulates many downstream target genes [40], and low levels of Hsp70, a gene with a key role in the repair mechanisms following cell injury [41]. The heat treatment induced some significant alteration in this gene profile. A similar decrease in the gene expression of the two competing genes Bcl-2 and BAX was observed 3 h following the heat shock, followed by a recovery to values similar to control. Regarding these two genes, it has been shown an increase in the expression of BAX and a decrease in Bcl-2 two hours after hyperthermia treatment at 42.5°C [42], [43], using HL60, HSB2 and Kasumi cell lines. This is in line with that occurring in physiological conditions due to the role of these genes [39]. In the present work, the use of a higher temperature, as 45°C, appears to create impairment in the behaviour of both genes probably due to a severe cytotoxic effect observed at this high temperature. Expression of p53 was greatly affected immediately after the heat shock, returned to normal values in the next few hours, however showing a tendency for a decrease, which might suggest a progressive impairment in coping with the inflicted cell injury. It is known that p53 protein has a key role in the heat shock reaction, acting as a transcription factor in cell cycle regulation, and loss of its function results in genetic instability and an impaired induction of apoptosis [40]. Regarding this, it appears that cell lines with lower levels of p53 protein have a decreased susceptibility to undergo apoptosis [20]. Hsp proteins can repair and restore the structures and functional integrity of damaged proteins, having a role in the protection against hyperthermia-induced apoptosis [40], [41], [44]. The present results showed that the expression of Hsp70 did not change immediately after the heat shock, but increased few hours later, attaining significantly higher values compared to control. This observation is in agreement with a previous report using human melanoma cell lines and the same temperature, with an augmentation in Hsp70 expression few hours after treatment [15]. Also, previous studies performed in hematopoietic and erythroleukaemia cells reported that, at 45°C, cells were able to induce Hsp70 only few hours after hyperthermia treatment [20], [21].
It is worth mentioning that, in vitro, some details in the experimental protocol might have a significant influence in the effects of the heat exposure. In this study, after the heat treatment, cells were kept for 48 hours in the same culture medium. Other authors have chosen to change the medium immediately after treatment [45]. Keeping the cells in the same medium after heat shock, would contribute to potentiate the harmful effects because of the longer contact time with toxic molecules liberated to the medium by damaged and dead cells.
Conclusion
In conclusion, the highly metastatic melanoma cell line B16-F10 is susceptible to hyperthermia treatment, at 45°C for 30 minutes. Immediately after the heat shock, and in the first 24 h, cells began a process of fast degradation, with decreased viability, alterations in morphology and a significant reduction of the number of adherent cells, most of them in late apoptosis/necrosis, suggesting significant inflicted toxic effects. During the same period, the heat treatment also caused evident changes in the expression of functional melanoma cell genes, and in genes associated with cell response to injury. A follow-up of two weeks after heat exposure showed that viability and number of live cells remained very low and, after few days, cells were in early apoptosis. Still, it is worth mentioning that heat-treated cultures maintained a low but relatively constant percentage of cells in S and G2/M phases long after heat exposure, suggesting the presence of metabolically active cells.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by Project FCT: PTDC/SAU-BEB/69497/2006 (“Hyperthermia treatment of experimental melanoma using a ferromagnetic cement”) and Faculty of Dental Medicine, University of Porto, Portugal. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Vertrees RA, Jordan JM, Zwischenberger JB. Hyperthermia and Chemotherapy: The Science. In: Helm CW, Edwards RP, editors. Intraperitoneal Cancer Therapy. 1st ed. Totowa, NJ: Humana Press; 2007. pp. 71–100. [Google Scholar]
- 2.Hildebrandt B, Wust P, Ahlers O, Dieing A, Sreenivasa G, et al. 1. Crit Rev Oncol Hematol 43; 2002. The cellular and molecular basis of hyperthermia. pp. 33–56. [DOI] [PubMed] [Google Scholar]
- 3.van der Zee J. 8. Ann Oncol 13; 2002. Heating the patient: a promising approach? pp. 1173–1184. [DOI] [PubMed] [Google Scholar]
- 4.Ito A, Tanaka K, Kondo K, Shinkai M, Honda H, et al. 3. Cancer Sci 94; 2003. Tumor regression by combined immunotherapy and hyperthermia using magnetic nanoparticles in an experimental subcutaneous murine melanoma. pp. 308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yanase M, Shinkai M, Honda H, Wakabayashi T, Yoshida J, et al. 4. Jpn J Cancer Res 89; 1998. Intracellular hyperthermia for cancer using magnetite cationic liposomes: an in vivo study. pp. 463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ito A, Tanaka K, Honda H, Abe S, Yamaguchi H, et al. 4. J Biosci Bioeng 96; 2003. Complete regression of mouse mammary carcinoma with a size greater than 15 mm by frequent repeated hyperthermia using magnetite nanoparticles. pp. 364–369. [DOI] [PubMed] [Google Scholar]
- 7.Matsuoka F, Shinkai M, Honda H, Kubo T, Sugita T, et al. Hyperthermia using magnetite cationic liposomes for hamster osteosarcoma. Biomagn Res Technol. 2004;2(1):3. doi: 10.1186/1477-044X-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawai N, Ito A, Nakahara Y, Futakuchi M, Shirai T, et al. 4. Prostate 64; 2005. Anticancer effect of hyperthermia on prostate cancer mediated by magnetite cationic liposomes and immune-response induction in transplanted syngeneic rats. pp. 373–381. [DOI] [PubMed] [Google Scholar]
- 9.Motoyama J, Yamashita N, Morino T, Tanaka M, Kobayashi T, et al. Hyperthermic treatment of DMBA-induced rat mammary cancer using magnetic nanoparticles. Biomagn Res Technol 6. 2008;2 doi: 10.1186/1477-044X-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shinkai M, Yanase M, Suzuki M, Hiroyuki H, Wakabayashi T, et al. Intracellular hyperthermia for cancer using magnetite cationic liposomes. J Magn Magn Mater. 1999;194(1–3):176–184. [Google Scholar]
- 11.Suzuki M, Shinkai M, Honda H, Kobayashi T. 2. Melanoma Res 13; 2003. Anticancer effect and immune induction by hyperthermia of malignant melanoma using magnetite cationic liposomes. pp. 129–135. [DOI] [PubMed] [Google Scholar]
- 12.Yanase M, Shinkai M, Honda H, Wakabayashi T, Yoshida J, et al. 7. Jpn J Cancer Res 89; 1998. Antitumor immunity induction by intracellular hyperthermia using magnetite cationic liposomes. pp. 775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanamori M, Ohmori K, Yasuda T, Yudoh K. 1. Cancer Detect Prev 27; 2003. Effects of hyperthermia and differentiation on cultured Dunn osteosarcoma cells. pp. 76–81. [DOI] [PubMed] [Google Scholar]
- 14.Nakayama J, Kageshita T, Nakashima M, Tsujisaki M, Imai K, et al. 3. Pigment Cell Res 9; 1996. Increase in shedding of intercellular adhesion molecule-1 in human malignant melanoma cell lines treated with hyperthermia in vitro. pp. 154–158. [DOI] [PubMed] [Google Scholar]
- 15.Milani V, Frankenberger B, Heinz O, Brandl A, Ruhland S, et al. 3. Int Immunol 17; 2005. Melanoma-associated antigen tyrosinase but not Melan-A/MART-1 expression and presentation dissociate during the heat shock response. pp. 257–268. [DOI] [PubMed] [Google Scholar]
- 16.Rong Y, Mack P. 1. Int J Hyperthermia 16; 2000. Apoptosis induced by hyperthermia in Dunn osteosarcoma cell line in vitro. pp. 19–27. [DOI] [PubMed] [Google Scholar]
- 17.Vorotnikova E, Ivkov R, Foreman A, Tries M, Braunhut SJ. 8. Int J Radiat Biol 82; 2006. The magnitude and time-dependence of the apoptotic response of normal and malignant cells subjected to ionizing radiation versus hyperthermia. pp. 549–559. [DOI] [PubMed] [Google Scholar]
- 18.Zhou M, Zhang A, Lin B, Liu J, Xu LX. 3. Int J Hyperthermia 23; 2007. Study of heat shock response of human umbilical vein endothelial cells (HUVECs) using cDNA microarray. pp. 225–258. [DOI] [PubMed] [Google Scholar]
- 19.Fukao H, Ikeda M, Ichikawa T, Inufusa H, Okada K, et al. 1–2. Clin Chim Acta 296; 2000. Effect of hyperthermia on the viability and the fibrinolytic potential of human cancer cell lines. pp. 17–33. [DOI] [PubMed] [Google Scholar]
- 20.Nijhuis EH, Poot AA, Feijen J, Vermes I. 2. Int J Radiat Biol 84; 2008. Hsp70- and p53-reponses after heat treatment and/or X-irradiation mediate the susceptibility of hematopoietic cells to undergo apoptosis. pp. 99–105. [DOI] [PubMed] [Google Scholar]
- 21.Sharif-Khatibi L, Kariminia A, Khoei S, Goliaei B. 8. Int J Hyperthermia 23; 2007. Hyperthermia induces differentiation without apoptosis in permissive temperatures in human erythroleukaemia cells. pp. 645–655. [DOI] [PubMed] [Google Scholar]
- 22.Babsky A, Hekmatyar SK, Gorski T, Nelson DS, Bansal N. 2. Int J Hyperthermia 21; 2005. Heat-induced changes in intracellular Na+, pH and bioenergetic status in superfused RIF-1 tumour cells determined by 23Na and 31P magnetic resonance spectroscopy. pp. 141–158. [DOI] [PubMed] [Google Scholar]
- 23.Oliveira Filho RS, Bevilacqua RG, Chammas R. 8. Braz J Med Biol Res 30; 1997. Hyperthermia increases the metastatic potential of murine melanoma. pp. 941–945. [DOI] [PubMed] [Google Scholar]
- 24.Morgan DM. Morgan DM, editor. Tetrazolium (MTT) Assay for Cellular Viability and Activity. Polyamine Protocols Methods in Molecular Biology. 1997. pp. 179–184. [DOI] [PubMed]
- 25.Mosmann T. 1–2. J Immunol Methods 65; 1983. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. pp. 55–63. [DOI] [PubMed] [Google Scholar]
- 26.Legrand C, Bour JM, Jacob C, Capiaumont J, Martial A, et al. 3. J Biotechnol 25; 1992. Lactate dehydrogenase (LDH) activity of the number of dead cells in the medium of cultured eukaryotic cells as marker. pp. 231–243. [DOI] [PubMed] [Google Scholar]
- 27.Elmore S. 4. Toxicol Pathol 35; 2007. Apoptosis: a review of programmed cell death. pp. 495–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poon IK, Hulett MD, Parish CR. 3. Cell Death Differ 17; 2010. Molecular mechanisms of late apoptotic/necrotic cell clearance. pp. 381–397. [DOI] [PubMed] [Google Scholar]
- 29.Brehmer M, Svensson I. 4. BJU Int 85; 2000. Heat-induced apoptosis in human prostatic stromal cells. pp. 535–541. [DOI] [PubMed] [Google Scholar]
- 30.Flanagan SW, Moseley PL, Buettner GR. 2. FEBS Lett 431; 1998. Increased flux of free radicals in cells subjected to hyperthermia: detection by electron paramagnetic resonance spin trapping. pp. 285–286. [DOI] [PubMed] [Google Scholar]
- 31.Halliwell B, Gutteridge JMC. Oxford New York. 936 p; 1999. Free radicals in biology and medicine. [Google Scholar]
- 32.Thannickal VJ, Fanburg BL. 6. Am J Physiol Lung Cell Mol Physiol 279; 2000. Reactive oxygen species in cell signaling. pp. L1005–1028. [DOI] [PubMed] [Google Scholar]
- 33.Grasso S, Scifo C, Cardile V, Gulino R, Renis M. 5. Exp Biol Med (Maywood) 228; 2003. Adaptive responses to the stress induced by hyperthermia or hydrogen peroxide in human fibroblasts. pp. 491–498. [DOI] [PubMed] [Google Scholar]
- 34.Slominski A, Tobin DJ, Shibahara S, Wortsman J. 4. Physiol Rev 84; 2004. Melanin pigmentation in mammalian skin and its hormonal regulation. pp. 1155–1228. [DOI] [PubMed] [Google Scholar]
- 35.Slominski A. 9. Exp Dermatol 18; 2009. Neuroendocrine activity of the melanocyte. pp. 760–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Medic S, Pearce RL, Heenan PJ, Ziman M. 2. Pigment Cell Res 20; 2007. Molecular markers of circulating melanoma cells. pp. 80–91. [DOI] [PubMed] [Google Scholar]
- 37.Goding CR. 20. A picture of Mitf in melanoma immortality. Oncogene 30; 2011. Commentary. pp. 2304–2306. [DOI] [PubMed] [Google Scholar]
- 38.Aydin IT, Hummler E, Smit NP, Beermann F. 1. Pigment Cell Melanoma Res 25; 2011. Coat color dilution in mice because of inactivation of the melanoma antigen MART-1. pp. 37–46. [DOI] [PubMed] [Google Scholar]
- 39.Zimmermann KC, Green DR. J Allergy Clin Immunol 108 (4 Suppl); 2001. How cells die: apoptosis pathways. pp. S99–103. [DOI] [PubMed] [Google Scholar]
- 40.Van Dyke T. 1. N Engl J Med 356; 2007. p53 and Tumor Suppression. pp. 79–81. [DOI] [PubMed] [Google Scholar]
- 41.Schmitt E, Gehrmann M, Brunet M, Multhoff G, Garrido C. 1. J Leukoc Biol 81; 2007. Intracellular and extracellular functions of heat shock proteins: repercussions in cancer therapy. pp. 15–27. [DOI] [PubMed] [Google Scholar]
- 42.Nijhuis EH, Poot AA, Feijen J, Vermes I. 8. Int J Hyperthermia 22; 2006. Induction of apoptosis by heat and gamma-radiation in a human lymphoid cell line; role of mitochondrial changes and caspase activation. pp. 687–698. [DOI] [PubMed] [Google Scholar]
- 43.Nijhuis EH, Le Gac S, Poot AA, Feijen J, Vermes I. 4. Int J Hyperthermia 24; 2008. Bax-mediated mitochondrial membrane permeabilization after heat treatment is caspase-2 dependent. pp. 357–365. [DOI] [PubMed] [Google Scholar]
- 44.Manjili MH, Wang XY, Park J, Macdonald IJ, Li Y, et al. 6. Int J Hyperthermia 18; 2002. Cancer immunotherapy: stress proteins and hyperthermia. pp. 506–520. [DOI] [PubMed] [Google Scholar]
- 45.Yonezawa M, Otsuka T, Matsui N, Tsuji H, Kato KH, et al. 3. Int J Cancer 66; 1996. Hyperthermia induces apoptosis in malignant fibrous histiocytoma cells in vitro. pp. 347–351. [DOI] [PubMed] [Google Scholar]