Abstract
Objectives
To compare the potential impact of rectal(RMB), vaginal(VMB) and bi-compartment(RVMB) (applied vaginally and protective during vaginal and anal intercourse) microbicides to prevent HIV in various heterosexual populations. To understand when a RMB is as useful than a VMB for females practicing anal intercourse(AI).
Methods
Mathematical model was used to assess the population-level impact (cumulative fraction of new HIV infections prevented(CFP)) of the 3 different microbicides in various intervention scenarios and prevalence settings. We derived the break-even RMB efficacy required to reduce a female’s cumulative risk of HIV infection by the same amount than a VMB.
Results
Under optimistic coverage (fast roll-out, 100% uptake), a 50% efficacious VMB used in 75% of sex acts in population without AI may prevent ~33%[27,42%] new total (males and females combined) HIV infections over 25 years. The 25-year CFP reduces to ~25%[20,32%] and 17% [13,23%] if uptake decreases to 75% and 50%, respectively. Similar loss of impact (by 25–50%) is observed if the same VMB is introduced in populations with 5–10% AI and for RRRAI=4–20. A RMB is as useful as a VMB (i.e. break-even) in populations with 5% AI if RRRAI=20, and in populations with 15–20% AI if RRRAI=4, independently of adherence as long as it is the same with both products. The 10-year CFP with a RVMB is 2-fold larger than for a VMB or RMB when AI=10% and RRRAI=10.
Conclusion
Even low AI frequency can compromise the impact of VMB interventions. RMB and RVMB will be important prevention tools for heterosexual populations.
Introduction
Research on vaginal microbicide (VMB) to prevent HIV infection is important because it is a biomedical intervention specifically designed to protect females(1). Until July 2010, none of the 1st generation of microbicide candidates (i.e. with non-specific activity against HIV) tested in large clinical trials had shown to protect against HIV(1–4). One clinical trial (CAPRISA-004) has demonstrated the effectiveness of a topical antiretroviral based vaginal microbicide (ARV-VMB), tenofovir 1% gel, against HIV acquisition among women in South-Africa(5;6)(Table 1). This was the first topical ARV-VMB with specific activity against HIV-1 (suppress viral replication) to be tested. This positive result needs to be confirmed in other trials before it can be licensed and used as a public health prevention tool. Many additional products designed to protect during vaginal and anal intercourse are currently at different stages of development and testing (4;7–9).
Table 1.
Summary of CAPRISA 004 trial results - HIV protection after 30 months follow-up(5)
| Main analysis | Effectiveness (95%CI) | Adherence | Crude efficacy§ |
|---|---|---|---|
| Intention to treat | 39% (6, 60) | 72% | 54.2% |
| Per protocol | 41% (7, 63) | ||
| Adjusted | 37% (6,58) | ||
| Sub-analysis | |||
| Rural | 43% (5, 67) | NA | |
| Urban | 26% (−59, 67) | NA | |
| High gel adherence | 54% (4,80) | >80% | <67.5% |
| Medium gel adherence | 38% (−67,77) | 50–80% | 47.5–76.0% |
| Low gel adherence | 28% (−40,64) | <50% | >56% |
| at 12 months follow-up | 50% | NA | |
| at 24 months follow-up | 40% | NA | |
The observed effectiveness in trials reflect the combination between the real (unmeasured) efficacy of the microbicide and the adherence to the product. Based on the information available, we can crudely estimate the “true” efficacy as the observed effectiveness divided by the observed adherence - The overall adherence during the trial was 72%, which means that the true efficacy could be around 54%, which is similar to the trial effectiveness estimate among high adherers.
The role of anal intercourse (AI) in the overall heterosexual HIV epidemic remains unclear. AI may be an important risk factor because the risk of HIV infection during unprotected receptive AI is much larger than during vaginal intercourse (VI)(10–14) and because the fraction of heterosexuals who engaged in AI at least once in their lifetime is substantial in different risk populations, countries, and time periods (WebSupplement Table S1)(11;15–17). AI may also be significantly underreported. For example, in one study, 3.5% of married men in Cotonou reported ever engaging in AI with a woman in face-to-face interviews compared to 17.5% in a pooling booth survey, a method designed to reduce social desirability bias(18).
Theoretical studies have also raised the concern that the practice of AI by trial participants may have reduced the effectiveness of VMB in large clinical trials(19–20). VMB use is currently limited to VI due to insufficient safety data on rectal use(19–21). However, data in animal studies indicate that tenofovir gel can protect during rectal challenges(22;23). In theory, it is biologically possible for a vaginally applied ARV-VMB gel to diffuse from the vaginal to the rectal linings and to protect during AI(5;24). Thus, the development of a rectal microbicide (RMB) or bi-compartmental microbicide that protect during vaginal and anal intercourse (RVMB) may eventually be possible and a useful HIV prevention tool for heterosexual populations. Previous mathematical modeling studies have assessed the potential impact of VMB and RMB in heterosexual and homosexual populations respectively(9;25–30). However, none have investigated the potential impact of RMB or RVMB in heterosexual population(31).
Our study aims to fill this gap by comparing the long-term population-level impact of VMB, RMB and RVMB in different heterosexual populations, HIV prevalence settings and intervention scenarios. First, we use a transmission dynamics model to assess the VMB intervention impact in populations without AI under various coverage scenarios. Then, we compare the “loss of VMB impact” due to AI in populations with AI and due to reduced coverage in populations without AI. Third, we assess the relative and incremental population-level impact of RMB/RVMB compared to VMB under different efficacy, adherence, anal sex assumptions. Finally, we derive the “break-even RMB efficacy” to quantify the usefulness of a RMB, compared to a VMB, at reducing the risk of HIV infection of females practicing AI.
Methods
Transmission dynamics model
To assess the MB population-level impact, we expanded a previously published deterministic model of heterosexually transmitted HIV infection and vaginal microbicide use to include anal intercourse, condom use and adherence to a vaginal or rectal microbicide(26). The model assumes random mixing between susceptible and infectious individuals and divides the population into three major classes: men, women not using and using microbicides, which are further stratified in susceptible, HIV infected, and AIDS states (WebAppendix A). Men and women who become sexually active join the community at constant rates, which are selected to balance the departure rate in a non-infected population (i.e. open but stable population). The gender-specific rates of HIV-infection, i.e. the forces of infection, for the different classes depend on the annual rate of new partner acquisition, the number of sex acts per partnership, the fraction of all sex acts, which are anal (a) and vaginal (1-a), and the HIV transmission probability per anal or vaginal sex act, the fraction of sex acts protected by condoms (c) or by microbicide during vaginal (γVMB) or/and anal ((γRMB) intercourse, and the HIV prevalence among the partners of opposite sex.
Microbicide intervention
Theoretically, depending on the product, a microbicide can protect directly against HIV (HIV efficacy) or indirectly by protecting against cofactor STI (STI efficacy) during anal or vaginal intercourse(32). Microbicide use can reduce susceptibility (acquisition) to HIV infection of uninfected women or reduce the infectiousness (transmission) of infected women to their male partners(3;5). In the CAPRISA-004 trial, the vaginal use of tenofovir 1% gel significantly reduced HIV incidence among women but did not reduce viral load of HIV female users who seroconverted and no resistance was observed during the trial(5). Although, ARV based microbicides are not necessarily expected to protect against cofactor STI, tenofovir gel significantly reduced HSV-2 incidence in the CAPRISA-004 trial(5). Data from the CAPRISA-004 trial suggest that vaginally applied tenofovir gel may have diffused from the women’s vaginal linings to the rectal linings (thereby explaining the increase in mild diarrhea seen in CAPRISA-004) opening the interesting, yet unproven, possibility that the gel could also protect during AI(24). It remains unknown if such microbicide would be equally protective during AI than VI although animal studies suggest that a MB gel could be equally effective during AI and VI(22;23).
Based on this information, we modelled three microbicides, which are assumed to reduce the female risk of HIV acquisition during receptive VI only (VMB), during RAI only (RMB), or during both (RVMB) with an efficacy of EVMB, ERMB, and ERVMB, respectively. The risks associated with drug-resistance and condom substitution were not considered. We modelled the increase in coverage by assuming that the microbicide is immediately adopted by a fraction k1 (speed of roll-out) of women in the population and by an additional fraction k (uptake) of females who newly enter the sexually active population annually. The parameter k determines the maximum long-term achievable coverage whereas k1 influences how fast it is achieved. We defined a fast (k1=k) and a slow roll-out(k1=k/2). The model is described fully in WebAppendix A.
Parameter assumptions and simulations
We defined ranges of values for the behavioural and biological parameters that are representative of different risk populations in Southern Africa and produced epidemics with HIV equilibrium prevalence between >0% and 35% (table 2A–B)(18;33–37). The gender-specific HIV transmission probability estimates per vaginal and anal act, and the relative increase in HIV acquisition risk during RAI (RRRAI=4 to 20) (WebAppendix C), are based on meta-analyses of observational studies(11–14). The limited data on HIV risk per insertive anal intercourse (IAI) indicates a lower risk than for RAI(10;11;14). We conservatively assumed a 2-fold increase in HIV risk during IAI (RRIAI) compared to insertive VI. Fewer studies report data on AI frequency than on the fraction of individuals who ever practiced AI(12). Overall, 6 to 10% of all unprotected sex acts reported by study participants (townships, STI clinics) in Cape Town were AI(12,17). Similarly 10% of all unprotected sex acts reported by FSW in Durban were AI(39). In previous multi-centre VMB trials, 2 % to 4% low-risk women reported at least one episode of AI in the month prior to enrolment(19;20;39;40). In comparison, between 1.2% and 6.3% of adults in France, Brazil, USA, Australia reported AI at their last sex (WebSupplement Table S1)(41–45). Thus, we explored scenarios with AI frequency of 0,2,5,10,15 and 20%. We also allow for a significant variation in the community rate of condom use, annual frequency of sex acts, annual number of sex partners, and condom efficacy (Table 2A).
Table 2.
Parameters and ranges
| Parameter | Description | Parameter values | |
|---|---|---|---|
| Ranges | References | ||
| A. Parameters sampled pre-intervention | |||
| βm | HIV acquisition risk for men per unprotected vaginal act | 0.0021 – 0.0068* | (13) |
| βw | HIV acquisition risk for women per unprotected vaginal act | 0.0019 – 0.0046* | (13) |
| 1/μ | Average time to remain sexually active | 30–40 years | |
| d | HIV-related mortality rates | 1/12–1/7 | (46; 47) |
| nw,, nm | Average number of sexual acts per year for women and men | 50–150 | (17; 28; 36; 39;40) |
| ρ | Average number of sexual partners per year for women and men | 0.5–2 | (16; 18; 26; 35–37) |
| c | Rate of condom use in general population; fraction of sex acts when a condom is used | 0–60% | (17; 36; 39; 48) |
| αc | Condom efficacy per act | 0.80–0.95 | (49) |
| RRRAI | Relative HIV- acquisition risk per receptive anal act compared to receptive vaginal act | 2, 4, 10,20 | (10–14) |
| RRIAI | Relative HIV- acquisition risk per insertive anal act compared to insertive vaginal act | 2 | (10; 11; 14;) |
| a | Frequency of anal intercourse, fraction of all (unprotected) sex acts which are anal intercourse | 0%, 2%, 5%, 10% 15%, 20% | WebSupplement table S1 |
| B. Characteristics of epidemics simulated | |||
| R0 | Basic reproductive rate | 1.00–1.65 | simulations |
| y* | Overall HIV equilibrium prevalence | >0–35% | simulations (5; 17; 33–34) |
| C. Intervention parameters | |||
| k | Uptake: fraction of new sexually active female entering the population using MB | 50%, 75%, 100% | assumed |
| k1 | Speed of roll-out: initial proportion of women in the population using MB | Fast k1=k Slow k1=k/2 |
|
| γRMB , γVMB | Adherence: fraction of sex acts protected by microbicide when using or not using condoms | 50%, 75% and [0–30]%1 , [60–90]%1 | Table 1(5) |
| EVMB | VMB efficacy: reduction in susceptibility per vaginal act | 30%, 50%, 75% and between 15 to 90% | Table 1 (5) |
| ERMB | RMB efficacy: reduction in susceptibility per anal act | 30%, 50%, 75% and between 15 to 90% | assumed |
| D. Intervention scenarios modelled | |||
| Optimistic coverage (For VMB, RMB, RVMB) | |||
| Uptake and roll-out | k1=k =100% | ||
| Efficacy | EVMB, ERMB, ERVMBvaried | ||
| Adherence | γRMB , γVMB= varied | ||
| Anal sex | a and RRRAIvaried | ||
| Alternative more realistic coverage (For VMB only) | |||
| Uptake and roll-out |
k=50%, 75%, 100% and k1= k/2 (slow roll-out) k=50%, 75% and k1=k (fast roll-out) |
||
| Efficacy | EVMB = 50% | ||
| Adherence | γVMB = 75% | ||
| Anal sex | a=0% | ||
80% CI of the pooled estimate(13); subscript w= females, subscript m= male, superscript p= microbicide user, RAI: receptive anal intercourse; IAI: insertive anal intercourse;
Ranges uniformly sampled
In the Caprisa-004 trial, the true efficacy of tenofovir gel against HIV remains uncertain. It could be over or underestimated since the estimated overall effectiveness (a reflection of the true efficacy and adherence) of tenofovir against HIV was 39%, after 30 months, under imperfect adherence (72% overall), and because tenofovir gel also protected against HSV-2 acquisition, which is a cofactor of HIV(38)(Table 1). Using this information, we predominantly explored efficacy around 30%, 50%, 75% and adherence around 50% and 75% but also varied them over a wider range (Table 2C). We assumed that the RVMB is equally effective during AI and VI. We explored an optimistic (k1=k=100%) and five alternative coverage scenarios with lower uptake (50%,75%), with slow and fast roll-out(Table 2D). The optimistic scenario serves as the reference when comparing the loss of impact due to AI and reduced coverage.
Monte Carlo sampling was used to randomly select different pre-intervention parameter sets from their predefined uniform ranges(Table 2A). The pre-intervention parameters were filtered to identify 1000 parameter sets that met predefined target criteria of i) basic reproductive number R0>1 in absence of intervention; and ii) equilibrium HIV-prevalence below 35% (Table 2B). The intervention is introduced in the different mature simulated HIV epidemics. The population-level effectiveness of the intervention is measured as the cumulative fraction of new HIV infections prevented over the period [0,T](CFP) following the start of the intervention. We report the median and the 90% uncertainty interval [90%UI], derived from the various parameter sets, which reflects the influence of the epidemiological conditions on the impact estimates.
Break-even efficacy ( )
The usefulness of RMB compared to VMB for females practicing AI was assessed by the break-even RMB efficacy ( )(Equation 1) which was derived from the formula of the cumulative risk of HIV infection over fixed time period, for women using a VMB or RMB during sex (CRpw; Equations B.1-B.2), and is embedded in the expressions for the force of infection of the deterministic model (WebAppendix B). Equation 1 determines the minimum RMB efficacy required to reduce a woman’s risk of HIV infection by the same amount than a VMB assuming that 100% of sex acts are with a HIV positive partner, the same adherence with both products, no condom substitution, and no reduction in HIV infectiousness due to microbicides use (WebAppendix B). Under these assumptions, the equivalence between a RMB and VMB only depends on the relative efficacy of the RMB (ERMB) and VMB (EVMB), the frequency of AI (a), and the increase in HIV risk during RAI(RRRAI) compared to the risk during receptive VI(βw); it is independent of the male increased in HIV risk during IAI(RRIAI), coverage and adherence as long as it is equal for both products.
| Equation 1 |
Results
Population-level impact of VMB by coverage level
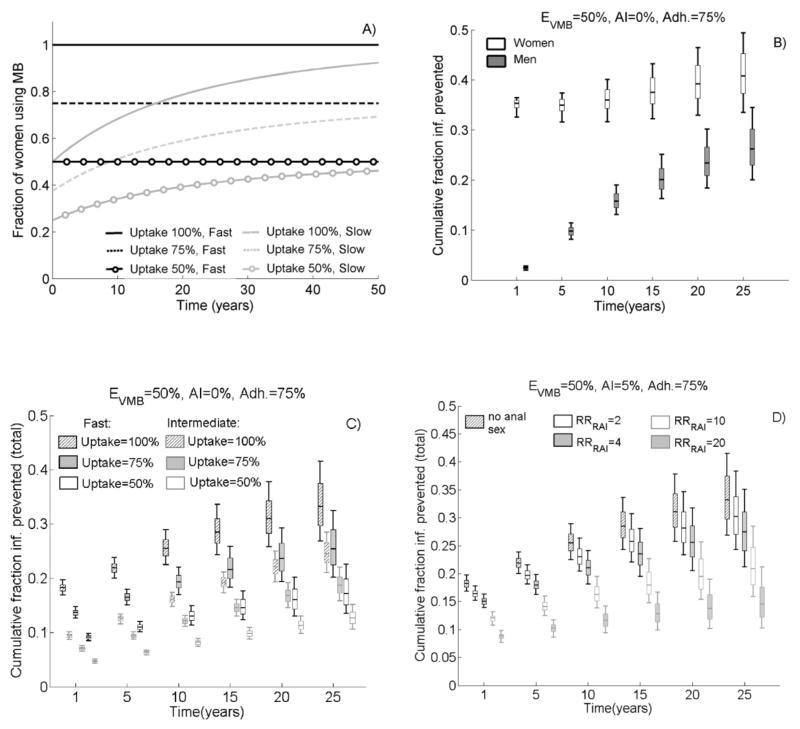
Figure 1A shows the fraction of female MB user (coverage) under different uptake and roll-out assumptions. With 50% uptake and slow roll-out, 25% and 40% of women use the MB after 1 and 25 years, respectively (low coverage). With 100% uptake and fast roll-out, all women immediately use MB (optimistic coverage). Figure 1B–D shows the cumulative fraction of new HIV infections prevented (CFP) over time following the MB introduction. Under optimistic coverage a 50% efficacious VMB, used in 75% of sex acts in populations without AI, is expected to prevent 35%[90%UI: 33,36%] and <3% new HIV infections in the first year among females and males, respectively(Figure 1B). However, the difference between men and women reduces over time solely due to “herd effects” (i.e. men are indirectly protected by female users) since the microbicide does not reduce the infectiousness of HIV positive females. As expected, the VMB impact is reduced if coverage decreases(Figure 1C). With fast roll-out, the 25-year total (men and women combined) CFP reduces from 33%[90%UI:27,42] to 25% [90%UI:20,32], and 17%[90%UI:13,23], respectively, when uptake decreases from 100% to 75% and to 50%(Figure 1C). Over ten years, only 8%[90%UI:7,9%] total HIV infections are prevented with low coverage (slow roll-out, 50% uptake).
Figure 1.
A) Fraction of women using MB over time (coverage) following the intervention start for different uptake and roll- out (fast, slow) assumptions; Cumulative fraction of new HIV infections prevented (CFP) over time among male and women not practicing AI (AI=0%) and under optimistic coverage (100% uptake, fast roll-out) (in B) and among the total population (male and female combined) (in C–D) following the introduction of a 50% efficacious VMB (EVMB=50%) used in 75% of sex acts (adh=75%). In C) nobody practice AI (AI=0%), uptake and roll-out are varied; In D) AI=5%, RRRAI are varied and assumes optimistic coverage. The box plots (median, 5th, 25th, 75th, 95th percentiles) reflect the variation in impact estimates due to the 1000 different parameters sets – (i.e. epidemiological conditions) explored.
Loss in VMB population-level impact due to anal intercourse
Under optimistic coverage, the median total 25-year CFP reduces from 33% in populations without AI to 27%, 21%, and 15% in populations with 5% AI if RRRAI=4, 10 and 20, respectively(Figure 1D). Thus, levels of only 5% AI could produce the same loss in VMB impact than a 25%–50% reduction in uptake(Figure 1C).
Relative population-level impact of RMB and VMB
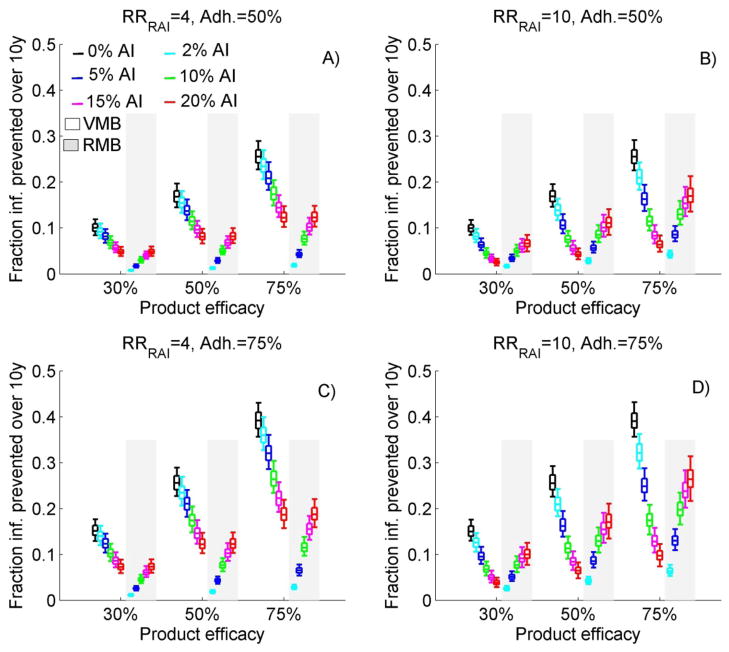
Figure 2A–D compares the total 10-year CFP of a VMB and RMB for different efficacy, adherence, AI frequency and RRRAI, under optimistic coverage. VMB impact reduces rapidly as AI frequency increases independently of the efficacy or adherence assumed. When AI frequency increases from 0% to 5%, 0% to 10% and 0% to 20%, the median 10-year CFP of VMB is reduced by ~18%,~32% and ~52% if RRRAI=4 or ~36%, ~55% and ~75% if RRRAI=10 respectively. To produce the same impact in populations practicing AI as in populations not practicing AI, the VMB adherence or efficacy needs to be considerably higher. To prevent a median of 10% new infections over ten years with a 30% efficacious VMB requires 50% adherence in absence of AI compared to 75% adherence in populations with 5% AI if RRRAI=10 (Figure 2D) or 10% AI if RRRAI=4 (Figure 2C). Alternatively, if RRRAI=10, a 75% or 50% efficacious VMB used in populations with 5% AI is not more effective than a 50% or 30% efficacious VMB in absence of AI, independently of the adherence level. Finally, RMB impact increases sharply as AI frequency increases. For example, a RMB which has the same efficacy and adherence level as a VMB is expected to prevent the same fraction of infections in populations with 20% AI if RRRAI=4 (Figure 2A,C) or in populations with approximately 10% AI if RRRAI=10 (Figure 2B,D).
Figure 2.
The total (men and females combined) cumulative fraction of new infections prevented by a VMB and RMB over ten years (10-year CFP) after the MB introduction assuming same adherence to both products, optimistic coverage, and various AI frequency and MB efficacy. In A) adherence level(adh)=50% and RRRAI=4; in B) adh=50% and RRRAI=10; in C) adh=75% and RRRAI=4; in D) adh=75% and RRRAI=10. Each box plot represents the median (5th, 25th, 75th, 95th percentiles) from 1000 simulations for each of the 36 scenarios per panel
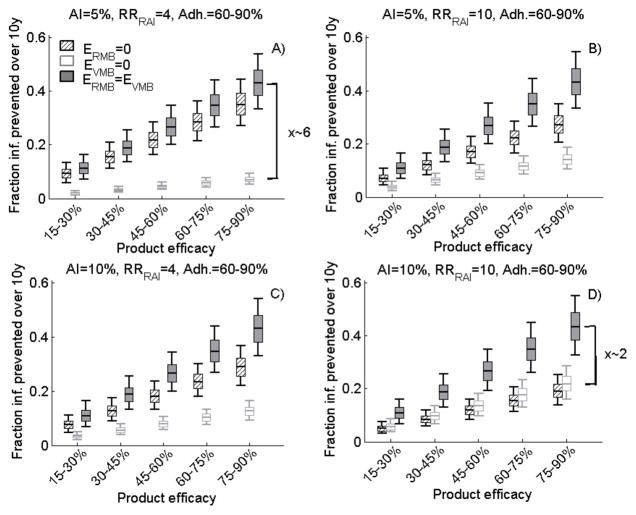
Incremental benefit of bi-compartmental RVMB
Figure 3 shows the incremental benefit of a bi-compartmental RVMB, which is equally efficacious during VI and AI compared to a VMB only or RMB only. Under optimistic coverage and 60–90% adherence, the 10-year CFP with a 45–60% efficacious RVMB is 27% [90%UI:20,35%]. The impact of the RVMB is insensitive to the frequency of AI or RRRAI. However, the incremental benefit of a RVMB compared to a single VMB (RMB) increases (decreases) with the frequency of AI or RRRAI. For example, the 10-year CFP of a bi-compartmental RVMB is 1.2 or ~6-fold larger than a single VMB or a RMB, respectively, in populations with 5% AI if RRRAI=4 (Figure 3A) but it is 2-fold larger than for single VMB or RMB in population with 10% AI if RRRAI=10 (Figure 3D). However, even under optimistic coverage, a 45–60% efficacious RVMB prevent less than 10% total new infections over 10 years if adherence is below 30%, independently of AI frequency and RRRAI (WebSupplement figure S2).
Figure 3.
The 10-year total (men and females combined) cumulative fraction of new infections prevented by a VMB only, RMB only, and RVMB (ERMB=EVMB) after the introduction of the MB intervention of various efficacy (from 15% to 90%) assuming 60–90% adherence to both products and optimistic coverage. In A) 5% AI and RRRAI=4; in B) 5% AI and RRRAI=10; in C) 10% AI and RRRAI=4; in D) 10% AI and RRRAI=10. The box plots (median, 5th, 25th, 75th, 95th percentiles) are derived from 10,000 different parameters sets.
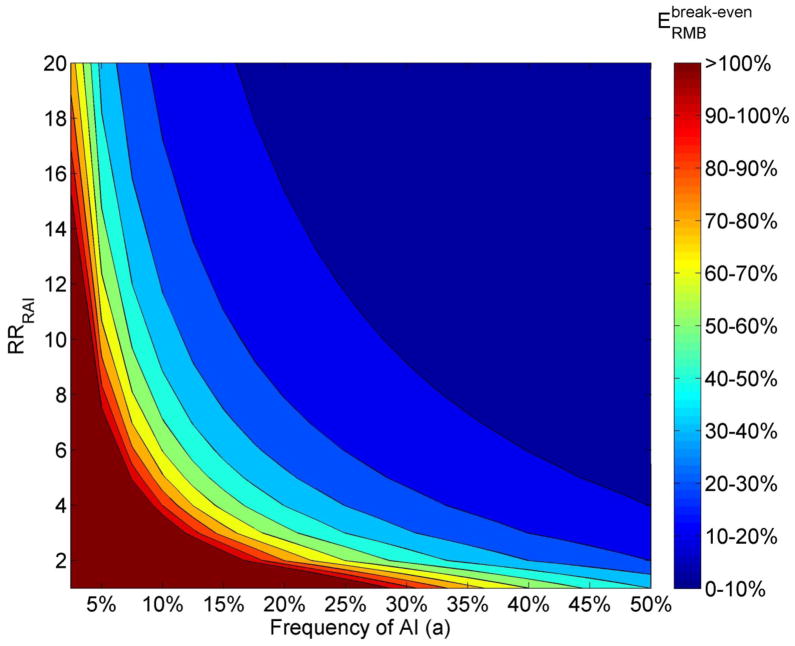
Usefulness of RMB to protect females practicing AI
Figure 4 shows the minimum, or break-even, efficacy ( ) required for a RMB to reduce female HIV risk by the same amount as a 40% efficacious VMB, in function of the AI frequency and RRRAI, if both products are used as frequently. Understandably, if half of the sex acts are AI, the break-even efficacy is 40% even if RRRAI=1. When the AI frequency is between 5–20%, the break-even efficacy strongly depends on RRRAI. For example, 40% efficacious RMB and VMB are equally useful in populations with 5% AI if RRRAI=18; with 10% AI if RRRAI=8; or with a 20% AI frequency if RRRAI=4. In many instances (e.g. a=7.5% if RRRAI >16, a=10% if RRRAI >12), a 20–30% efficacious RMB can even be more useful than a 40% efficacious VMB. However, with less than 2.5% AI, a RMB is unlikely to be as useful as a 40% efficacious VMB, unless the RMB is 1.8-fold ( ) more efficacious than the VMB (i.e. ) and RRRAI=20. These results are independent of the adherence assumed as long as it is the same for both products. From equation 1, we can show that, for a fixed RRRAI and AI frequency, the break-even is approximately independent of EVMB. Of note, the break-even conditions (in term of AI frequency and RRRAI) are similar t0 those observed for the population-level impact derived with the dynamical model (Figure 2) because of the proportionate mixing assumption.
Figure 4.
A) Contour plot representing the minimum efficacy ( ) required for a RMB to be as useful as a 40% efficacious VMB for a woman practicing AI with a HIV positive partner in function of the AI frequency (a) and increased HIV risk during RAI (RRRAI). The results are based on equation 1, which assumes same adherence with both products and βw=0.003. The region above the efficacy boundary indicates the conditions when the RMB can be less efficacious than the VMB and still be as useful – which also means that the 40% efficacious RMB is more useful than the 40% efficacious VMB. The region below the 40% boundary indicates when the RMB needs to be more efficacious than the VMB to be as useful.
Discussion
Our study on the relative impact of VMB, RMB and RVMB in heterosexual populations provides valuable information for product developers and policy makers.
First, we estimated that in populations not practicing AI, a 50% efficacious VMB may prevent a median of ~8% [90%UI:7,9%] total (male and female) new HIV infections over ten years with slow roll-out and 50% uptake (coverage increasing from 25% to 35% after 10 years) compared to ~16% [90%UI:15,17%] with 100% uptake(Figure 1C). We showed that the influence of AI frequency is as important as coverage when assessing the population-level impact of MB interventions since a 5–10% AI frequency could produce a similar loss in VMB impact as a 25%–50% reduction in coverage if RRRAI=4–20(Figure 1C-D).
Our results are useful to determine the desirable characteristics that a MB must have to be of public health use in different populations (i.e. “How good is good enough”)(31). To produce the same 10-year CFP as a 50% efficacious VMB in populations without AI, under the same conditions of use, a VMB would have to be 75% efficacious in populations with 5% AI if RRRAI=10(Figure 2). A RVMB may be ~2-fold more effective than a RMB or VMB in populations with more than 10% AI and RRRAI=10(Figure 3). Thus, a dual protection seems a desirable MB characteristic.
We showed that AI can reduce the impact of VMB to the extent that RMB can become as useful as VMB, for a wide range of plausible AI and RRRAI, despite being used in much fewer sex acts overall than a VMB(Figure 2,4). Our break-even efficacy analysis suggested that, in many instances when both products are used as frequently, even a slightly less efficacious RMB could be more useful than a VMB, especially in populations with more than 15–20% AI. Below this, the relative (incremental) benefit of RMB (RVMB) compared to VMB strongly depends on the RRRAI assumed.
In this analysis, we explored various VMB coverage and adherence scenarios. Although, the optimistic coverage will likely overestimate the potential impact of a microbicide intervention, especially on the short to medium term(50–52), they are unlikely to influence the relative comparison between RMB, VMB and RVMB. The optimistic scenario helps to appreciate the maximum impact that a MB with fixed efficacy can have and to determine the very minimum MB efficacy required to be useful. For example, a 30–45% RVMB would have a modest impact if adherence was less than 30% even under optimistic coverage (WebSupplement figure S2). Adherence is an important determinant of the potential success of microbicide interventions. Although, overall gel adherence in the CAPRISA-004 trial was high at 72%, this may over-estimate the potential achievable adherence-level in populations in real life setting(5). How often a microbicide will be used will depend, among others things, on the delivery system (e.g. coital or daily gel, or slow release ring), individual preferences, availability, acceptability and cost of the product(53–55).
We did not investigate the potential impact of condom substitution, where women switch from more effective condom to a potentially less effective microbicide, and assumed the same adherence with RMB and VMB. Condom substitution can worsen the HIV epidemic if it is frequent, especially with product of poor to moderate efficacy, or if initial condom use is high prior to the VMB introduction(27;28;31;56). Given that condom use is sometimes less frequently reported during anal than vaginal intercourse, a RMB may have the additional advantage of minimizing the risk of condom substitution especially in populations with high rates of condom use during VI (e.g. FSWs with commercial clients)(57;58). On the other hand, a coital topical RMB may be used less frequently than a VMB because AI may not always be planned. However, adherence could be improved with slow release RMB and especially with bi-compartmental RVMB since they would only need to be applied vaginally to protect during AI(53). In the CAPRISA-004 trial, the gel needed to be applied 12 hours before and after each sex act which may have protected during multiple sex acts during this 24-hours window period(5). The measurable effectiveness of tenofovir in the CAPRISAL-004 trial may also be due to the fact that very few women reported AI or perhaps (although premature to conclude) because the gel also protected during AI, despite being vaginally applied.
The precision of our model predictions is limited because available estimates of HIV risk during URAI and UIAI are imprecise and because many studies report the proportion of individuals who ever engaged in AI over fixed time periods but do not report the frequency of unprotected AI and VI, which is required to more precisely assess the future role of RMB and VMB in specific populations(12). Ideally information on AI should be collected using interviewing techniques to reduce social desirability bias(18;35). In our model, we have assumed random mixing between those who practice and do not practice anal intercourse. Data on mixing patterns, i.e. who is having AI with whom, would also be valuable to better understand the contribution of this high-risk practice to the heterosexual HIV epidemic.
Our results suggest that even low AI frequency can seriously compromise the impact of VMB interventions. The development of RMB and bi-compartment RVMB is essential HIV prevention among heterosexual populations.
Supplementary Material
Key messages.
Anal intercourse(AI) frequency of only 5–10% can reduce the impact of VMB intervention among heterosexuals by a similar extent as a 25–50% reduction in coverage.
A RMB can prevent more infections than an equally efficacious VMB despite being used in fewer sex acts under a wide range of plausible assumptions.
Considering realistic level of AI frequency is as important as considering realistic coverage when assessing the potential impact of microbicide interventions.
More precise data on the frequency of AI is required to better understand the preventive role of RMB/RVMB against HIV in different heterosexual populations and risk groups.
Acknowledgments
Funding
None
Footnotes
Licence for Publication statement
”The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd to permit this article (if accepted) to be published in STI and any other BMJPGL products and sublicences such use and exploit all subsidiary rights, as set out in our licence http://group.bmj.com/products/journals/instructions-for-authors/licence-forms.”
Competing Interests
None declared
Authors’ contribution
MCB and DD designed the analysis. DD developed the model and performed the simulations. DD and MCB analyzed the results. MCB drafted the first version of the manuscript. BM and SSAK provided input on different MB trial results. All authors helped with the parameterization of the model, interpretation of the results, and revised all drafts.
References
- 1.McCoy S, Watts C, Padian N. Preventing HIV infection: turning the tide for young women. Lancet. 2010;376:1281–1282. doi: 10.1016/S0140-6736(10)61309-8. [DOI] [PubMed] [Google Scholar]
- 2.Padian NS, Buvé A, Balkus J, et al. Biomedical interventions to prevent HIV infection: evidence, challenges, and way forward. Lancet. 2008;372(9638):585–599. doi: 10.1016/S0140-6736(08)60885-5. [DOI] [PubMed] [Google Scholar]
- 3.Mc Cormack S, Hayes R, Lacey C, et al. Microbicide and HIV prevention. BMJ. 2001;322:410–413. doi: 10.1136/bmj.322.7283.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minces LR, McGowan I. Advances in the development of microbicides for the prevention of HIV infection. Curr Infect Dis Rep. 2010;12(1):56–62. doi: 10.1007/s11908-009-0076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and Safety of Tenofovir Gel, an antiretroviral microbcide, for the prevention of HIV infection in women. Science Express. 2010;329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baleta A. Antiretroviral gel shows promise against HIV. Lancet. 2010;376(9738):304. doi: 10.1016/s0140-6736(10)61123-3. [DOI] [PubMed] [Google Scholar]
- 7.Alliance for Microbicide Development. [Date accessed July 31st 2010];Pipeline update of microbicide and PrEP candidates [Internet] Available from: www.microbicide.org/uploads/3/1/2/1/3121935/microbicide_pipeline_update_1_oct_2009.pdf.
- 8.McGowan I. Rectal microbicides: A new focus for HIV prevention. Sex Transm Infect. 2008;84:413–417. doi: 10.1136/sti.2008.031328. [DOI] [PubMed] [Google Scholar]
- 9.Hendrix CW, Cao YJ, Fuchs EJ. Topical microbicides to prevent HIV: Clinical drug development challenges. Annual Review of Pharmacology and Toxicology. 2009;49:349–375. doi: 10.1146/annurev.pharmtox.48.113006.094906. [DOI] [PubMed] [Google Scholar]
- 10.Baggaley RF, White RG, Boily M. HIV transmission risk through anal intercourse: systematic review, meta-analysis and implications for HIV prevention. Int J Epidemiol. 39(4):1048–63. doi: 10.1093/ije/dyq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baggaley RM, White RG, Boily M. Infectiousness of HIV-infected homosexual men in the era of HAART. AIDS. 2010;24(15):2418–20. doi: 10.1097/QAD.0b013e32833dbdfd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boily M-C, Baggaley R, Masse B. The role of heterosexual anal intercourse for HIV transmission in developing countries: are we ready to draw conclusions? Sex Transm infect. 2009;85(6):408–410. doi: 10.1136/sti.2009.037499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boily M-C, Baggaley RF, Wang L, et al. Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. Lancet Infect Dis. 2009;9(2):118–29. doi: 10.1016/S1473-3099(09)70021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin F, Jansson J, Law M, et al. Per-contact probability of HIV transmission in homosexual men in Sydney in the era of HAART. AIDS. 2010;24:907–913. doi: 10.1097/QAD.0b013e3283372d90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdool Karim SS, Ramjee G. Anal sex and HIV transmission in women. Am J Public Health. 1998;88:1265–1266. doi: 10.2105/ajph.88.8.1265-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matasha E, Ntembelea T, Mayaud P, et al. Sexual and reproductive health among primary and secondary school pupils in Mwanza, Tanzania: need for intervention. AIDS Care. 1998;10(5):571–82. doi: 10.1080/09540129848433. [DOI] [PubMed] [Google Scholar]
- 17.Kalichman S, Cain D, Simbayi L. Heterosexual anal intercourse among community and clinical settings in Cape Town, South Africa. Sex Transm Iinfect. 2009;5(6):411–5. doi: 10.1136/sti.2008.035287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minani I, Alary M, Lowndes CM, et al. Higher levels of HIV-related risky behaviour reported in Polling Booth Surveys (PBS) compared to Face-to-Face Interviews in a General Population Survey (GPS) in Cotonou-Benin (West Africa). Abstract OS1.8.03. ISSTDR; London. 2009. [Google Scholar]
- 19.McGowan I, Taylor DJ. Heterosexual anal intercourse has the potential to cause a significant loss of power in vaginal microbicide effectiveness studies. Sex Transm Dis. 2010;37(12):10–13. [PubMed] [Google Scholar]
- 20.Masse BR, Boily MC, Dimitrov D, et al. Efficacy dilution in randomized placebo-controlled vaginal microbicide trials. ETE. 2009;6:5. doi: 10.1186/1742-7622-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGowan I, Anton P. Rectal microbicides. [Internet] Current opinion in HIV and AIDS. 2008 Sep;3(5):593–8. doi: 10.1097/COH.0b013e32830891cf. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19373027. [DOI] [PubMed] [Google Scholar]
- 22.Parikh U, Dobard C, Sharma S, et al. Complete protection from repeated vaginal SHIV exposures in macaques by a topical gel containing Tenofovir alone or with emtricitabine. J Virol. 2009;83(20):10358–65. doi: 10.1128/JVI.01073-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cranage M, Sharpe S, Herrera C, et al. Prevention of SIV rectal transmission and priming of T cell responses in macaques after local pre-exposure application of Tenofovir gel. PLoS Med. 2008;5(8):e157. doi: 10.1371/journal.pmed.0050157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McNeil DGJ. Advance on AIDS raises questions as well as joy. The New York Time. 2010 http://www.nytimes.com/2010/07/27/health/27AIDS.html.
- 25.Breban R, McGowan I, Topaz EJC, et al. Modeling the potential impact of rectal microbicides to reduce HIV transmission in bathhouses. Math Biosci Eng. 2008;3(3):459–466. doi: 10.3934/mbe.2006.3.459. [DOI] [PubMed] [Google Scholar]
- 26.Dimitrov D, Masse M, Boily MC. Who Will Benefit from a Wide-Scale Introduction of Vaginal Microbicides in Developing Countries? Statistical Communications in Infectious Diseases. 2010;2(1):Article 4. doi: 10.2202/1948-4690.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foss AM, Vickerman PT, Alary M, et al. How much could a microbicide’s sexually transmitted infection efficacy contribute to reducing HIV risk and the level of condom use needed to lower risk? Model estimates. Sex Transm Inf. 2009;85:276–282. doi: 10.1136/sti.2008.032458. [DOI] [PubMed] [Google Scholar]
- 28.Foss AM, Vickerman PT, Heise L, et al. Shifts in condom use following microbicide introduction: should we be concerned? AIDS. 2003;44:1227–1237. doi: 10.1097/00002030-200305230-00015. [DOI] [PubMed] [Google Scholar]
- 29.Wilson DP, Coplan P. Mathematical models and health economic aspects of microbicides. Curr Opin HIV AIDS. 2008;3(5):587–592. doi: 10.1097/COH.0b013e328305b959. [DOI] [PubMed] [Google Scholar]
- 30.Weber J, Desai K, Darbyshire J on The Behalf Of The Microbicides Development. The development of vaginal microbicides for the prevention of HIV transmission. PLoS Medicine. 2005;2(5):392–395. doi: 10.1371/journal.pmed.0020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boily M-C. Population-level impact of microbicides to prevent HIV: The efficacy that matters. International 2010 Microbicides Conference. Pittsburgh Symposium presentation; 2010. p. 176. [Google Scholar]
- 32.Boily M-C, Desai K, Masse B, et al. The incremental role of male circumcision on HIV infection through its protective effect against other sexually transmitted infections: from efficacy to effectiveness to population-level impact. Sex Transm inf. 84(Suppl 2):ii28–34. doi: 10.1136/sti.2008.030346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buvé A, Caraël M, Hayes RJ, et al. Multicentre study on factors determining differences in rate of spread of HIV in sub-Saharan Africa: methods and prevalence of HIV infection. AIDS. 2001;15(Suppl 4):S5–14. doi: 10.1097/00002030-200108004-00002. [DOI] [PubMed] [Google Scholar]
- 34.Hargreaves JR, Bonell CP, Morison LA, et al. Explaining continued high HIV prevalence in South Africa: socioeconomic factors, HIV incidence and sexual behaviour change among a rural cohort, 2001–2004. AIDS. 2007;21(Suppl 7):S39–48. doi: 10.1097/01.aids.0000300534.97601.d6. [DOI] [PubMed] [Google Scholar]
- 35.Langhaug L, Cheung Y, Pascoe S, et al. How you ask really matters: randomised comparison of four sexual behaviour questionnaire delivery modes in Zimbabwean youth. Sex Transm Inf. 2010;87(2):165–173. doi: 10.1136/sti.2009.037374. [DOI] [PubMed] [Google Scholar]
- 36.Ferry B, Carael M, Buvé A, et al. Comparison of key parameters of sexual behaviour in four African urban populations with different levels of HIV infection. AIDS. 2001;15(Suppl 4):S41–50. doi: 10.1097/00002030-200108004-00005. [DOI] [PubMed] [Google Scholar]
- 37.Todd J, Cremin I, McGrath N, et al. Reported number of sexual partners: comparison of data from four African longitudinal studies. Sex Transm inf. 2009;85(Suppl 1):i72–i80. doi: 10.1136/sti.2008.033985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Celum C. The interaction between herpes simplex virus and human immunodeficiency virus. Herpes. 2004;11(Suppl 1):36A–45A. [PubMed] [Google Scholar]
- 39.Van Damme L, Ramjee G, Alary M, et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet. 2002;360 (9348):971–977. doi: 10.1016/s0140-6736(02)11079-8. [DOI] [PubMed] [Google Scholar]
- 40.Skoler-Karpoff S, Ramjee G, Ahmed K, et al. Efficacy of carraguard for prevention of HIV infection in women in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1977–87. doi: 10.1016/S0140-6736(08)61842-5. [DOI] [PubMed] [Google Scholar]
- 41.Laumann E, Gagnon J, Michael T, et al. The social organization of sexuality: sexual practices in the United States. University of Chicago Press; Chicago: 1994. [Google Scholar]
- 42.Messiah A, Pelletier A. Partner-specific sexual practices among heterosexual men and women with multiple partners: results from the French National survey, ACSF. Analyse des comportements sexuels en France. Arch Sex Behav. 1996;25(3):233–47. doi: 10.1007/BF02438163. [DOI] [PubMed] [Google Scholar]
- 43.Silveira M, Beria J, Horta B, et al. Factors associated with risk behaviors for sexually transmitted disease/AIDS among urban Brazilian women: a population-based study. Sex Transm Dis. 2002;9:536–41. doi: 10.1097/00007435-200209000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Leonard L, Ross M. The last sexual encounter: the contextualization of sexual risk behaviour. Int J STD AIDS. 1997;8(10):643–5. doi: 10.1258/0956462971918788. [DOI] [PubMed] [Google Scholar]
- 45.O’Connor C, Wen L, Rissel C, et al. Sexual behaviour and risk in Vietnamese men living in metropolitan Sydney. Sex Transm Inf. 2007;83(2):147–50. doi: 10.1136/sti.2006.021394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Collaborative Group on AIDS Incubation and HIV Survival. Time from HIV-1 seroconversion to AIDS and death before widespread use of highly active antiretroviral therapy: a collaborative re-analysis. Collaborative Group on AIDS Incubation and HIV Survival including the CASCADE EU Concerted Action. Lancet. 2000;355:1131–1137. [PubMed] [Google Scholar]
- 47.San Andres Rebollo FJ, Rubio Garcia R, Castilla Catalan JP, et al. Mortality and survival in a cohort of 1, 115 HIV-infected patients (1989–97) An Med Interna. 2004;21:523–532. doi: 10.4321/s0212-71992004001100002. [DOI] [PubMed] [Google Scholar]
- 48.Allen S, Meinzen-derr J, Kautzman M, et al. Sexual behavior of HIV discordant couples after HIV counseling and testing. AIDS. 2003;17(5):733–40. doi: 10.1097/00002030-200303280-00012. [DOI] [PubMed] [Google Scholar]
- 49.Foss AM, Hossain M, Vickerman PT, et al. A systematic review of published evidence on intervention impact on condom use in sub-Saharan Africa and Asia. Sex Transm Inf. 2007;83(7):510–6. doi: 10.1136/sti.2007.027144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lissouba P, Taljaard D, Rech D, et al. A model for the roll-out of comprehensive adult male circumcision services in African low-Income settings of high HIV incidence: The ANRS 12126 Bophelo Pele project. PLoS Medicine. 2010;7(7):e1000309. doi: 10.1371/journal.pmed.1000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cleary S. Equity and efficiency in scaling up access to HIV-related interventions in resource-limited settings. Curr Opin HIV AIDS. 2010;5(3):210–4. doi: 10.1097/COH.0b013e3283384a6f. [DOI] [PubMed] [Google Scholar]
- 52.Verma R, Shekhar A, Khobragade S, et al. Scale-up and coverage of Avahan: a large-scale HIV-prevention programme among female sex workers and men who have sex with men in four Indian states. AIDS. 2004;(Suppl 3):S21–5. doi: 10.1136/sti.2009.039115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Geonnotti A, Katz D. Compartmental transport model of microbicide delivery by an intravaginal ring. J Pharm Sci. 2010;99(8):3514–21. doi: 10.1002/jps.22120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coly A, Gorbach P. Microbicide acceptability research: recent findings and evolution across phases of product development. Curr Opin HIV AIDS. 2008;3(5):581–6. doi: 10.1097/COH.0b013e32830aba00. [DOI] [PubMed] [Google Scholar]
- 55.Greene E, Batona G, Hallad J, et al. Acceptability and adherence of a candidate microbicide gel among high-risk women in Africa and India. Cult Heatlh Sex. 2010;12(7):739–54. doi: 10.1080/13691051003728599. [DOI] [PubMed] [Google Scholar]
- 56.Karmon E, Potts M, Getz WM. Microbicides and HIV: help or hindrance? J Acquir Immune Defic Syndr. 2003;34(1):71–75. doi: 10.1097/00126334-200309010-00011. [DOI] [PubMed] [Google Scholar]
- 57.Halperin D. Heterosexual anal intercourse: prevalence, cultural factors, and HIV infection and other health risks, Part I. AIDS Patient Care STDs. 1999;13:717–30. doi: 10.1089/apc.1999.13.717. [DOI] [PubMed] [Google Scholar]
- 58.Leichliter J, Chandra A, Liddon N, et al. Prevalence and correlates of heterosexual anal and oral sex in adolescents and adults in the United States. J Infect Dis. 2007;196:1852–59. doi: 10.1086/522867. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.