Abstract
As part of an ongoing effort to expand the genetic alphabet for in vitro and eventual in vivo applications, we have synthesized a wide variety of predominantly hydrophobic unnatural base pairs and evaluated their replication in DNA. Collectively, the results have led us to propose that these base pairs, which lack stabilizing edge-on interactions, are replicated via a unique intercalative mechanism. Here, we report the synthesis and characterization of three novel derivatives of the nucleotide analog dMMO2, which forms an unnatural base pair with the nucleotide analog d5SICS. Replacing the para-methyl substituent of dMMO2 with a furanyl substituent (yielding dFMO) has a dramatically negative effect on replication, while replacing it with a methoxy (dDMO) or with a thiomethyl group (dTMO), improves replication in both steady-state assays and during PCR amplification. Thus, dTMO-d5SICS, and especially dDMO-d5SICS, represent significant progress toward the expansion of the genetic alphabet. To elucidate the structure-activity relationships governing unnatural base pair replication, we determined the solution structure of duplex DNA containing the parental dMMO2-d5SICS pair, and also used this structure to generate models of the derivative base pairs. The results strongly support the intercalative mechanism of replication, reveal a surprisingly high level of specificity that may be achieved by optimizing packing interactions, and should prove invaluable for the further optimization of the unnatural base pair.
Keywords: unnatural base pair, genetic alphabet, DNA replication, polymerase, intercalation
Introduction
The natural genetic alphabet relies on the selective pairing of the four natural nucleotides, which is governed by a combination of hydrogen-bonding (H-bonding)[1] and shape complementarity.[2–4] However, a priori there is no reason that these forces should be unique in their ability to mediate base pairing. With the long term goal of expanding the genetic code, we[5–15] and others[3,16–22] have explored the development of unnatural nucleotides bearing nucleobase analogs that pair via hydrophobic and packing forces. Among the most promising predominantly hydrophobic base pairs that we have identified is that formed between dMMO2 and d5SICS (dMMO2-d5SICS, Figure 1a).[8] dMMO2-d5SICS is synthesized (by insertion of each unnatural triphosphate opposite the other in the template)[23] and then extended (by insertion of the next correct dNTP) with relatively high efficiency and fidelity by diverse polymerases,[13] including the exonuclease deficient Klenow fragment of E. coli DNA polymerase I (Kf).
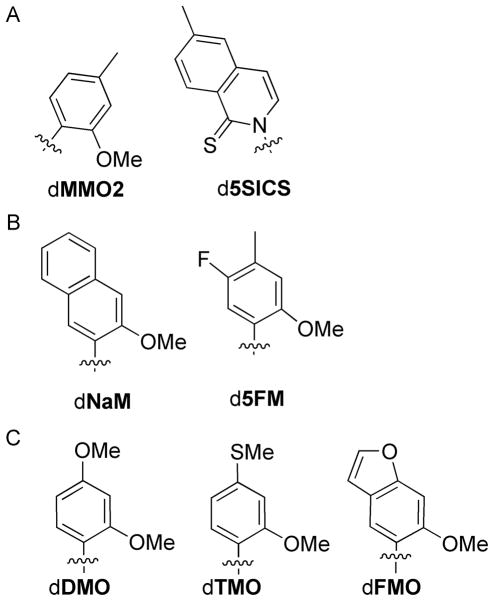
Figure 1.
Unnatural nucleotides used in current study. Only nucleobases are shown; sugars and phosphates have been omitted for clarity.
The step that most limits the replication of DNA containing dMMO2-d5SICS is the insertion of dMMO2TP opposite d5SICS in the template. In general, we have found that the rates of insertion are most sensitive to triphosphate derivatization; thus our efforts to optimize dMMO2-d5SICS have focused on modification of dMMO2 (with the goal of optimizing the insertion of dMMO2TP opposite d5SICS). Because previous studies showed that the methoxy and sulfur substituents at the position ortho to the glycosidic linkage are essential for efficient extension of the nascent unnatural base pair,[6–8] our efforts focused specifically on meta and para derivatizations of dMMO2.[9,10] Indeed, we already demonstrated that both d5NaMTP and d5FMTP (Figure 1b) are inserted opposite d5SICS with higher efficiency and fidelity than dMMO2TP, and that dNaM-d5SICS is sufficiently well recognized for expansion of the genetic alphabet in vitro.[9] However, we anticipate that one of the most interesting in vitro applications of an expanded genetic alphabet will be the use of an unnatural base pair to site specifically modify DNA or RNA in a format consistent with enzymatic synthesis, and one limitation of dNaM is that the second aromatic ring precludes derivatization at the position most commonly used to attach linkers (i.e. the C5 position of natural pyrimidines[24]). While other positions might be found to derivatize dNaM with linkers, modifications of dMMO2 that improve replication without blocking the C5 position are desirable.
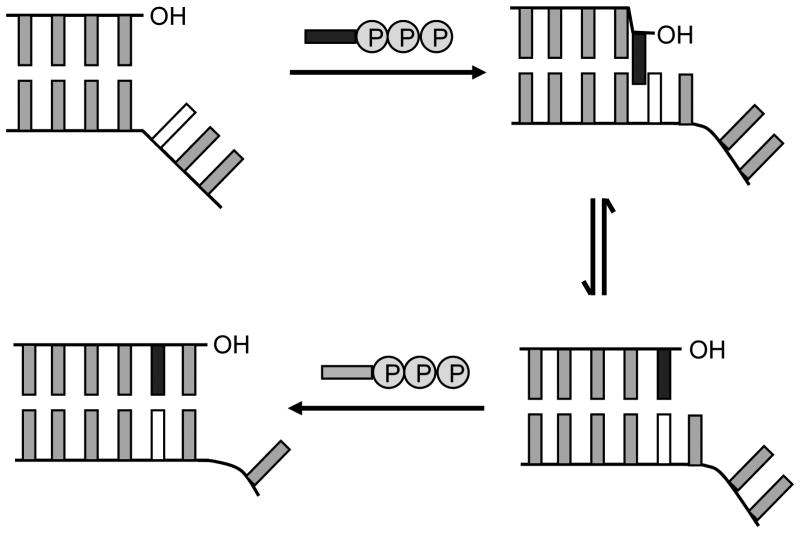
Previous kinetic[5–11] and structural[12] studies have prompted us to propose that the predominantly hydrophobic unnatural base pairs are replicated via a unique mechanism involving partial interstrand intercalation (Figure 2). In this mechanism, the unnatural triphosphates are recognized by at least partial intercalation of their nucleobases into the polymerase-bound template strand between the nucleobase of their cognate unnatural nucleotide and a flanking nucleobase. This mode of insertion likely results from the high hydrophobic packing and stacking potential of the unnatural nucleobases and the absence of interactions that favor edge-to-edge pairing, and also suggests that increased packing within the major groove underlies the more efficient insertion of dNaMTP and d5FMTP opposite d5SICS, relative to dMMO2TP. Importantly, the model also suggests that de-intercalation is required to position the primer terminus appropriately for continued extension, which is also favored by H-bond formation between a polymerase-based donor and the ortho substituents of d5SICS and dMMO2,[6–8] explaining why they are essential for extension. Thus, a subtle balance between intercalation and de-intercalation is required for the unnatural base pair to be synthesized and extended efficiently. (Despite the requirement of both intercalation and de-intercalation, for simplicity, we refer to this mechanism as the “intercalative mechanism.”).
Figure 2.
Intercalative model for unnatural base pair replication.[9] The unnatural nucleobase in the template is shown in white and the natural or unnatural nucleobase of the incoming dNTP is shown in black.
To test the intercalative mechanism of replication and to continue our efforts to optimize the dMMO2-d5SICS unnatural base pair, we now report the kinetic and structural characterization of base pairs formed between d5SICS and three dMMO2 derivatives that have been modified at the meta and/or para positions: dDMO, dTMO, and dFMO (Figure 1c). Complete steady-state kinetic analysis, as well as the efficiency and fidelity of PCR amplification show that derivatization with a thiomethyl, or especially with a methoxy substituent, significantly improves replication: dTMO-d5SICS and dDMO-d5SICS are replicated more efficiently than dMMO2-d5SICS. Unlike dNaM, the C5 position of dTMO and dDMO is available for derivatization, making them amenable for uses involving the site-specific modification of DNA. Surprisingly, the furan substituent of dFMO dramatically reduces the efficiency of each step of replication. To help understand these trends in replication, we also report the solution structure of dMMO2-d5SICS in duplex DNA, along with models of the derivative base pairs. The data strongly supports the intercalative model of replication and provides a rationale for the observed variations in the recognition of the dMMO2 derivatives.
Results
Unnatural base pair design and evaluation
The dDMO and dTMO nucleotides were designed to probe the effects of heteroatom substitution in the developing major groove at the primer terminus while leaving the C5 position unsubstituted. In contrast, like dNaM, the C5 position of dFMO is substituted, and this analog was designed to introduce a more rigidly positioned heteroatom while simultaneously increasing the potential for nucleobase packing. Oligonucleotides containing the unnatural nucleotides were synthesized to act as templates or primers so that both synthesis and extension of each unnatural base pair could be evaluated independently. Kinetic analyses were performed under steady-state conditions using Kf, and second order rate constants (efficiency, kcat/KM) were determined (individual kcat and KM values are reported in Supporting Information). PCR amplification was also performed to further evaluate the unnatural base pair and to gauge the potential practical utility. The unnatural base pairs are generally referred to as dY-dX, when no strand context is implied, while dY:dX is used to refer specifically to the strand context with dY in the primer strand and dX in the template strand.
Unnatural base pair synthesis - Insertion of dMMO2TP analogs opposite d5SICS
To characterize the effects of major groove substitution, we first characterized the rate at which the dMMO2TP analogs are inserted opposite d5SICS in the template (Table 1). For comparison, dMMO2TP itself is inserted with a second order rate constant of 3.6 × 105 M−1min−1.[8] We found that dDMOTP and dTMOTP are inserted 5-fold more efficiently, resulting entirely from a decreased apparent KM for unnatural triphosphate binding. However, insertion of dFMOTP opposite d5SICS is more than 10-fold less efficient than insertion of dMMO2TP, due to changes in both the apparent kcat and KM. A complete characterization of mispair synthesis with d5SICS in the template was reported previously.[8]
Table 1.
Second order rate constants (kcat/KM) for Kf–mediated synthesis and extension of dMMO2 or an analog paired opposite d5SICS in the template[a]
| Sequence Context I
|
Sequence Context II
|
||||||
|---|---|---|---|---|---|---|---|
| 5′–dTAATACGACTCACTATAGGGAGAY | 5′–dTAATACGACTCACTATAGGGAGCY | ||||||
| 3′–dATTATGCTGAGTGATATCCCTCTXGCTAGGTTACGGCAGGATCGC
|
3′–dATTATGCTGAGTGATATCCCTCGXTCTAGGTTACGGCAGGATCGC
|
||||||
| X | Y | Synthesis (M−1min−1) | Extension (M−1min−1) | X | Y | Synthesis (M−1min−1) | Extension (M−1min−1) |
| dT | dA | 3.2 × 108 | 1.7 × 108 | dT | dA | 3.3 × 108 | 3.1 × 108 |
| d5SICS | dMMO2[b] | 3.6 × 105 | 1.9 × 106 | d5SICS | dMMO2[c] | 4.8 × 105 | 3.2 × 105 |
| dDMO | 1.7 × 106 | 7.8 × 106 | dDMO | 5.0 × 105 | 1.6 × 106 | ||
| dTMO | 1.6 × 106 | 9.8 × 105 | dTMO | Not measured | Not measured | ||
| dFMO | 2.5 × 104 | 5.4 × 104 | dFMO | Not measured | Not measured | ||
| d5SICS[b] | 2.7 × 104 | < 1.0 × 103 [d] | d5SICS[c] | 2.4 × 105 | <1.0 × 103 [d] | ||
| dA[b] | 2.2 × 104 | 1.0 × 104 | dA[c] | 5.9 × 104 | 3.6 × 103 | ||
| dC[b] | < 1.0 × 103 [d] | 4.2 × 103 | dC[c] | < 1.0 × 103 [d] | < 1.0 × 103 [d] | ||
| dG[b] | 1.3 × 105 | 4.9 × 103 | dG[c] | 1.9 × 105 | 6.5 × 102 | ||
| dT[b] | 1.3 × 104 | 4.0 × 105 | dT[c] | 8.5 × 103 | 3.3 × 105 | ||
Unnatural base pair synthesis - Insertion of d5SICSTP opposite dMMO2 analogs
To characterize the recognition of the unnatural nucleotides in the template, we examined the efficiencies with which Kf inserts d5SICSTP (Table 2). For reference, d5SICSTP is inserted opposite dMMO2 with an efficiency of 4.7 × 107 M−1min−1, and it is inserted opposite itself in the template with an efficiency of 1.2 × 105 M−1min−1. Insertion of natural dNTPs opposite dMMO2 in the template is not detectable (kcat/KM < 1.0 × 103 M−1min−1), except in the case of dATP, which is inserted with moderate efficiency (kcat/KM = 1.0 × 105 M−1min−1).[8] We found that insertion of d5SICS opposite dDMO is 3-fold less efficient than opposite dMMO2. However, dDMO also directs the synthesis of the mispair with itself or that with dA less efficiently than does dMMO2, without significantly increasing the synthesis efficiencies of any of the other mispairs.
Table 2.
Second order rate constants (kcat/KM) for Kf–mediated synthesis and extension of d5SICS paired opposite dMMO2 or an analog in the template[a]
| Sequence Context I
|
Sequence Context II
|
||||||
|---|---|---|---|---|---|---|---|
| 5′–dTAATACGACTCACTATAGGGAGAY |
5′–dTAATACGACTCACTATAGGGAGCY |
||||||
| 3′–dATTATGCTGAGTGATATCCCTCTXGCTAGGTTACGGCAGGATCGC | 3′–dATTATGCTGAGTGATATCCCTCGXTCTAGGTTACGGCAGGATCGC | ||||||
| X | Y | Synthesis (M−1min−1) | Extension (M−1min−1) | X | Y | Synthesis (M−1min−1) | Extension (M−1min−1) |
| dMMO2 | d5SICS[b] | 4.7 × 107 | 6.7 × 105 | dMMO2 | d5SICS[c] | 6.6 × 107 | 1.7 × 106 |
| dMMO 2[b] | 1.2 × 105 | 5.3 × 103 | dMMO2[c] | 1.8 × 106 | < 1.0 × 103 [d] | ||
| dA[b] | 1.0 × 105 | 4.6 × 104 | dA[c] | 1.7 × 106 | 1.1 × 104 | ||
| dC[b] | < 1.0 × 103 [d] | 1.2 × 106 | dC[c] | 3.0 × 103 | 4.4 × 105 | ||
| dG[b] | < 1.0 × 103 [d] | < 1.0 × 103 [d] | dG[c] | 7.9 × 103 | < 1.0 × 103 [d] | ||
| dT[b] | < 1.0 × 103 [d] | 6.6 × 105 | dT[c] | 5.2 × 103 | 2.0 × 106 | ||
| dDMO | d5SICS | 1.5 × 107 | 2.6 × 106 | dDM O | d5SICS | 9.7 × 107 | 1.3 × 106 |
| dDMO | 7.1 × 104 | < 1.0 × 103 [d] | dDMO | 1.6 × 105 | < 1.0 × 103 [d] | ||
| dA | 8.3 × 104 | 1.1 × 105 | dA | 8.4 × 105 | 6.9 × 103 | ||
| dC | < 1.0 × 103 [d] | 1.3 × 106 | dC | < 1.0 × 103 [d] | 1.1 × 106 | ||
| dG | < 1.0 × 103 [d] | < 1.0 × 103 [d] | dG | < 1.0 × 103 [d] | < 1.0 × 103 [d] | ||
| dT | 2.5 × 103 | 2.4 × 105 | dT | 1.8 × 103 | 9.7 × 104 | ||
| dTMO | d5SICS | 2.7 × 107 | 1.9 × 106 | ||||
| dTMO | Not measured | 8.3 × 103 | |||||
| dA | 8.5 × 105 | 5.3 × 104 | |||||
| dC | 2.5 × 103 [e] | 2.2 × 105 | |||||
| dG | 3.0 × 103 | < 1.0 × 103 [d] | |||||
| dT | 1.5 × 103 | 3.6 × 104 | |||||
| dFMO | d5SICS | 4.9 × 105 | 9.1 × 103 | ||||
| dFMO | < 1.0 × 103 [d] | < 1.0 × 103 [d] | |||||
| dA | 7.1 × 105 | 7.0 × 103 | |||||
| dC | 7.5 × 103 [e] | 4.9 × 103 | |||||
| dG | 9.0 × 103 | < 1.0 × 103 [d] | |||||
| dT | 2.2 × 104 | 5.2 × 103 | |||||
In all cases “X” is the nucleotide in the template. For synthesis, “Y” corresponds to the triphosphate inserted, and for extension, it corresponds to the nucleotide at the nascent primer terminus.
Taken from reference [8].
Taken from reference [9].
Below limit of detection.
Kinetic parameters were calculated based on n+2 product, as dCTP is inserted slowly against the unnatural base pair and then efficiently against dG, the next nucleotide in the template.
We found that insertion of d5SICSTP opposite dTMO is 2-fold less efficient than opposite dMMO2. Interestingly, the thiomethyl substituent significantly increases the rate at which dATP is inserted, while only slightly increasing the rates at which the other mispairs are synthesized. Surprisingly, incorporating the major groove oxygen atom as a restrained furan (i.e. dFMO), as opposed to a free methyl ether (i.e. dDMO), dramatically reduces the efficiency of d5SICSTP insertion (by 100-fold). While dFMO does not direct Kf to synthesize the self pair (kcat/KM < 1.0 × 103 M−1min−1), it does direct the relatively more efficient insertion of each natural triphosphate.
Unnatural base pair extension - Extension of dMMO2 analogs paired opposite d5SICS
Efficient and high fidelity replication of DNA containing the unnatural base pair also requires efficient continued primer elongation after incorporation of the unnatural nucleotide, and inefficient primer extension after incorporation of an incorrect nucleotide. We first examined the efficiencies with which Kf extends primers terminating with a dMMO2 analog paired opposite d5SICS or a natural nucleotide by insertion of dCTP opposite dG (Table 1). For comparison, Kf extends dMMO2:d5SICS (primer:template) with a second order rate constant of 1.9 × 106 M−1min−1. Changing the major groove substituent from the methyl group of dMMO2 to the methoxy group of dDMO results in a 4-fold increase in extension efficiency. However, the thiomethoxy and furanyl substituents result in approximately 2- and 40-fold reduced efficiencies. Extension efficiencies of primers terminating with a natural nucleotide paired opposite d5SICS have been reported previously.[8]
Unnatural base pair extension - Extension of d5SICS paired opposite dMMO2 analogs
We next examined unnatural base pair extension with the dMMO2 analogs in the template paired opposite either the correct d5SICS nucleotide or one of the incorrect unnatural or natural nucleotides at the primer terminus (Table 2). For comparison, Kf extends primers terminating with d5SICS paired opposite dMMO2 with an efficiency of 6.7 × 105 M−1min−1. Kf does not efficiently extend primers terminating with the dMMO2 self pair or the dG:dMMO2 mispair; however, the mispairs with dA, dT, and especially dC are extended more efficiently.[8] We found that primers terminating with d5SICS paired opposite dDMO are extended 4-fold more efficiently than when paired opposite dMMO2. Primers terminating with the dDMO self pair are extended less efficiently than those terminating with the dMMO2 self pair. As with dMMO2, primers terminating with dG paired opposite dDMO are not extended at a detectable rate, while primers terminating with dA are extended slightly faster, and primers terminating with dT or dC are extended slower. Extension of d5SICS:dTMO is 3-fold faster than extension of d5SICS:dMMO2. Again, primers terminating with dG paired opposite dTMO are not extended, while the dA:dTMO and dA:dMMO2 mispairs are extended with similar efficiencies, and the mispairs with dT or dC paired opposite dTMO are extended an order of magnitude slower than when paired opposite dMMO2. Surprisingly, the d5SICS:dFMO pair is extended 70-fold less efficiently than d5SICS:dMMO2. Moreover, all of the mispairs between a natural nucleotide and dFMO are extended with rates slower than 7.8 × 103 M−1min−1, revealing that both correct pairs and mispairs with dFMO in the template are extended only poorly by Kf.
dDMO-d5SICS replication as a function of sequence context
The steady-state kinetic data described above suggest that Kf recognizes dDMO-d5SICS better than dMMO2-d5SICS or the other derivatized unnatural base pairs. Because the practical utility of an unnatural base pair depends on its sequence-independent replication, we examined replication of dDMO-d5SICS in a second sequence context, hereafter referred to as sequence context II. In this context the unnatural nucleotide is positioned in the template between a 3′-dG and a 5′-dT (Tables 1 and 2), as opposed to between a 3′-dT and a 5′-dG as in the context examined above, hereafter referred to a context I.
For comparison, Kf inserts dMMO2TP opposite d5SICS in sequence context II with the same efficiency as in context I (~4 × 105 M−1min−1).[9] We found that sequence context has a slightly larger effect on dDMOTP insertion, with a 3-fold lower efficiency in context II than in context I (Table 1). Thus, while dDMOTP is inserted opposite d5SICS in context I better than dMMO2TP, the two triphosphates are inserted with the same efficiency in context II. Sequence context also has a larger effect on the synthesis of d5SICS:dDMO than on that of d5SICS:dMMO2, in this case the efficiency of synthesis is increased more than 6-fold, to the remarkable efficiency of 9.7 × 107 M−1min−1, which is the most efficient rate for the synthesis of any unnatural base pair identified to date. In fact this efficiency is only marginally less than that for a natural base pair in the same sequence context. While the efficiencies of mispairing with dDMO (i.e. self pair formation) and dA are also increased, they remain more than two-orders of magnitude less efficient, and the mispairs resulting from dCTP or dGTP insertion remain undetectable.
The effect of sequence context on the Kf-mediated extension of dDMO-d5SICS was also examined. For comparison, Kf extends dMMO2:d5SICS in context II approximately 6-fold less efficiently than in context I.[9] However, it generally extends each mispair with lower efficiency, as well. We find that dDMO:d5SICS is also extended 5-fold less efficiently in context II. In contrast, Kf extends d5SICS:dMMO2 in context II 3-fold more efficiently than in context I, while it extends each mispair less efficiently, with the exception of dT:dMMO2, which it extends approximately 3-fold more efficiently.[9] We find that Kf extends the d5SICS:dDMO heteropair with similar efficiencies in both sequence contexts. Similar efficiencies were also observed for the extension of the mispairs with dG, dC, and dT paired opposite dDMO in the template, but surprisingly, extension of the mispair with dA is an order of magnitude less efficient in context II than in context I.
Generality of unnatural base-pair recognition
While derivatization of the nucleobase scaffold commonly results in large effects on the recognition of the nucleotide as a triphosphate, modifications to the templating nucleotide are typically less perturbative.[9,10] Thus, it is surprising that Kf recognizes dFMO in the template so poorly, relative to dMMO2 or dTMO, both during unnatural base pair synthesis and extension. To determine whether this observation is specific for Kf, or whether it is inherent to the unnatural base pair itself, we characterized the ability of another A family polymerase, Taq, as well as a more diverged B family polymerase, exonuclease-negative Vent, to insert d5SICSTP opposite dMMO2, dTMO, or dFMO (Table 3). We found that Taq and Vent insert d5SICSTP opposite dMMO2 with an efficiency of 3.5 × 106 and 9.9 × 106 M−1min−1, respectively.[13] These two polymerases insert the same triphosphate opposite dTMO in the template with similar efficiencies of ~6 × 106 M−1min−1. However, just as observed with Kf, the efficiency of d5SICSTP insertion opposite dFMO by either Taq or Vent is greatly reduced relative to insertion opposite either dMMO2 or dTMO. Thus, for all three polymerases, d5SICSTP is inserted opposite dMMO2 and dTMO with similar efficiencies, but it is inserted opposite dFMO with an efficiency that is approximately two-orders of magnitude reduced. These results suggest that the factors disfavoring dFMO recognition are inherent to the unnatural base pair.
Table 3.
Second order rate constant for incorporation of d5SICSTP against X in the template by different polymerases.[a]
PCR amplification of DNA containing the unnatural base pairs
We recently showed that DNA containing dMMO2-d5SICS or dNaM-d5SICS in a variety of sequence contexts is PCR amplified with good efficiency and fidelity using multiple thermostable polymerases, including exonuclease-positive Deep Vent.[15] To further characterize the effects of the major groove modifications, the Deep Vent-mediated PCR amplification of DNA containing dDMO-d5SICS, dTMO-d5SICS, or dFMO-d5SICS was characterized to determine the amplification efficiency (fold-amplification of strand) and fidelity (percentage of strands that retain the unnatural base pair per doubling) (Table 4 and Figure S2; templates range in size from 134 to 149 nucleotides). As predicted by the steady-state data, dDMO-d5SICS is amplified with highest efficiency and fidelity, followed by dTMO-d5SICS, and then dFMO-d5ICS which is replicated with lower efficiency and fidelity than is dMMO2-d5SICS. With template D1, where the unnatural base pair is flanked by a natural dG-dC and dA-dT, dDMO-d5SICS is amplified with virtually natural like efficiency and fidelity. To examine the sequence dependence of amplification, dDMO-d5SICS was further characterized with templates D2-D6 (see Supporting Information for full sequences). As expected, both efficiency and fidelity decreased slightly with increasing dG-dC content of the flanking DNA, as it does with natural sequences,[25,26] but the fact that it remained high in the randomized sequence context of duplex D6 suggests that the efficiencies and fidelities are generally reasonable in different sequence contexts.
Table 4.
PCR efficiency and fidelity.[a]
| Template | Base pair amplified | Amplification | Fidelity[b] |
|---|---|---|---|
| D1[c] | dMMO2-d5SICS | 224 | 99.4 |
| D1 | dDMO-d5SICS | 397 | 99.8 |
| D1 | dTMO-d5SICS | 364 | 99.1 |
| D1 | dFMO-d5SICS | 121 | 91.9 |
| D7[c] | dA-dT | 556 | - |
| D2 | dDMO-d5SICS | 69 | 95.7 |
| D3 | dDMO-d5SICS | 130 | 97.9 |
| D4 | dDMO-d5SICS | 78 | 90.7 |
| D5[c] | dMMO2-d5SICS | 25 | 97.1 |
| D5 | dDMO-d5SICS | 12 | 94.4 |
| D6[c] | dMMO2-d5SICS | 52 | 92.9 |
| D6 | dDMO-d5SICS | 109 | 97.6 |
Conditions: 1 ng of the DNA template; dNTPs/dXTPs = 600/400 μM, 6 mM MgSO4, 0.03 U/μL of the enzyme, 8 min extension, 14 cycles.
Calculated from sequencing data as described in Supporting Information.
Taken from reference [15].
Structures of the unnatural base pairs
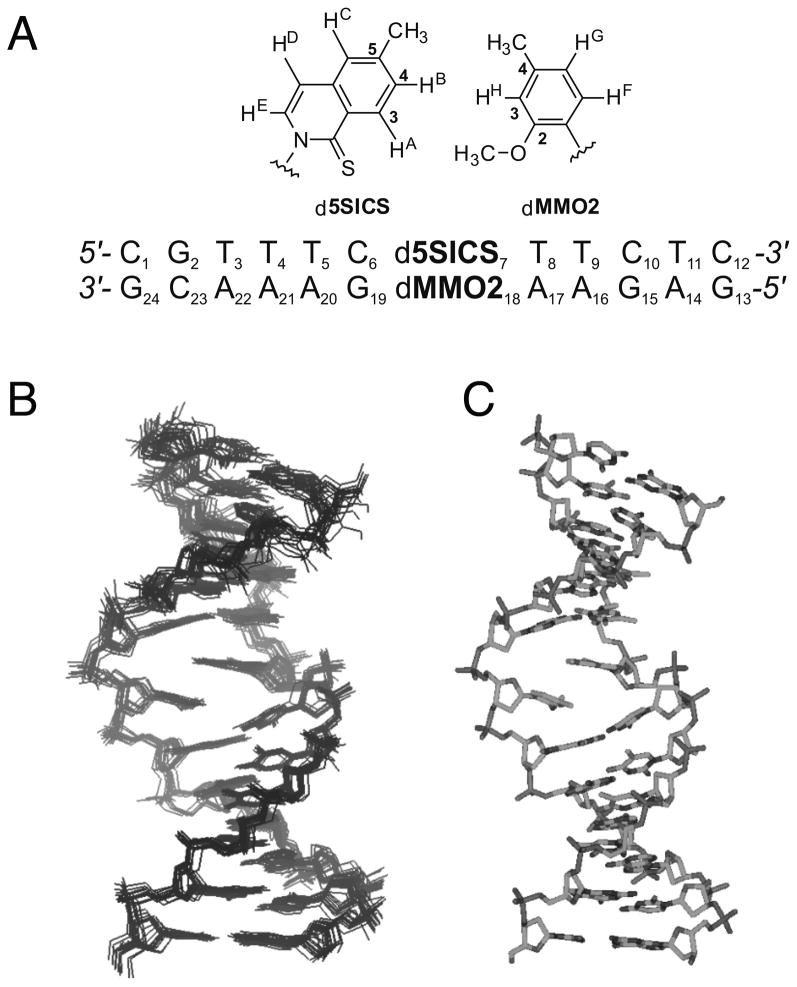
To help elucidate the factors underlying unnatural base pair recognition, we determined the NMR structure of a 12-mer duplex containing d5SICS and dMMO2 at the complementary positions 7 and 18 within the duplex (Figure 3a). Resonance assignments for the duplex followed conventional NOESY based methods.[27] The NOESY and DQF-COSY spectra suggest that the unnatural base pair adopts a single, well defined structure, with only small distortions localized to the region of the unnatural base pair. Following standard protocols,[28] a family of 15 structures were generated and used to generate an average structure (Figure 3b and c). In the average structure, both nucleobases of the unnatural base pair are positioned within the interior of a B-form duplex. Key cross-peaks in the NOESY spectra that support this conclusion include: d5SICS7 HD to dC6 H5, dC6 H6, and dC6 C1′H; dMMO218 CH3 to dG19 H8; d5SICS7 CH3 to A17 H8; and dMMO218 CH3/OCH3 to dG10 H8, in addition to cross-strand NOEs between d5SICS7 HB and dMMO218 CH3/OCH3 and HH (see Figures S3 and S4). However, slight distortions of the duplex, relative to a canonical B-form duplex, were apparent at the site occupied by the unnatural base pair. Specifically, relative to a canonical B-form duplex, the C1′-C1′ distance within the unnatural base pair is elongated ~3 Å, and the nucleobases are inclined ~10°, tilted ~30°, and tipped ~5°, with an increase in rise of ~1.5 Å. The deoxyribose rings of the d5SICS7-dMMO218 adopt a C2′-endo conformation, with an average sugar pucker (pseudorotation phase angle) of 137° (Figure S6).
Figure 3.
a) Sequence of the duplex characterized by NMR and structure of d5SICS-dMMO2 with atoms labeled. b) Family of structures and c) average structure generated from the NMR data.
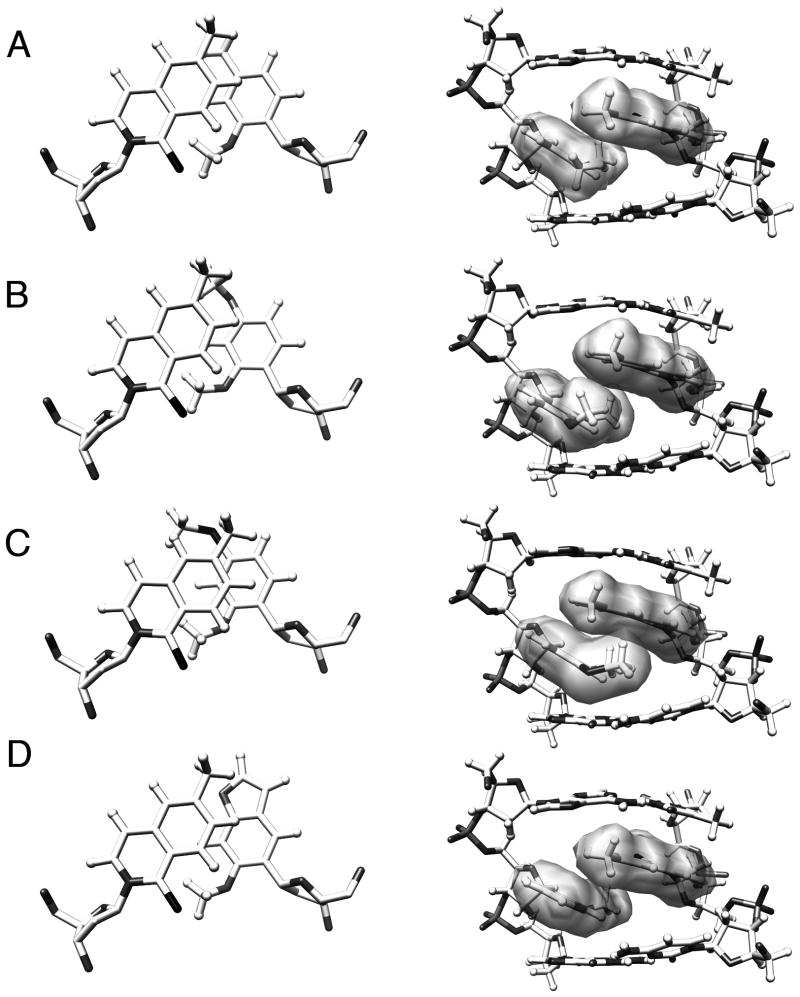
The structure clearly reveals that the unnatural nucleobases pair via partial interstrand intercalation (Figure 4a). While d5SICS7 stacks well with dT8, it is not well packed with dC6, and instead reaches across the duplex and partially intercalates into the opposite strand between dMMO218 and dA17. Correspondingly, the nucleobase moiety of dMMO218 appears to stack rather poorly with both dA17 and dG19, and instead packs with d5SICS7 from the opposite strand. This mode of pairing appears to induce an approximately 5 Å stagger of the d5SICS7 nucleobase relative to that of dMMO218. The ortho sulfur and methoxy groups are oriented into the minor groove of the duplex, as predicted based on the expected anti geometry of the nucleotides, which is confirmed by cross-strand NOEs between dMMO218 CH3/OCH3 and d5SICS7 HB, between d5SICS7 HB and dMMO218 HH, as well as the absence of NOEs from HC, HD and HE of d5SICS7 to any proton of dMMO218. As further support of this nucleotide geometry, the aromatic protons giving rise to sequential NOEs between aromatic and C1′ protons along each strand include d5SICS7 HE and dMMO218 HF. The methoxy group of dMMO218 rotates out of planarity with the aromatic ring to achieve favorable van der Waals contact with the polarizable sulfur group of d5SICS7. This orientation necessarily places the ring substituted methyl groups of d5SICS7 and dMMO218 in close contact in the major groove. The sum of these interactions provides favorable hydrophobic packing but drives the nucleobase of dMMO218 out of planarity with dT17 and dG19. Within the d5SICS7-dMMO218 pair, the aromatic rings are oriented such that C4 of d5SICS7 is positioned nearly directly over C3 of dMMO218 (Figure 4a).
Figure 4.
Structure of unnatural base pairs viewed along helix axis (left, with sugar hydrogens omitted for clarity) or from the major groove (right). a) The NMR structure of the dMMO2-d5SICS base pair, and b – d) the models generated from the parental base pair for dDMO-d5SICS, dTMO-d5SICS, and dFMO-d5SICS, respectively. A color version is provided in Supporting Information.
We next used the structure of d5SICS7-dMMO218 as a starting point to model the structures of the derivative base pairs in the same 12-mer duplex. Suitable parameters for the derivative nucleotides (dDMO, dTMO, and dFMO) were generated using DFT calculations (B3LYP/6-31G*),[29] and then dMMO218 was replaced and the resulting duplex was subjected to unconstrained minimization for up to 5000 steps in the Sander module of AMBER,[30] until the energy converged (Figure 4b – d). Like the parental unnatural base pair, each derivative base pair shows a similar level of interstrand intercalation. While the increased major groove bulk of dFMO18 appears to introduce some additional local perturbations, none of the structures predict significant distortions relative to the structure of DNA containing the parental base pair. The minor groove interactions between the methoxy and sulfur groups are conserved in all of the structures. While the overall structure of the base pairs in the major groove is also conserved, with the para-substituent of the dMMO2 analog stacking against the methyl/aromatic portion of d5SICS7, the models reveal differences in the stacking interactions that result from derivatization. The para-methoxy group of dDMO18 appears to rotate so that the methyl group packs against the methyl group of d5SICS7 and the oxygen lone pairs are oriented into the major groove. The increased size and hydrophobicity of the sulfur substituent of dTMO appears to preclude packing of the methyl group with d5SICS7, and instead the sulfur atom packs with d5SICS7 and the hydrophobic methyl group is oriented into the major groove. In contrast to dDMO, the cyclic aryl-ether bond of dFMO is unable to rotate and thus the lone pairs of the oxygen atom are forced toward the methyl group of d5SICS7. Furthermore, packing with the flanking dC6-dG19 pair isolates this oxygen and precludes it from potentially engaging in stabilizing interactions with water or metal ions within the major groove.
Discussion
The effort to expand the genetic alphabet is predicated on the availability of an unnatural base pair that is well replicated and transcribed, and preferably also suitable for modification such that it may be used to enzymatically produce site-specifically modified DNA and/or RNA. The data reveal that dDMO-d5SICS is better replicated by Kf than is the parental base pair, dMMO2-d5SICS. In the steady-state experiments, dMMO2TP insertion opposite d5SICS limits replication, and the ortho methoxy group of dDMO increases the rate of this step, at least in sequence context I. In the opposite strand context, where increases in efficiency are less critical (as it is already very efficient), d5SICSTP is inserted opposite dDMO slightly less efficiently than it is opposite dMMO2 in sequence context I, but slightly faster in context II. In fact, the insertion of d5SICSTP opposite dDMO in context II is the most efficient reported for an unnatural base pair. Moreover, in both sequence contexts examined, extension of dDMO-d5SICS is more efficient than that of dMMO2-d5SICS by approximately a factor of four, except in the case of the extension with d5SICS in the primer in sequence context II, where both unnatural base pairs were extended with similar efficiencies. In addition, no mispairs between the unnatural or natural nucleotides and dDMO are synthesized more efficiently than those with dMMO2, and in fact, most are synthesized less efficiently. Finally, the mispairs with dDMO are also generally extended less efficiently than those with dMMO2, except for the mispairs with dA in sequence context I and dC in sequence context II. These individual steps combine to make dDMO-d5SICS replication significantly higher fidelity than dMMO2-d5SICS (Table 5).
Table 5.
Minimum single step and overall replication fidelities.[a]
| Primer[b] (Y) | Template[b] (X) | minimum synthesis fidelity[a] | minimum extension fidelity[a] | minimum replication fidelity[a] | |||
|---|---|---|---|---|---|---|---|
| context I | context II | context I | context II | context I | context II | ||
| d5SICS | dMMO2 | 390 (dMMO2, dA) | 37 (dMMO2, dA) | 0.56 (dC) | 0.85 (dT) | 7100 (dA) | 5900 (dA) |
| dMMO2 | d5SICS | 2.8 (dG) | 2.0 (d5SICS, dG) | 4.8 (dT) | 1.0 (dT) | 130 (dT) | 56 (dT) |
| d5SICS | dDMO | 180 (dA, dDMO) | 120 (dA) | 2 (dC) | 1.2 (dC) | 4300 (dA) | 23000 (dA) |
| dDMO | d5SICS | 13 (dG) | 2.1 (d5SICS, dG) | 20 (dT) | 4.8 (dT) | 2600 (dT) | 280 (dT) |
| d5SICS | dTMO | 32 (dA) | N/A | 8.6 (dC) | N/A | 1100 (dA) | N/A |
| dTMO | d5SICS | 12 (dG) | N/A | 2.5 (dT) | N/A | 300 (dT) | N/A |
| d5SICS | dFMO | 0.69 (dA) | N/A | 1.3 (dA) | N/A | 0.90 (dA) | N/A |
| dFMO | d5SICS | 0.19 (dG) | N/A | 0.14 (dT) | N/A | 0.26 (dT) | N/A |
Minimum synthesis and extension fidelity represent the ratio of second order rate constants for the synthesis or extension of the correct unnatural pair to the most efficiently synthesized mispair (shown in parentheses). Minimum replication fidelity corresponds to the product of the minimum fidelities for synthesis and extension (relative to the most efficiently replicated mispair, shown in parentheses).
The improved recognition of dDMO-d5SICS relative to the other unnatural base pairs, including dMMO2-d5SICS, is also apparent in the PCR data. Importantly, the efficiencies and fidelities of dDMO-d5SICS amplification appear to be sufficient for in vitro applications.[31] For example, dDMO-d5SICS appears to be uniquely suited for the site specific labeling of DNA (and possibly RNA[11]) within a format compatible with PCR (or transcription). Along with analogous modifications of (d)5SICS, this should allow the site-specific modification of DNA and RNA with two different functional groups, which should be useful for variety of in vitro applications, including SELEX with an expanded genetic alphabet,[32] as well as biophysical studies that rely on the modification of DNA with multiple biophysical probes.
The mechanism by which DNA polymerases replicate predominantly hydrophobic unnatural base pairs is of great interest for designing better base pairs, as well as for understanding the range of activities possible with these important enzymes. It has been suggested that shape complementarity is important;[2–4] however, it is critical to define in what context it is manifest (i.e. the mode of pairing). Shape complementarity is usually evoked within a natural, Watson-Crick-like mode of pairing, where two in-plane nucleobases interact in an edge-on manner. Each natural base pair thus adopts a similar shape that is thought to be uniquely well accommodated by DNA polymerases.[2–4] In contrast, the model proposed here (Figure 2) evokes a different mode of base pairing, where instead of interacting edge-to-edge, where little to no stabilization is available, the nucleobases partially interstrand intercalate during base pair synthesis, which is likely driven by their high stacking potential. However, extension of the nascent unnatural base pair requires de-intercalation to position the primer terminus 3′-OH appropriately for continued elongation. While de-intercalation is favored by a stabilizing H-bond between the polymerase and the ortho substituents of the nucleobase analogs,[33–38] the model emphasizes the balance of intercalation propensity that must be possessed by the pairing nucleobases: they must intercalate sufficiently for synthesis, but not so much that extension is inhibited. This model nicely explains a large body of previously reported kinetic[5–10] and structural data.[12]
The solution structure of the parental dMMO2-d5SICS pair, as well as the derivative model structures of the dDMMO-d5SICS, dTMO-d5SICS, and dFMO-d5SICS pairs in duplex DNA supports the intercalative model of replication (Figure 2). The structures clearly reveal that the nucleotides are accommodated within a B-form duplex, adopt anti-orientations about their glycosidic bonds, and importantly, pair in an intercalative manner. The data further reveal that the stacking interface between the nucleobases is comprised of the methyl group and proximal portion of the associated aromatic ring of d5SICS and the para substituent of dMMO2 or a dMMO2 analog. It should be emphasized that the structures suggest that the unnatural nucleobase analogs only partially intercalate, they do not fully insert into the opposite strand due to their size and the constraints imposed by the duplex (nonetheless, we refer to the interaction as intercalation for simplicity). Importantly, it is clear that the various substituents examined are predicted to be positioned within the stacking interface between the unnatural nucleobases, which accounts for their effects on replication. It should also be emphasized that the structural data is based on the analogs embedded within a duplex, and not at a primer terminus bound to a DNA polymerase. However, the fact that at least some of the specific interactions involved in base pair recognition are inherent to the base pair and not dependent on the polymerase supports the interpretation of the structure in terms of replication.
The structural models highlight the importance of how the different substituents affect the partitioning of the unnatural nucleobases between intercalated and de-intercalated states, which appear to be required for synthesis and extension, respectively. In the intercalated state, the major groove substituents form a central part of the nucleobase packing interface, but upon de-intercalation, these substituents are more solvent exposed in a more traditional-like major groove. The models suggest that the more efficient replication of dDMO-d5SICS results from an optimized balance of forces governing the stability of the intercalated and de-intercalated states. Synthesis is likely favored by optimized packing interactions between the major groove methyl groups of dDMO and d5SICS. In addition, the structure adopted by dDMO orients the oxygen lone pairs toward the major groove, where upon deintercalation, they may engage in stabilizing interactions with proximal water molecules and/or metals, thus favoring unnatural base pair extension. While anisole is generally not a strong metal ligand or H-bond acceptor due to electron delocalization, both interactions are favored when the conjugation is disrupted by rotation,[39–42] as is observed in the modeled structure of dDMO-d5SICS. The increased substituent size of dTMO appears to induce subtle structural changes without any significant affect on replication. In contrast, the cyclic structure of dFMO appears to force the oxygen lone pairs directly into the hydrophobic interface between the nucleobases, which is likely destabilizing.[43–45] Moreover, if the furanyl oxygen is solvated as the free triphosphate,[46,47] then this stabilizing solvation will be lost upon insertion without being replaced with any other favorable interactions. Moreover, de-intercalation is expected to force the hydrophobic methines further into the hydrophilic major groove, which is likely further destabilizing. Thus, with the aid of the structural models, the intercalative mechanism nicely explains the relatively large effects of the modifications on unnatural base pair synthesis and extension.
Conclusion
We have identified dDMO-d5SICS as an unnatural base pair that is better replicated than the parental dMMO2-d5SICS pair. In addition structural studies support an intercalative model of replication, as previously proposed based on kinetic data[9] and help to explain the observed effects of the various modifications. The intercalative mode of pairing is likely not limited to the analogs examined in the present work. Indeed, it is similar to that observed in the DNA zipper motif, where alternating natural nucleobases are interdigitated, as opposed to interacting in an edge-on manner.[48–56] Moreover, a similar mode of pairing has been observed previously by our group,[12] as well as by the Leumann group[57] with unnatural nucleotides bearing large aromatic nucleobase analogs. However, in these cases the extended aromatic surface area of the nucleobase analogs likely makes intercalation or extrusion from the duplex the only viable options. The intercalative mode of pairing observed between d5SICS and the dMMO2 derivatives occurs despite their potential in-plane accommodation. It is likely that such an intercalative mode of pairing is common to all analogs that are incapable of engaging in stabilizing edge-on interactions. It is also possible that some mispairs between natural nucleotides may be synthesized in a similar manner. Regardless of its potential contribution to the replication of natural DNA, the elucidation of the intercalative mode of pairing should prove invaluable for the further optimization of the unnatural base pairs as well as for our understanding of the potential substrate repertoires of DNA polymerases in general.
Experimental Section
Synthetic Methods
dFMO and dTMO were synthesized as described in Supporting Information and dDMO was synthesized as described previously.[7] Briefly, the corresponding arylbromides were lithiated and coupled to 3,5-di-(tert-butyldimethylsilyloxy)-2-deoxy-erythropentofuranose (Scheme S1). After deprotection with TBAF, anomeric mixtures of nucleosides were obtained, and the β-anomer was purified by column chromatography. Nucleosides were converted to triphosphates by POCl3 treatment in the presence of proton sponge, followed by reaction with tributylammonium pyrophosphate. Phosphoramidites of FMO and TMO were obtained from the free nucleosides by 5′ DMT protection and reaction with 2-cyanoethyl N,N-diisopropylchlorophosphoramidite. Oligonucleotides were synthesized by standard solid phase synthesis on controlled pore glass supports. Experimental details together with characterization of all nucleosides, phosphoramidites, oligonucleotides, and triphosphates are provided in the Supporting Information.
Kinetic Assays
Primer oligonucleotides were 5′-radiolabeled with T4 polynucleotide kinase (New England Biolabs) and [γ-33P]-ATP (Amersham Biosciences) and annealed to template oligonucleotides by heating to 95 °C followed by slow cooling. Reactions were initiated by adding of 5 μL of 2× dNTP solution to a 5 μL solution containing polymerase (0.15–1.23 nM) and 40 nM primer-template in reaction buffer (see Supporting Information for details). After incubation at 25 °C (Kf) or 50 °C (Taq and Vent) for 3–10 min the reactions were quenched with 20 μl of loading dye (95% formamide, 20 mM EDTA, bromophenol blue, and xylene cyanole), reaction products were resolved by 15% polyacrylamide gel electrophoresis, and gel band intensities corresponding to the extended and unextended primers were quantified by phosphorimaging (Storm Imager, Molecular Dynamics) and Quantity One software (Bio-Rad). Plots of kobs versus triphosphate concentration were fit to a Michaelis-Menten equation using the program Origin (Microcal Software) to determine Vmax and KM. kcat was determined from Vmax by normalizing by the total enzyme concentration. Each reaction was run in triplicate and standard deviations for both KM and kcat were determined (see Tables S1–S4). Representative raw kinetic data are shown in Figure S1.
PCR amplification
DNA duplexes used as templates in PCR amplification reactions were synthesized as described previously.[15] PCR amplification of duplexes D1–D6 (see Table 4 in the main text for details and Supporting Information for sequences) was carried out in 1× ThermoPol reaction buffer (New England Biolabs) with the following modifications: 6.0 mM MgSO4, 0.6 mM each natural dNTP, 0.4 mM each unnatural triphosphate, 1 μM each primer (see Supporting Information for sequences), and 0.03 unit/μL of DeepVent (exo+) in an iCycler Thermal Cycler (Bio-Rad) with a total volume of 25 μL under the following thermal cycling conditions: 94 °C, 30 s; 48 °C, 30 s; 65 °C, 8 min, 14 cycles. Upon completion, PCR products were purified utilizing the PureLink™ PCR purification kit (Invitrogen), quantified by fluorescent dye binding (Quant-iT dsDNA HS Assay kit, Invitrogen) and sequenced on 3730 DNA Analyzer (Applied Biosystems) to determine the fidelity of unnatural base pair replication (see Supporting Information and reference [15] for details).
Structural studies
Lyophilized duplex DNA containing the dMMMO2-d5SICS unnatural base pair was dissolved in buffer containing 10 mM sodium phosphate, pH 7.0, 100 mM NaCl, and 0.1 mM EDTA in 99.99% D2O to a final analyte concentration of 2 mM. All NMR spectra were acquired at 25 °C to resolve as much cross peak overlap as possible on a Varian Inova 500 MHz spectrometer. Proton resonance assignments were made according to established procedures. NOESY spectra with a mixing time of 300 ms were collected with a spectral width of 5913 Hz, 2048 complex points in t2 and 512 t1 increments (zero filled to 2048 on processing); for each t1 value 64 scans were averaged using a recycle delay of 2 s. The approach for computing the structure for the d5SICS-dMMO2 duplex was patterned as described.[28,58] Forcefield parameters for d5SICS and dMMO2 were calculated using Gaussian 98.[29] All energy minimization and restrained molecular dynamics (rMD) calculations were performed with the SANDER module of AMBER 9.[30] A total of 373 constraints were applied (including Watson-Crick hydrogen bonding constraints, 346 NMR-derived distance restraints and torsion restraints for each sugar moiety) during rMD. Structures of duplexes containing d5SICS:dDMO, d5SICS:dTMO, and d5SICS:dFMO shown in Figure 4 were modeled from the NMR determined d5SICS:dMMO2 structure. Briefly, each nucleobase (DMO, TMO, FMO) was subjected to DFT calculations to obtain charge distribution and geometrical parameters. These analogs were used to replace dMMO2 in the NMR structure and each duplex was then minimized (unconstrained) for up to 5000 steps in the Sander module of AMBER,[30] until the energy converged.
Acknowledgments
Funding for this work was provided by the National Institutes of Health (GM060005). The NMR facility at USD was established with a grant from the NSF-MRI program (0417731).
Footnotes
Supporting information for this article is available on the WWW under http://www.chemeurj.org/ or from the author
Contributor Information
Prof. Tammy J. Dwyer, Email: tdwyer@sandiego.edu.
Prof. Floyd E. Romesberg, Email: floyd@scripps.edu.
References
- 1.Watson JD, Crick FHC. Nature. 1953;171:737–738. doi: 10.1038/171737a0. [DOI] [PubMed] [Google Scholar]
- 2.Goodman MF. Proc Natl Acad Sci USA. 1997;94:10493–10495. doi: 10.1073/pnas.94.20.10493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morales JC, Kool ET. Nat Struct Biol. 1998;5:950–954. doi: 10.1038/2925. [DOI] [PubMed] [Google Scholar]
- 4.Krueger AT, Kool ET. Curr Opin Chem Biol. 2007;11:588–594. doi: 10.1016/j.cbpa.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMinn DL, Ogawa AK, Wu Y, Liu J, Schultz PG, Romesberg FE. J Am Chem Soc. 1999;121:11585–11586. [Google Scholar]
- 6.Yu C, Henry AA, Romesberg FE, Schultz PG. Angew Chem Int Ed. 2002;41:3841–3844. doi: 10.1002/1521-3773(20021018)41:20<3841::AID-ANIE3841>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 7.Matsuda S, Leconte AM, Romesberg FE. J Am Chem Soc. 2007;129:5551–5557. doi: 10.1021/ja068282b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leconte AM, Hwang GT, Matsuda S, Capek P, Hari Y, Romesberg FE. J Am Chem Soc. 2008;130:2336–2343. doi: 10.1021/ja078223d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seo YJ, Hwang GT, Ordoukhanian P, Romesberg FE. J Am Chem Soc. 2009;131:3246–3252. doi: 10.1021/ja807853m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seo YJ, Romesberg FE. ChemBioChem. 2009;10:2394–2400. doi: 10.1002/cbic.200900413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seo YJ, Matsuda S, Romesberg FE. J Am Chem Soc. 2009;131:5046–5047. doi: 10.1021/ja9006996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuda S, Fillo JD, Henry AA, Rai P, Wilkens SJ, Dwyer TJ, Geierstanger BH, Wemmer DE, Schultz PG, Spraggon G, Romesberg FE. J Am Chem Soc. 2007;129:10466–10473. doi: 10.1021/ja072276d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang GT, Romesberg FE. J Am Chem Soc. 2008;130:14872–14882. doi: 10.1021/ja803833h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leconte AM, Romesberg FE. In: Prot Engineer. RajBhandary CKaUL., editor. Springer-Verlag; Berlin: 2009. pp. 291–314. [Google Scholar]
- 15.Malyshev DA, Seo YJ, Ordoukhanian P, Romesberg FE. J Am Chem Soc. 2009:14620–14621. doi: 10.1021/ja906186f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitsui T, Kitamura A, Kimoto M, To T, Sato A, Hirao I, Yokoyama S. J Am Chem Soc. 2003;125:5298–5307. doi: 10.1021/ja028806h. [DOI] [PubMed] [Google Scholar]
- 17.Hirao I. Curr Opin Chem Biol. 2006;10:622–627. doi: 10.1016/j.cbpa.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 18.Hirao I, Kimoto M, Mitsui T, Fujiwara T, Kawai R, Sato A, Harada Y, Yokoyama S. Nat Methods. 2006;3:729–735. doi: 10.1038/nmeth915. [DOI] [PubMed] [Google Scholar]
- 19.Hirao I, Mitsui T, Kimoto M, Yokoyama S. J Am Chem Soc. 2007;129:15549–15555. doi: 10.1021/ja073830m. [DOI] [PubMed] [Google Scholar]
- 20.Kimoto M, Kawai R, Mitsui T, Yokoyama S, Hirao I. Nucleic Acids Res. 2009;37:e14. doi: 10.1093/nar/gkn956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiaramonte M, Moore CL, Kincaid K, Kuchta RD. Biochemistry. 2003;42:10472–10481. doi: 10.1021/bi034763l. [DOI] [PubMed] [Google Scholar]
- 22.Kincaid K, Kuchta RD. Nucleic Acids Res. 2006;34 doi: 10.1093/nar/gkl632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Unnatural triphosphate insertion opposite an unnatural base in the template obviously results in the synthesis of the unnatural base pair, thus unnatural triphosphate insertion and unnatural base pair synthesis in a specific primer:template strand context are used interchangeably throughout this manuscript.
- 24.Hocek M, Fojta M. Org Biomol Chem. 2008;6:2233–2241. doi: 10.1039/b803664k. [DOI] [PubMed] [Google Scholar]
- 25.Hansen LL, Justensen J. In: PCR Primer: A Laboratory Manual. Diffenbach CW, Dveksler GS, editors. Cold Spring Harbor Laboratory Press; Woodbury, NY: 2003. pp. 226–235. [Google Scholar]
- 26.Arezi B, Xing W, Sorge JA, Hogrefe HH. Anal Biochem. 2003;321:226–235. doi: 10.1016/s0003-2697(03)00465-2. [DOI] [PubMed] [Google Scholar]
- 27.Hare DR, Wemmer DE, Chou SH, Drobny G, Reid BR. J Mol Biol. 1983;171:319–336. doi: 10.1016/0022-2836(83)90096-7. [DOI] [PubMed] [Google Scholar]
- 28.Smith JA, Gomez-Paloma L, Case DA, Chazin WJ. Magnet Res Chem. 1996;34:S147–S155. [Google Scholar]
- 29.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA, Jr, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Baboul AG, Stefanov BB, Liu G, Liashenko A, Piskorz A, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara AA, Gonzalez CC, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Gonzalez C, Head-Gordon M, Replogle ES, Pople JA. Gaussian 98, Revision A.7. Gaussian, Inc; Pittsburgh, PA: 1998. [Google Scholar]
- 30.Case DA, Darden TA, Cheatham TEI, Simmerling CL, Wang RE, Duke RE, Luo R, Crowley M, Walker RC, Zhang W, Wang B, Hayik S, Roitberg A, Seabra G, Wong KF, Paesani F, Wu X, Brozell SR, Tsui V, Gohlke H, Yang L, Tan C, Mongan J, Hornak V, Cui G, Beroza P, Mathews DH, Schafmeister C, Ross WS, Kollman PA. AMBER 9. University of California; San Francisco: 2006. [Google Scholar]
- 31.Klussmann S. The Aptamer Handbook: Functional Oligonucleotides and Their Applications. Wiley-VCH; Weinheim, Germany: 2006. [Google Scholar]
- 32.Keefe AD, Cload ST. Curr Opin Chem Biol. 2008;12:448–456. doi: 10.1016/j.cbpa.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 33.Morales JC, Kool ET. J Am Chem Soc. 1999;121:2323–2324. doi: 10.1021/ja983502+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morales JC, Kool ET. J Am Chem Soc. 2000;122:1001–1007. doi: 10.1021/ja993464+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y, Waksman G. Protein Sci. 2001;10:1225–1233. doi: 10.1110/ps.250101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spratt TE. Biochemistry. 2001;40:2647–2652. doi: 10.1021/bi002641c. [DOI] [PubMed] [Google Scholar]
- 37.Meyer AS, Blandino M, Spratt TE. J Biol Chem. 2004;279:33043–33046. doi: 10.1074/jbc.C400232200. [DOI] [PubMed] [Google Scholar]
- 38.McCain MD, Meyer AS, Schultz SS, Glekas A, Spratt TE. Biochemistry. 2005;44:5647–5659. doi: 10.1021/bi047460f. [DOI] [PubMed] [Google Scholar]
- 39.Nobeli I, Yeoh SL, Price SL, Taylor R. Chem Phys Lett. 1997;280:196–202. [Google Scholar]
- 40.Reimann B, Buchhold K, Barth HD, Brutschy B, Tarakeshwar P, Kim KS. J Chem Phys. 2002;117:8805–8822. [Google Scholar]
- 41.Becucci M, Pietraperzia G, Pasquini M, Piani G, Zoppi A, Chelli R, Castellucci E, Demtroeder W. J Chem Phys. 2004;120:5601–5607. doi: 10.1063/1.1648635. [DOI] [PubMed] [Google Scholar]
- 42.Ribblett JW, Sinclair WE, Borst DR, Yi JT, Pratt DW. J Phys Chem A. 2006;110:1478–1483. doi: 10.1021/jp052832v. [DOI] [PubMed] [Google Scholar]
- 43.Friedman RA, Honig B. Biophys J. 1995;69:1528–1535. doi: 10.1016/S0006-3495(95)80023-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reid KSC, Lindley PF, Thornton JM. FEBS Lett. 1985;190:290–213. [Google Scholar]
- 45.Egli M, Sarkhel S. Acc Chem Res. 2007;40:197–205. doi: 10.1021/ar068174u. [DOI] [PubMed] [Google Scholar]
- 46.Glidewell C, Zakaria CM, Ferguson G. Acta Crystallogr Sect C-Cryst Struct Comm. 1996;52:1305–1309. [Google Scholar]
- 47.Nakanaga T, Ito F. J Phys Chem A. 1999;103:5440–5445. [Google Scholar]
- 48.Chou SH, Zhu LM, Reid BR. J Mol Biol. 1994;244:259–268. doi: 10.1006/jmbi.1994.1727. [DOI] [PubMed] [Google Scholar]
- 49.Shepard W, Cruse WBT, Fourme R, de la Fortelle E, Prange T. Structure. 1998;6:849–861. doi: 10.1016/s0969-2126(98)00087-2. [DOI] [PubMed] [Google Scholar]
- 50.Chou SH, Tseng YY. J Mol Biol. 1999;285:41–48. doi: 10.1006/jmbi.1998.2318. [DOI] [PubMed] [Google Scholar]
- 51.Spackova NA, Berger I, Sponer J. J Am Chem Soc. 2000;122:7564–7572. [Google Scholar]
- 52.Chou SH, Chin KH. J Mol Biol. 2001;314:139–152. doi: 10.1006/jmbi.2001.5131. [DOI] [PubMed] [Google Scholar]
- 53.Chou SH, Chin KH. J Mol Biol. 2001;312:753–768. doi: 10.1006/jmbi.2001.4962. [DOI] [PubMed] [Google Scholar]
- 54.Chou SH, Chin KH, Wang AHJ. Nucleic Acids Res. 2003;31:2461–2474. doi: 10.1093/nar/gkg367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kondo J, Umeda S-i, Fujita K, Sunami T, Takenaka A. J Synchrot Radiat. 2004;11:117–120. doi: 10.1107/s0909049503023562. [DOI] [PubMed] [Google Scholar]
- 56.Sunami T, Kondo J, Hirao I, Watanabe K, Miura K-i, Takenaka A. Acta Crystallogr Sect D. 2004;60:90–96. doi: 10.1107/s0907444903024703. [DOI] [PubMed] [Google Scholar]
- 57.Johar Z, Zahn A, Leumann CJ, Jaun B. Chem Eur J. 2008;14:1080–1086. doi: 10.1002/chem.200701304. [DOI] [PubMed] [Google Scholar]
- 58.Pfaff DA, Clarke KM, Parr TA, Cole JM, Geierstanger BH, Tahmassebi DC, Dwyer TJ. J Am Chem Soc. 2008;130:4869–4878. doi: 10.1021/ja7103608. [DOI] [PubMed] [Google Scholar]