Abstract
The partial pressure of oxygen constitutes an important factor in the regulation of human erythrocyte physiology, including control of cell volume, membrane structure, and glucose metabolism. Because band 3 is thought to be involved in all three processes, and since hemoglobin (Hb) binding to the cytoplasmic domain of band 3 (cdb3) is strongly oxygen-dependent, the possibility arises that the reversible association of deoxyhemoglobin (deoxyHb) with cdb3 might constitute an O2-dependent sensor that mediates O2-regulated changes in erythrocyte properties. While several lines of evidence support this hypothesis, a major opposing argument lies in the fact that the deoxyHb binding sequence on human cdb3 is not conserved. Moreover, no effect of O2 pressure on Hb-band 3 interactions has ever been demonstrated in another species. To explore whether band 3-Hb interactions might be widely involved in O2-dependent regulation of erythrocyte physiology, we undertook to characterize the effect of O2 on band3-Hb interactions in the mouse. We report here that murine band 3 binds deoxyHb with significantly greater affinity than oxyHb, despite the lack of significant homology within the deoxyHb binding sequence. We further map the deoxyHb binding site on murine band 3 and show that deletion of the site eliminates deoxyHb binding. Finally, we identify mutations in murine cdb3 that either enhance or eliminate its affinity for murine deoxyHb. These data demonstrate that despite a lack of homology in the sequences of both murine band 3 and murine Hb, a strong oxygen-dependent association of the two proteins has been conserved.
Considerable evidence exists to demonstrate that multiple erythrocyte properties are regulated by the partial pressure of oxygen to which the red cells are exposed. Among the functions thought to be controlled by O2 levels are glucose metabolism, cell volume and hydration, and membrane structure (1–5). Erythrocyte glucose consumption occurs primarily via glycolysis in deoxygenated cells, but upon exposure to O2 a considerable fraction of the cell’s glucose is channeled into the pentose phosphate pathway (6–8). The importance of this regulatory switch has been argued to lie in the heightened need for reductants during periods of elevated exposure to O2 to protect the cell against oxidative stress (2, 9). Thus, by activating the pentose phosphate pathway upon erythrocyte oxygenation, the cell is assured of sufficient NADPH for both glutathione reduction and maintenance of Hb in its reduced state.
What is the evidence for band 3-deoxyHb interactions in this regulation? Data from other labs and our own show that the glycolytic enzymes bind avidly to the NH2-terminus of band 3 (10–14). These data also demonstrate that deoxyHb (but not oxyHb) competes avidly for this enzyme binding site on human band 3 (3), and that upon red cell deoxygenation, the vast excess of deoxygenated Hb competitively displaces glycolytic enzymes from the membrane (15–16). Because the catalytic properties of the glycolytic enzymes are significantly altered upon association with band 3 (13, 17–19), reversible displacement of these enzymes by deoxyHb can explain the O2-dependent switch in red cell metabolism. However, as noted above, the lack of homology between the deoxyHb binding site on human and other mammalian band 3 orthologs raises questions about the validity of the proposed regulatory mechanism.
Evidence for the role of band 3-deoxyHb interactions in regulation of red cell volume/hydration by oxygen pressure is also mounting. To facilitate volume modulation during transit through regions of hypotonic/hypertonic stress, erythrocytes are equipped with an array of cotransporters that can reverse either cell swelling or cell shrinkage upon activation (20–22). Importantly, the K+/Cl− cotransporter (KCC) in human erythrocytes increases in activity ~20-fold during erythrocyte oxygenation (23). Moreover, this O2-dependent regulation occurs only in whole cells and Hb-containing ghosts, but not in white ghosts or whole cells treated with CO to block O2 binding (24–25). Together with data showing a sigmoidal dependence of K+/Cl− cotransport on O2 pressure (i.e. similar to the sigmoidal dependence of Hb saturation on O2 pressure), the results suggest that Hb must participate in the O2-dependent switch in KCC activity (22, 26). Because band 3 constitutes the only established binding site for deoxyHb on the membrane (3), participation of band 3 in the O2-triggered KCC regulation has frequently been proposed (21–22, 27). Similarly, an O2-dependent change in sickle cell cation transport (termed Psickle) has been observed, as have O2-triggered changes in the activities of the Na+/K+/2Cl− cotransporter and the Na+/H+ antiporter (28–30). However, once again, the absence of homology in the critical band 3-deoxyHb binding site casts doubt on the universality of the participation of band 3 in the proposed mechanism.
Finally, evidence is emerging that human erythrocytes might also modulate their membrane structural properties in response to changes in O2 tension. During their ~120 day lifespan, red blood cells continuously squeeze through capillaries or sinusoids which are less than half their cell diameters. Their ability to recover their biconcave shape following exit from these depends at least in part on interactions between the plasma membrane and its underlying spectrin-based membrane skeleton (31). Importantly, band 3 constitutes a major anchor for the spectrin skeleton on the membrane and ankyrin performs the major bridging function that connects band 3 to spectrin (32). Since the band 3-ankyrin interaction has recently been shown to be O2-sensitive (Stefanovic M. and Low P. unpublished data), the possibility arises that displacement of ankyrin from band 3 by deoxyHb might serve as an O2 switch that can confer O2 sensitivity on red cell rheology.
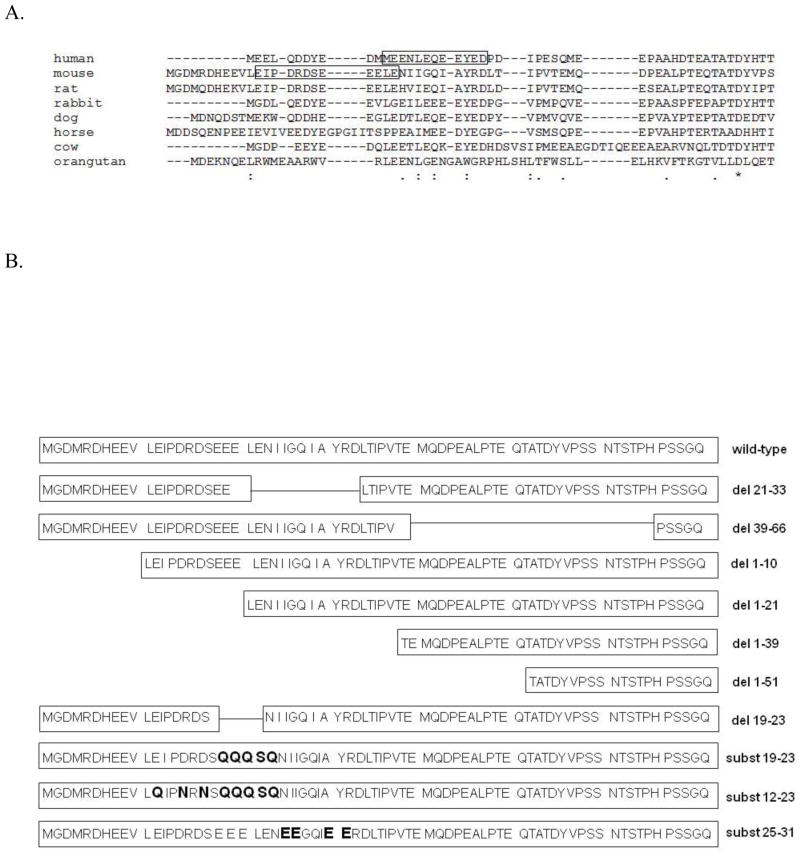
Figure 1A shows the sequence on human band 3 that was previously demonstrated to bind human deoxyHb (boxed residues). Not only does deletion of this sequence abrogate deoxyHb binding to human band 3, but mutations on either side of this sequence have also been found to elevate the affinity of deoxyHb for human band 3 so dramatically that deoxyHb remains bound even at supraphysiological O2 pressures (3). Thus, the deoxyHb binding site appears to enable the reversible O2-dependent association of band 3 and deoxyHb precisely over the physiological range of O2 pressures. To explore whether this O2-dependent binding function might have been maintained in other mammalian species despite obvious differences in primary structure, we have examined whether murine band 3 might display preferential affinity for murine oxyHb or deoxyHb. We have also mutated many of the residues within the NH2-terminus of murine band 3 to determine whether murine Hb affinity might be affected. We report here that a nonhomologous sequence near the NH2-terminus of murine band 3 selectively binds murine deoxyHb and that mutations within and adjacent to this sequence generate band 3 mutants with either high or very low affinity for mouse deoxyHb.
Figure 1.
A) Alignment of the NH2-terminus of band 3 from different mammalian erythrocytes. The experimentally established deoxyhemoglobin binding sites for human and murine band 3 are framed in boxes. The sequence alignment was performed using the CLUSTAL W program (http://workbench.sdsc.edu/). Asterisks (*) indicate complete sequence conservation; (:) - conservation of strong groups; (.) - conservation of weak groups. B) Description of the mutant cytoplasmic domains of band 3 used in this study. For simplicity, only the first 70 amino acids of the 398 residue mouse cdb3 are shown. Deletions are denoted as (del) and substitutions as (subst). The substituted residues are printed in bold letters.
EXPERIMENTAL PROCEDURES
Materials
Protease inhibitors were purchased from Research Products International Corp. All protein concentration steps were performed by ultrafiltration using Vivaspin tubes from GE Healthcare Life Sciences. Dialysis was performed with dialysis bags from Spectrum Laboratories Inc. Protein concentration was measured with MicroBCA protein assay from Thermo Scientific. All others materials and reagents were purchased from SIGMA Aldrich.
GFP fusion constructs
One assay for measuring the affinity of murine deoxyHb for murine band 3 involved preparation of a fusion protein between the cytoplasmic domain of band 3 (cdb3) and GFP, and measuring the quenching of this fusion construct upon its association with Hb. The GFP fusion vector employed was a generous gift from Dr. David Thompson, Purdue University. GFP, containing a histidine tag (His8) tag attached to its own COOH-terminus was fused to the COOH-terminus of wild type and mutant cdb3 proteins. Murine cdb3 DNA (encoding amino acids 1 to 398) was PCR-amplified using a forward primer containing an NdeI cleavage site followed by a start codon (5′-cat atg ggg gac atg cgg gac cac-3′) and kidney cdb3 (lacking first 79 amino acids) using this forward primer 5′-gcg cat atg gac cag agg aac cag-3′; the reverse primer was: 5′-ctc gag aaa gat ccg gcc tgt gcg-3′. Because the GFP gene has an additional NdeI cleavage site in its sequence, we mutated this site with conservative substitutions, yielding a final construct containing only the single NdeI site in the multiple cloning site. The wild type and kidney murine cdb3 sequences were fused to the NH2-terminus of GFP proteins between the NdeI and XhoI enzymatic sites. The amplified DNA product was first ligated into a pGEM-T easy vector (Promega) and then excised with NdeI and XhoI from the pGEM-T. After ligating the product into the GFP-fusion vector, the final construct was sequenced to assure the absence of unwanted mutations.
Wild-type murine cdb3 cDNA lacking GFP and His tags was prepared by PCR amplification using the same parent cDNA described above and inserted in pGEM-T vector. After digestion with NdeI and HindIII, the sequence was inserted into a pT-7.7 vector for protein expression.
Site-directed mutagenesis. All mutated proteins were prepared using a QuickChange® mutagenesis kit (Stratagene) following the manufacturer’s instructions. The sense primers used in this study were as follows: for deletion of residues 1-51 (del 1-51): 5′-gga gat ata cat atg aca gcc aca gac tac gtc-3′; for deletion of residues 1-38 (del1-38): 5′-ggg tcc tgc atc tcg gtc ata tgt ata tct cc-3′; for deletion of residues 1-21 (del 1-21): 5′-gga gat ata cat atg ctg gag aac ata ata gga cag ata gc-3′; for deletion of residues 1-10: 5′-gat ata cat atg ctg gag atc ccag-3′; for deletion of residues 19-23: 5′-gat cga gac agc aac ata ata ggac-3′; for deletion of residues 21-33: 5′-cga gac agc gaa gaa cta acc atcc-3′; for deletion of residues 39-66: 5′-cta acc atc cct gt g agc tcc ggt ca-3′; for substitution of residues19-23: 5′-cca gat cga gac agc caa caa caa tcg cag aac ata ata gga cag-3′; for substitution of residues 12-23: 5′-gga agt gct gca gat ccc aaa tcg aaa cag cca aca ac-3′; for substitution of residues 25-31: 5′-gaa ctg gag aac gaa gaa gga cag ata gaa gat aga gac cta acc-3′. The 12-23 substitution mutant was prepared using the 19-23 substitution mutant DNA as the template. All other constructs were made using the wild-type cdb3–GFP fusion DNA as a template. Sequences of the final products were all confirmed by DNA sequencing.
Expression and purification of proteins
GFP fusion proteins were expressed using BL21(DE3)pLysS bacteria strains (Invitrogen) in 2XYT media at 28°C for 2 to 3 hours. The collected pellet was stored overnight at −80°C and then thawed in lysis buffer (20mM NaH2PO4, 500mM NaCl, 15mM imidazole, 1 μM AEBSF, pH 7.5) at room temperature and lysed using a French press (SLM-Aminco). The bacterial lysate was clarified by centrifugation at 17,000xg on a Sorval SS-35 rotor for 30 minutes, and the supernatant was filtered and loaded onto a nickel-affinity column equilibrated in lysis buffer (GE Healthcare Life Sciences). The column was first washed with lysis buffer containing 40mM imidazole and then eluted with the same buffer containing 250mM imidazole.
Proteins lacking GFP fusion constructs and affinity tags were expressed in BL21(DE3)pLysS bacteria (Invitrogen) in 2XYT media at 29°C for 3 to 4 hours. Bacterial pellets were lysed in 20mM Tris, 1mM EDTA, 0.2% Triton X-100, 0.2% β-mercaptoethanol, pH 7, and wild type and mutated cdb3 proteins were purified by anion exchange chromatography and hydrophobic column chromatography, as described previously (3).
Hemoglobin purification and handling
Hemoglobin for all cdb3-GFP binding studies was obtained from erythrocytes of C57BL/6J mice, as described previously (33). Hemoglobin from C57BL/6 mice is highly homogeneous and does not polymerize when stored as other murine hemoglobins do (34). When larger quantities of blood were required for the HEMOX analyses, hemoglobins from C57BL/6J and BALB/cJ were mixed in a 1.2:1 ratio prior to analysis.
For Hb isolation, whole blood was pelleted and the buffy-coat and plasma were removed by repeated washing in PBS (137mM NaCl, 2.7mM KCl, 8.1mM NaH2PO4, and 1.5mM KH2PO4, pH 7.4). The packed red blood cells were stored at 4°C for one week to begin depletion of 2, 3- diphosphoglycerate (2, 3-DPG), after which the cells were lysed in 5 volumes of cold water and spun at 20,000xg. To remove any residual 2,3-DPG, the supernatant was first dialyzed for 12h in 1M NaCl, then 0.1M NaCl, and finally in 10mM BisTris acetate, pH 6.5. Hemoglobin concentration was determined by measuring absorbance at 540nm using an extinction coefficient of 14.17 (35). Deoxygenation was performed by flushing the Hb solution with humidified argon gas until the change in absorption spectrum confirmed deoxygenation of the sample.
Fluorescence resonance energy transfer analysis of deoxyHb affinity for band 3
All fluorescence measurements were obtained on an AMINCO-Bowman luminescence spectrometer. The excitation wavelength for analysis of cdb3-GFP fusion protein solutions was set at 386nm and the emission spectrum was scanned from 470 to 550 nm. Both murine cdb3 mutants (05μM) and hemoglobin (1 μM) were dialyzed against 10mM BisTris acetate buffer, pH 6.5 at room temperature before analysis of binding. Samples were incubated for 5 min at room temperature prior to measurement of the GFP quenching by any bound oxyHb. The emission spectrum of a fully oxygenated solution of cdb3-GFP and Hb was recorded first, after which the solution was deoxygenated and the emission spectrum recorded again. In most cases, reversibility of the process was confirmed by re-oxygenating the sample. In addition, a control sample containing pure GFP (not fused to cdb3), was analyzed to allow subtraction of the inner filter effect using the following formula:
where F is the fluorescence of the GFP fused protein or pure GFP in the presence of deoxyHb.
Equilibrium binding analysis of deoxyHb affinity for band 3
To measure the equilibrium dissociation constant for the interaction of deoxyHb with different mutated forms of cdb3-GFP, increasing concentrations of deoxyHb were incubated for 45 min at room temperature with cdb3-GFP (or one of its mutants) in BisTris acetate buffer, after which the intensity of GFP fluorescence was measured as described above. Fluorescence quenching was then calculated using the above equation on three separate replicates at each deoxyHb concentration (on two different days), and the mean and standard deviations were determined at each deoxyHb concentration. GraphPad Prism software version 4.00 for Windows was then used to fit the plot of fluorescence quenching as a function of deoxyHb concentration to a simple single site noncooperative binding curve and calculate the equilibrium dissociation constant.
Analysis of deoxyHb affinity for band 3 by measurement of oxygen dissociation curves
Preferential binding of deoxyHb over oxyHb to cdb3 can be assessed by measuring the shift in the oxygen dissociation curve of Hb in the presence of cdb3. For this purpose, hemoglobin and wild-type or mutant cdb3 were dialyzed against 10 mM BisTris acetate buffer, pH 6.5, and twenty nmol of Hb were incubated with 10 nmol cdb3 for 1 min at 37°C. 20μl of 20% BSA solution) and 10 μl antifoam solutions (TCS, Southampton, PA) were then added to the cdb3/Hb solution and oxygen-Hb dissociation curves were generated on a HEMOX analyzer (TCS) according to manufacturer’s instructions. The effect of cdb3 on Hb affinity for oxygen is reported as a shift in the P50 value for Hb.
Data analysis
All average results are presented as mean ± standard error. The graphs were made using GraphPad Prism version 4.00 for Windows, GraphPad Software, San Diego California USA, www.graphpad.com.
RESULTS
Murine deoxyHb exhibits higher affinity for murine cdb3 than murine oxyHb
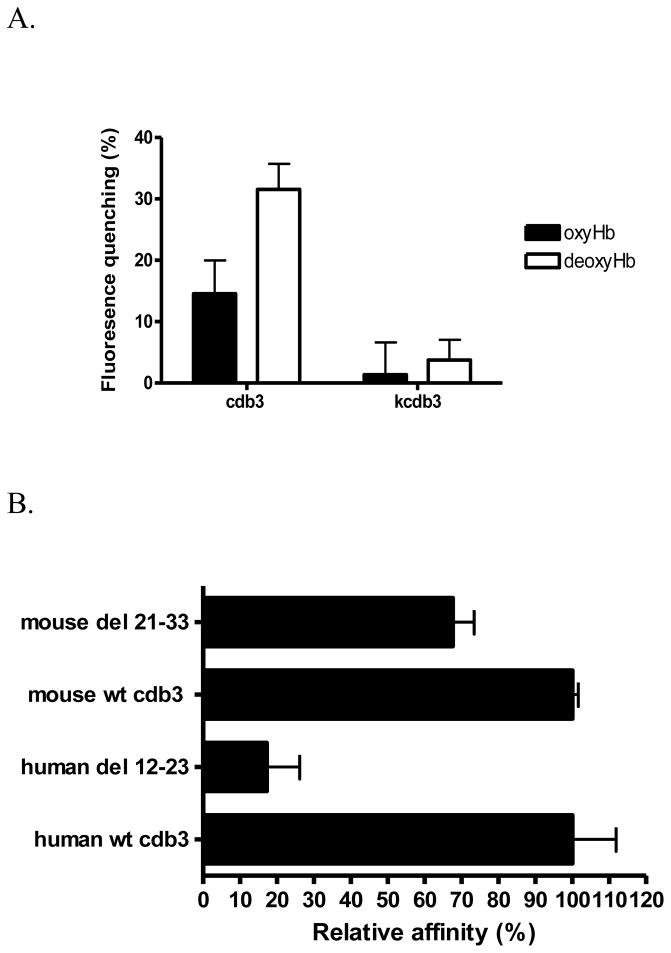
As discussed in the Introduction, modulation of cdb3-hemoglobin interactions by O2 has been proposed to account for oxygen control of several erythrocyte properties, including cell hydration, glucose metabolism and membrane structure (1–3). However, since the sequence on human band 3 responsible for Hb binding (i.e., the NH2-terminus of band 3) is not highly conserved (see Fig. 1A), the question naturally arose whether the deoxyHb-band 3 interaction could constitute the O2 sensor that would mediate O2 effects on erythrocyte physiology across species. To directly test this hypothesis and based on our crystallographic data (36) that showed both termini N- and C-terminus in very close proximity, we chose to generate a fusion protein between the COOH-terminus of murine cdb3 and GFP, and examine the binding of oxyHb and deoxyHb to this fusion construct by quantitating the quenching of GFP fluorescence. As seen in Fig. 2A, murine Hb displays O2-dependent binding to murine cdb3, with deoxyHb exhibiting higher affinity for cdb3 than oxyHb. As a negative control, the kidney spliceoform of murine cdb3 (kcdb3) that lacks the first 79 amino acids including the Hb binding site, displays little affinity for Hb under either oxy- or deoxygenated conditions. These results indicate that O2 tension constitutes a modulator of cdb3-Hb interactions in the mouse despite the lack of significant homology in the region previously shown to bind Hb in the human.
Figure 2.
A) Relative affinity of murine oxy- and deoxyHb (1μM) for murine cdb3 (0.5μM) determined by fluorescence quenching. Because the kidney spliceoform of cdb3 (kcdb3) lacks the entire NH2-terminus (residues 1-79), it was used as a nonbinding (negative) control. B) Effect of deletion from murine cdb3 of the sequence homologous to the deoxyHb binding site on human cdb3 on the affinity of the mutated murine cdb3 for murine deoxyHb. Based on the sequence alignment in panel A, the sequence in murine cdb3 (residues 21-33) found to be most homologous to the deoxyHb binding site on human cdb3 (residues 12-23) was deleted and its relative binding affinity for murine deoxyHb was examined by measuring the shift in the oxygen dissociation curve in the presence of cdb3. For comparison, the relative affinity of human deoxyHb for human cdb3 is also shown.
Deoxyhemoglobin binding site on mouse cdb3 is primarily located within the first 21 residues of the anion transporter
The sequence alignment showed residues 21-33 of murine cdb3 to be most similar to amino acids 12-23 of human cdb3, i.e. the deoxyHb binding site on human cdb3 (see ref. 3, as indicated by the box in Fig. 1A). To test whether residues 21-33 of murine cdb3 indeed comprise the deoxyHb binding site on murine cdb3, these residues were deleted and deoxyHb binding affinity was analyzed by measuring O2 dissociation curve. As seen in Fig. 2B only a small reduction in deoxyHb binding ensued, suggesting that residues 21-33 of murine cdb3 do not contain the major residues involved in Hb binding.
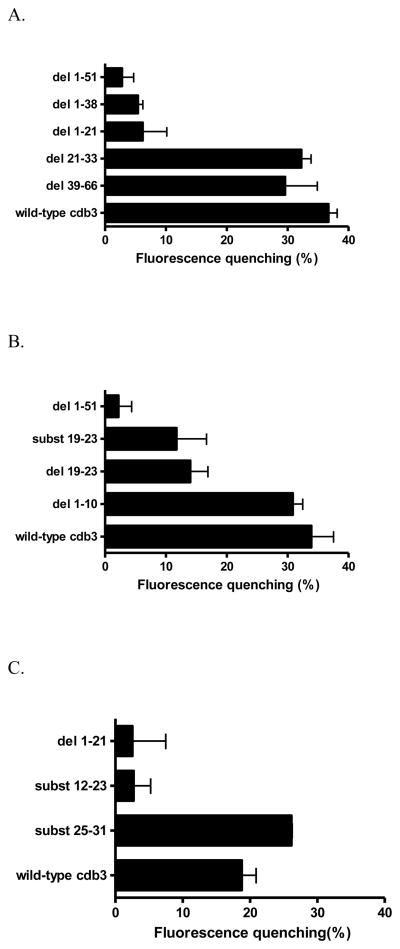
To begin to identify those residues that might be critical for deoxyHb binding in the mouse, we prepared a series of deletion and substitution mutants near the NH2-terminus of murine cdb3 (Fig. 1B) and re-examined each mutant’s affinity for deoxyHb. As seen in Fig. 3A (see also the raw data in Fig. S3), deletion of either amino acids 21-33 or 39-66 exerts little effect on GFP fluorescence, suggesting that residues 21 to 66 do not participate significantly in deoxyHb binding. In contrast, a dramatic decrease in fluorescence quenching is seen upon deletion of any NH2–terminal fragment containing residues 1-21. These data suggest that the murine deoxyHb binding site resides within the first 21 amino acids of the cdb3 polypeptide (Fig. 3A).
Figure 3.
A) Comparison of the relative affinities of different murine cdb3 mutants for murine deoxyHb. For analysis of deoxyHb binding affinity, the COOH-terminus of each cdb3 was fused to GFPuv and the binding of deoxyHb was assayed by quantitating the quenching of GFPuv fluorescence upon deoxyHb binding. In all cases, the inner filter effect due to Hb absorption of GFPuv fluorescence was subtracted (see Methods). As seen, mutant proteins that lack the first 21 amino acids do not bind deoxyHb (i.e. GFP fluorescence is high for del 1-51, del 1-38, an del 1-21), but when these first 21 residues are present (wild-type, del 21-33, del 39-66), binding affinity is high. A 1-51 deletion mutant was used as a negative control, since it exhibits no affinity for deoxyHb. See Fig. 1 for description of the mutations. B) Localization of the deoxyHb binding site on murine cdb3 to residues 12-23. C) Examination of substitution mutations within the binding region comprising residues 12-23 of cdb3.
The deoxyhemoglobin binding site on murine cdb3 resides between amino acids 12 and 23
Based on the fact that cdb3-deoxyHb binding is predominantly electrostatic in nature (14, 37), and since the negatively charged amino acids E19EYED23 were shown to play a critical role in human deoxyHb binding to human cdb3 (3), we next decided to explore whether the murine sequence E19EELE23 might be similarly involved in murine deoxyHb binding. For this purpose, we either deleted the sequence (del19-23) or replaced each acidic residue with its uncharged amide counterpart (subst19-23, Fig. 1B). Moreover, because the first 10 amino acids in murine cdb3 also contain four acidic residues (see Fig. 1B), we also investigated whether deletion of these residues might similarly reduce deoxyHb binding (del 1-10). The results of these studies are presented in Fig. 3B. As shown in the bar graphs, removal or replacement of acidic amino acids between amino acids 19 and 23 (see del19-23, and subst19-23) yields a cdb3 with significantly reduced affinity for deoxyHb, suggesting that negatively charged residues within the 19-23 peptide contribute prominently to deoxyHb binding. Surprisingly, however, deletion of residues 1-10 with their accompanying four acidic residues exerts little effect on deoxyHb affinity (Fig. 3B, del1-10). Taken together, these findings suggest that the binding site of murine deoxyHb on murine cdb3 does not reside within the first 10-mer fragment, but rather is centered in the directly adjacent region comprising residues 19-23 of mouse cdb3.
Because removal or replacement of the four acidic amino acids between residues 19 and 23 resulted in only 60% reduction in deoxyHb binding (Fig. 3B), and since the deoxyHb binding peptide in human band 3 comprised 7–12 amino acids, we elected to determine whether elimination of additional negative charges near the NH2-terminus of cdb3 might further reduce deoxyHb affinity. For this purpose, all 7 acidic amino acids between residues 12 and 23 were converted to their corresponding amides (Fig. 1B, subst12-23), and deoxyHb binding was again examined. As seen in Fig. 3C, removal of the aforementioned additional negative charges further lowered cdb3 affinity for deoxyHb to 15% of normal. These data suggest that the major docking site of murine deoxyHb on murine cdb3 resides primarily between amino acids 12 and 23.
Identification of a mutant murine cdb3 with higher affinity for murine deoxyHb than wild type murine cdb3
The finding that certain mutations in human cdb3 led to a polypeptide with little affinity for deoxyHb, while other mutations generated a band 3 with such high affinity that even supraphysiological pressures of O2 could not promote its dissociation from deoxyHb (3) suggests that the O2 dependence of Hb-cdb3 binding occurs precisely over the physiological range of oxygen pressures. To determine whether murine cdb3 might have a similar sensitivity to physiological O2, we looked for mutations in cdb3 that might elevate its deoxyHb affinity to a level that would prevent deoxyHb dissociation even at supraphysiological O2 pressures. Identification of such a mutation would support the contention that O2 regulation of Hb binding in the mouse was also tuned to occur over physiologically relevant O2 pressures. Initial attempts to generate a murine cdb3 with elevated deoxyHb affinity were guided by the mutations in human cdb3 that produced the undesirable excessive affinity. Surprisingly, deletion of neither of the murine sequences (del1-10, del39-66), that corresponded with the human sequences (i.e. residues 1-10 or 29-52) whose deletion yielded a cdb3 with extraordinarily high affinity for deoxyHb, generated a murine cdb3 with elevated affinity for deoxyHb. However, guided by the fact that the interaction between cdb3 and deoxyHb is electrostatic in nature, we hypothesized that replacement of some of the amino acids in the sequence 25-31 with negative residues might generate a cdb3 with increased affinity for deoxyHb (Fig. 1B, subst25-31). As seen in Fig. 3C, deoxyHb binding to the subst25-31 mutant yielded the strongest fluorescence quenching yet observed with murine proteins, indicating that addition of these charged residues promotes an unusually high affinity for murine deoxyHb. The apparent absence of a similar sequence in nature suggests that higher cdb3-deoxyHb affinity is not functionally desirable.
Confirmation of the deoxyHb binding site on murine cdb3 by analysis of the equilibrium dissociation constant
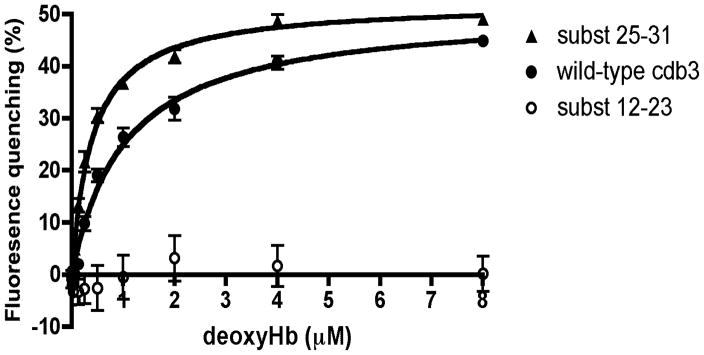
Fluorescence resonance energy transfer is strongly dependent on the distance and orientation between the fluorescence donor and the associated quencher. For example, if the distance between donor and quencher were to decrease as a consequence of a mutation in cdb3, an increase in quenching might be expected even in the absence of a change in affinity. To confirm that alterations in donor to quencher distance and/or orientation did not bias the above data on cdb3-deoxyHb relative affinity, we measured the equilibrium dissociation constants of the three most critical forms of cdb3 for murine deoxyHb: namely, i) wild-type cdb3, ii) the cdb3 mutant with the lowest affinity for deoxyHb (subst 12-23), and iii) the cdb3 mutant with the highest affinity for deoxyHb (subst 25-31). As shown in Figure 4, the cdb3 mutant that previously showed no affinity in the GFP quenching assay also showed no affinity in the equilibrium binding study. Moreover, the mutant that displayed the highest affinity for deoxyhemoglobin in the quenching assay also displayed the highest affinity in the equilibrium binding assay, lowering the equilibrium dissociation constant for deoxyhemoglobin from 1.04 μM (wild type band 3) to 0.39 μM for the mutated band 3. These data confirm that the relative affinities of the three major forms of cdb3 for deoxyHb correspond to their relative extents of quenching upon addition of deoxyHb.
Figure 4.
Evaluation of equilibrium binding affinity of deoxyHb for various isoforms of cdb3. Increasing concentrations of deoxyHb were incubated for 45 min at room temperature with cdb3-GFP or one of its mutants and the equilibrium binding constant was calculated as described in the Methods (n=3). Derived Kd values were 1.04 ± 0.14 μM for wild type cdb3-GFP and 0.39 ± 0.03 μM for the residues 25-31 substitution mutant. The 12-23 substitution mutant displayed no measurable affinity for deoxyHb.
Confirmation of the deoxyHb binding site on murine cdb3 by analysis of Hb-oxygen dissociation curves
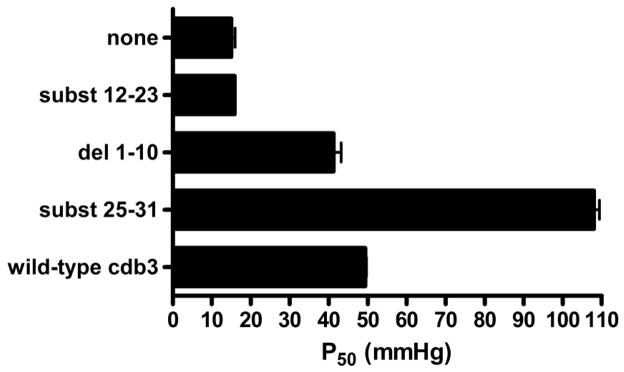
To confirm the above deoxyHb binding site analyses, we determined the binding affinity of our various murine cdb3 mutants using a second and independent method. In this method, the ability of cdb3 to shift the oxygen dissociation curve for Hb to higher O2 pressures is used as a measure of the affinity of cdb3 for deoxyHb (3). Thus, the greater the shift in the P50 values of Hb (i.e. the O2 pressure where Hb is half saturated with oxygen) to higher O2 pressures, the greater the affinity of the mutant cdb3 for deoxyHb (3, 38). In agreement with results from the FRET assay, the low affinity mutant, subst12-23, did not affect the O2 dissociation curve of Hb, but rather displayed the same P50 value as Hb alone (Fig. 5). In contrast, the mutant that exhibited the greatest quenching capacity in the FRET assay also showed the largest right shift of the Hb-O2 dissociation curve, confirming its exaggerated affinity for cdb3. As anticipated from the FRET data, removal of the first 10 amino acids from the NH2-terminus of mouse cdb3 did not induce an elevated affinity for mouse Hb; i.e. in contradistinction to the impact of the same deletion in human cdb3.
Figure 5.
Confirmation of the deoxyHb binding site on murine cdb3 by evaluation of the effect of various NH2-terminal mutants of cdb3 on the Hb-O2 dissociation curve. Hb-O2 binding curves were measured for Hb alone, or Hb in the presence of wild-type cdb3, del(1-10), del(1-21), subst(12-23), or subst(25-31), and the affinity of deoxyHb for cdb3 was determined by analysis of the oxygen pressure where the murine Hb became 50% saturated with O2 (P50).
DISCUSSIONS
Considerable effort has been devoted to characterizing the interaction between human deoxyHb and human band 3 (3, 14, 38–40), even to the point of mapping the amino acids involved in binding (3) and solving the crystal structure of a complex of deoxyHb with the NH2-terminus of band 3 (38). The results collectively suggest that the NH2-terminus of band 3 inserts into the 2, 3-DPG binding cavity in the center of the deoxyHb tetramer, with residues 12-23 of band 3 establishing the major contact with amino acids along the walls of the deoxyHb cleft (41–42). Since exposure to O2 induces a “T” to “R” state transition that closes the central cationic cavity in deoxyHb, oxygenation of Hb leads to ejection of band 3 from the cavity, causing dissociation of oxyHb from the membrane (38). We and others have hypothesized that this reversible association of deoxyHb with band 3 might directly or indirectly (via an induced conformational change in the anion transporter) alter the functional properties of proteins associated with band 3 (1, 21–22). Because deoxyHb competes directly with glycolytic enzymes (19) and ankyrin (Stefanovic M. and Low P. unpublished data) for binding to cdb3, a mechanism whereby changes in O2 might modulate both erythrocyte metabolism and membrane structural properties can be readily envisioned. However, an explanation of how deoxyHb binding could regulate K+/Cl− and Na+/K+/2Cl− cotransport, Na+/H+ antiport, or Psickle is much more difficult to perceive. While a variety of pathways can be proposed, we offer the following hypothesis as one that is supported by some experimental data. We propose that binding of deoxyHb to the cytoplasmic domain of band 3 induces a conformational change in the protein’s membrane-spanning domain and that this conformational change is communicated to associated ion transporters via interfacial interactions. Evidence that significant communication occurs between the cytoplasmic and membrane-spanning domains of band 3 derives from studies showing that: i) association of Hb with cdb3 affects stilbene disulfonate binding to an externally exposed site on the membrane-spanning domain of band 3 (43–44), ii) binding of Hb (45) or ankyrin (46) to cdb3, or phosphorylation of cdb3 (47) alters anion transport through the membrane-spanning domain of the polypeptide, and iii) hemichrome binding to cdb3 induces autologous antibody binding to an external epitope on band 3 (48–50). If the conformational changes that mediate these functional alterations in the membrane-spanning domain of band 3 are also “sensed” by associated ion transporters, O2 regulation of K+/Cl− and Na+/K+/2Cl− cotransport, Na+/H+ antiport, and Psickle might be envisioned.
To evaluate whether the same O2 regulatory mechanism might apply broadly among mammalian species, we explored whether the O2-dependent Hb binding in humans might also occur in mice. Based on the alignment of the NH2-termini of human and mouse cdb3s, the predicted murine Hb binding site for deoxyHb was predicted to reside between residues 21 and 33 (Fig. 1A). However, when this sequence was deleted from murine cdb3, deoxyHb was found to display normal affinity for cdb3. In contrast, when residues 12-23 were mutated, Hb affinity was significantly compromised. Thus, although the binding site of Hb on murine cdb3 differs from that in humans, the ability of physiological changes in O2 pressure to modulate Hb binding has been conserved. This suggests that O2 modulation of red cell properties might constitute an important regulatory mechanism that is required for adequate function of mammalian erythrocytes.
Curiously, mutations in human band 3 that generated a cdb3 with abnormally high affinity for deoxyHb were also not conserved in the mouse. Thus, whereas deletion of residues 1-10 or 29-52 in human cdb3 promoted such affinity for deoxyHb that its dissociation could not be induced even at supraphysiological O2 pressures, mutation of the same residues in the mouse had little effect on deoxyHb binding. In contrast, introduction of negatively charged residues directly adjacent to the Hb binding site on murine cdb3 was found to yield a cdb3 with greatly elevated Hb affinity, as demonstrated by both FRET and oxygen dissociation curve analysis (Fig. 3C and 4, subst25-31). Thus, the murine erythrocyte like the human erythrocyte appears to have a finely tuned O2 regulatory system that responds to changes in O2 pressure precisely over the physiological range of O2 tensions.
Finally, the question naturally arises whether malfunction of this O2-triggered switch in erythrocyte properties might have medical consequences. That is, if a pathological condition or mutation were to cause sustained association of deoxyHb with band 3, would an observable “constitutively on” phenotype emerge? While insufficient information currently exists to provide an unequivocal answer to this question, several observations argue that it may ultimately be answered in the affirmative. Thus, a diversity of mutations in the central cavity of deoxyHb, where acidic amino acids are replaced with neutral or cationic residues, have been reported to lead to an activation in K+/Cl− cotransport (51–52). If the consequent change in cdb3 affinity were to be communicated through the membrane-spanning domain of band 3 to the KCC, the change in K+/Cl− cotransport might be explained. Similarly, patients with mutant hemoglobins characterized by reduced O2 affinities have been reported to have elevated 2,3-DPG levels in their erythrocytes (53). While other explanations are again possible (54), it is also conceivable that increased binding of deoxyHb to cdb3 might lead to the constitutive displacement of glycolytic enzymes from band 3 and the consequent production of elevated 2,3-DPG. Along the same lines, patients with compromised erythrocyte oxygenation due to poor gas exchange in the lungs (e.g. chronic obstructive pulmonary disease (55), cystic fibrosis (56), asthma (57), and chronic cardiac insufficiency (58)) have been frequently reported to have erythrocytes with atypical deformabilities and/or morphologies. While other explanations are again possible, it should not be ignored that deoxyHb displacement of ankyrin from band 3 might contribute to these structural abnormalities. Finally, humans and dogs that have been exposed to high altitude/hypoxia are commonly observed to produce erythrocytes with prolonged filtration times (59–60). Because the slower filtration of their erythrocytes can be reversed with pentoxifylline, a drug that regulates erythrocyte hydration through control of ion transporters (61–62), it is plausible that the altered rheology of these individuals’ red cells might derive from sustained deoxyHb-band 3 interactions leading to an abnormal ion balance.
Regardless of whether any of the above pathologies are impacted by deoxyHb-band 3 interactions, the observation that an O2 regulatory switch that controls these interactions is conserved in anucleate erythrocytes, despite considerable variability in the primary structure of band 3, suggests that the ability to regulate erythrocyte properties with O2 is useful and that deviations from this, if they have accrued, appear to be inconsistent with life.
Supplementary Material
Acknowledgments
The authors thank Dr. Robert Franco and Mary Palascak for the assistance with the HEMOX instrument (Department of Internal Medicine, Division of hematology-oncology, University of Cincinnati, Cincinnati, Ohio). We greatly appreciate the gift of the GFPuv vector from Dr. David Thompson (Purdue University, Department of Chemistry). Also, we really appreciate Dr. Alan Rebar insights on this matter.
The abbreviations used are
- cdb3
cytoplasmic domain of band 3
- Hb
hemoglobin
- deoxyHb
deoxyhemoglobin
- GFP
green fluorescence protein
- KCC
K+/Cl− cotransporter
- AEBSF
4-(2-Aminoethyl) benzenesulfonyl fluoride
- 2,3-DPG
2, 3-diphosphoglycerate
Footnotes
This work was supported by National Institutes of Health grant GM24417-33.
SUPPORTING INFORMATION AVAILABLE. SDS-PAGE and CD analysis show that mouse cdb3 and mutant proteins are pure and folded correctly. Also, the raw spectra of fluorescence quenching by deoxyHb are shown in supplemental figure 3. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Barvitenko NN, Adragna NC, Weber RE. Erythrocyte signal transduction pathways, their oxygenation dependence and functional significance. Cell Physiol Biochem. 2005;15:1–18. doi: 10.1159/000083634. [DOI] [PubMed] [Google Scholar]
- 2.Castagnola M, Messana I, Sanna MT, Giardina B. Oxygen-linked modulation of erythrocyte metabolism: state of the art. Blood Transfus. 2010;8(Suppl 3):s53–58. doi: 10.2450/2010.009S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chu H, Breite A, Ciraolo P, Franco RS, Low PS. Characterization of the deoxyhemoglobin binding site on human erythrocyte band 3: implications for O2 regulation of erythrocyte properties. Blood. 2008;111:932–938. doi: 10.1182/blood-2007-07-100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamasaki N, Asakura T, Minakami S. Effect of oxygen tension on glycolysis in human erythrocytes. J Biochem. 1970;68:157–161. doi: 10.1093/oxfordjournals.jbchem.a129341. [DOI] [PubMed] [Google Scholar]
- 5.Rapoprot I, Berger H, Rapoport SM, Elsner R, Gerber G. Response of the glycolysis of human erythrocytes to the transition from the oxygenated to the deoxygenated state at constant intracellular pH. Biochim Biophys Acta. 1976;428:193–204. doi: 10.1016/0304-4165(76)90120-3. [DOI] [PubMed] [Google Scholar]
- 6.Kinoshita A, Tsukada K, Soga T, Hishiki T, Ueno Y, Nakayama Y, Tomita M, Suematsu M. Roles of hemoglobin Allostery in hypoxia-induced metabolic alterations in erythrocytes: simulation and its verification by metabolome analysis. J Biol Chem. 2007;282:10731–10741. doi: 10.1074/jbc.M610717200. [DOI] [PubMed] [Google Scholar]
- 7.Lewis IA, Campanella ME, Markley JL, Low PS. Role of band 3 in regulating metabolic flux of red blood cells. Proc Natl Acad Sci U S A. 2009;106:18515–18520. doi: 10.1073/pnas.0905999106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Messana I, Orlando M, Cassiano L, Pennacchietti L, Zuppi C, Castagnola M, Giardina B. Human erythrocyte metabolism is modulated by the O2-linked transition of hemoglobin. FEBS Lett. 1996;390:25–28. doi: 10.1016/0014-5793(96)00624-2. [DOI] [PubMed] [Google Scholar]
- 9.Giardina B, Messana I, Scatena R, Castagnola M. The multiple functions of hemoglobin. Crit Rev Biochem Mol Biol. 1995;30:165–196. doi: 10.3109/10409239509085142. [DOI] [PubMed] [Google Scholar]
- 10.Chu H, Low PS. Mapping of glycolytic enzyme-binding sites on human erythrocyte band 3. Biochem J. 2006;400:143–151. doi: 10.1042/BJ20060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higashi T, Richards CS, Uyeda K. The interaction of phosphofructokinase with erythrocyte membranes. J Biol Chem. 1979;254:9542–9550. [PubMed] [Google Scholar]
- 12.Murthy SN, Liu T, Kaul RK, Kohler H, Steck TL. The aldolase-binding site of the human erythrocyte membrane is at the NH2 terminus of band 3. J Biol Chem. 1981;256:11203–11208. [PubMed] [Google Scholar]
- 13.Tsai IH, Murthy SN, Steck TL. Effect of red cell membrane binding on the catalytic activity of glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1982;257:1438–1442. [PubMed] [Google Scholar]
- 14.Low PS. Structure and function of the cytoplasmic domain of band 3: center of erythrocyte membrane-peripheral protein interactions. Biochim Biophys Acta. 1986;864:145–167. doi: 10.1016/0304-4157(86)90009-2. [DOI] [PubMed] [Google Scholar]
- 15.Campanella ME, Chu H, Low PS. Assembly and regulation of a glycolytic enzyme complex on the human erythrocyte membrane. Proc Natl Acad Sci U S A. 2005;102:2402–2407. doi: 10.1073/pnas.0409741102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campanella ME, Chu H, Wandersee NJ, Peters LL, Mohandas N, Gilligan DM, Low PS. Characterization of glycolytic enzyme interactions with murine erythrocyte membranes in wild-type and membrane protein knockout mice. Blood. 2008;112:3900–3906. doi: 10.1182/blood-2008-03-146159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrison ML, Rathinavelu P, Arese P, Geahlen RL, Low PS. Role of band 3 tyrosine phosphorylation in the regulation of erythrocyte glycolysis. J Biol Chem. 1991;266:4106–4111. [PubMed] [Google Scholar]
- 18.Low PS, Allen DP, Zioncheck TF, Chari P, Willardson BM, Geahlen RL, Harrison ML. Tyrosine phosphorylation of band 3 inhibits peripheral protein binding. J Biol Chem. 1987;262:4592–4596. [PubMed] [Google Scholar]
- 19.Low PS, Rathinavelu P, Harrison ML. Regulation of glycolysis via reversible enzyme binding to the membrane protein, band 3. J Biol Chem. 1993;268:14627–14631. [PubMed] [Google Scholar]
- 20.Cossins AR, Gibson JS. Volume-sensitive transport systems and volume homeostasis in vertebrate red blood cells. J Exp Biol. 1997;200:343–352. doi: 10.1242/jeb.200.2.343. [DOI] [PubMed] [Google Scholar]
- 21.Ellory JC, Gibson JS, Stewart GW. Pathophysiology of abnormal cell volume in human red cells. Contrib Nephrol. 1998;123:220–239. doi: 10.1159/000059915. [DOI] [PubMed] [Google Scholar]
- 22.Gibson JS, Cossins AR, Ellory JC. Oxygen-sensitive membrane transporters in vertebrate red cells. J Exp Biol. 2000;203:1395–1407. doi: 10.1242/jeb.203.9.1395. [DOI] [PubMed] [Google Scholar]
- 23.Drew C, Ball V, Robinson H, Clive Ellory J, Gibson JS. Oxygen sensitivity of red cell membrane transporters revisited. Bioelectrochemistry. 2004;62:153–158. doi: 10.1016/j.bioelechem.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Khan AI, Drew C, Ball SE, Ball V, Ellory JC, Gibson JS. Oxygen dependence of K(+)-Cl− cotransport in human red cell ghosts and sickle cells. Bioelectrochemistry. 2004;62:141–146. doi: 10.1016/j.bioelechem.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Muzyamba MC, Speake PF, Gibson JS. Oxidants and regulation of K(+)-Cl(−) cotransport in equine red blood cells. Am J Physiol Cell Physiol. 2000;279:C981–989. doi: 10.1152/ajpcell.2000.279.4.C981. [DOI] [PubMed] [Google Scholar]
- 26.Speake PF, Gibson JS. Urea-stimulated K-Cl cotransport in equine red blood cells. Pflugers Arch. 1997;434:104–112. doi: 10.1007/s004240050369. [DOI] [PubMed] [Google Scholar]
- 27.Gibson JS, Ellory JC. Red cell membrane transport in health and disease. 1. Springer-Verlag; Berlin: 2003. [Google Scholar]
- 28.Gibson JS, Khan A, Speake PF, Ellory JC. O2 dependence of K+ transport in sickle cells: the effect of different cell populations and the substituted benzaldehyde 12C79. Faseb J. 2001;15:823–832. doi: 10.1096/fj.00-0177com. [DOI] [PubMed] [Google Scholar]
- 29.Motais R, Garcia-Romeu F, Borgese F. The control of Na+/H+ exchange by molecular oxygen in trout erythrocytes. A possible role of hemoglobin as a transducer. J Gen Physiol. 1987;90:197–207. doi: 10.1085/jgp.90.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muzyamba MC, Cossins AR, Gibson JS. Regulation of Na+-K+-2Cl− cotransport in turkey red cells: the role of oxygen tension and protein phosphorylation. J Physiol. 1999;517(Pt 2):421–429. doi: 10.1111/j.1469-7793.1999.0421t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bennett V, Gilligan DM. The spectrin-based membrane skeleton and micron-scale organization of the plasma membrane. Annu Rev Cell Biol. 1993;9:27–66. doi: 10.1146/annurev.cb.09.110193.000331. [DOI] [PubMed] [Google Scholar]
- 32.Chang SH, Low PS. Identification of a critical ankyrin-binding loop on the cytoplasmic domain of erythrocyte membrane band 3 by crystal structure analysis and site-directed mutagenesis. J Biol Chem. 2003;278:6879–6884. doi: 10.1074/jbc.M211137200. [DOI] [PubMed] [Google Scholar]
- 33.Jelkmann W, Baufer C. What is the best method to remove 2,3-diphosphoglycerate from hemoglobin? Anal Biochem. 1976;75:382–388. doi: 10.1016/0003-2697(76)90092-0. [DOI] [PubMed] [Google Scholar]
- 34.Riggs A. Hemoglobin Polymerization in Mice. Science. 1965;147:621–623. doi: 10.1126/science.147.3658.621-a. [DOI] [PubMed] [Google Scholar]
- 35.van Kampen EJ, Zijlstra WG. Spectrophotometry of hemoglobin and hemoglobin derivatives. Adv Clin Chem. 1983;23:199–257. doi: 10.1016/s0065-2423(08)60401-1. [DOI] [PubMed] [Google Scholar]
- 36.Zhang D, Kiyatkin A, Bolin JT, Low PS. Crystallographic structure and functional interpretation of the cytoplasmic domain of erythrocyte membrane band 3. Blood. 2000;96:2925–2933. [PubMed] [Google Scholar]
- 37.Chetrite G, Cassoly R. Affinity of hemoglobin for the cytoplasmic fragment of human erythrocyte membrane band 3. Equilibrium measurements at physiological pH using matrix-bound proteins: the effects of ionic strength, deoxygenation and of 2,3-diphosphoglycerate. J Mol Biol. 1985;185:639–644. doi: 10.1016/0022-2836(85)90076-2. [DOI] [PubMed] [Google Scholar]
- 38.Walder JA, Chatterjee R, Steck TL, Low PS, Musso GF, Kaiser ET, Rogers PH, Arnone A. The interaction of hemoglobin with the cytoplasmic domain of band 3 of the human erythrocyte membrane. J Biol Chem. 1984;259:10238–10246. [PubMed] [Google Scholar]
- 39.Cassoly R. Quantitative analysis of the association of human hemoglobin with the cytoplasmic fragment of band 3 protein. J Biol Chem. 1983;258:3859–3864. [PubMed] [Google Scholar]
- 40.Weber RE, Voelter W, Fago A, Echner H, Campanella E, Low PS. Modulation of red cell glycolysis: interactions between vertebrate hemoglobins and cytoplasmic domains of band 3 red cell membrane proteins. Am J Physiol Regul Integr Comp Physiol. 2004;287:R454–464. doi: 10.1152/ajpregu.00060.2004. [DOI] [PubMed] [Google Scholar]
- 41.O’Donnell S, Mandaro R, Schuster TM, Arnone A. X-ray diffraction and solution studies of specifically carbamylated human hemoglobin A. Evidence for the location of a proton- and oxygen-linked chloride binding site at valine 1 alpha. J Biol Chem. 1979;254:12204–12208. [PubMed] [Google Scholar]
- 42.Richard V, Dodson GG, Mauguen Y. Human deoxyhaemoglobin-2,3-diphosphoglycerate complex low-salt structure at 2.5 A resolution. J Mol Biol. 1993;233:270–274. doi: 10.1006/jmbi.1993.1505. [DOI] [PubMed] [Google Scholar]
- 43.Salhany JM, Cassoly R. Kinetics of p-mercuribenzoate binding to sulfhydryl groups on the isolated cytoplasmic fragment of band 3 protein. Effect of hemoglobin binding on the conformation. J Biol Chem. 1989;264:1399–1404. [PubMed] [Google Scholar]
- 44.Salhany JM, Cordes KA, Gaines ED. Light-scattering measurements of hemoglobin binding to the erythrocyte membrane. Evidence for transmembrane effects related to a disulfonic stilbene binding to band 3. Biochemistry. 1980;19:1447–1454. doi: 10.1021/bi00548a028. [DOI] [PubMed] [Google Scholar]
- 45.Ducis I, Kandrach A, Racker E. Stimulation of 32Pi transport into human erythrocyte ghosts and reconstituted vesicles by Mg2+ and hemoglobin. J Biol Chem. 1988;263:8544–8550. [PubMed] [Google Scholar]
- 46.Kay MM, Bosman GJ, Lawrence C. Functional topography of band 3: specific structural alteration linked to functional aberrations in human erythrocytes. Proc Natl Acad Sci U S A. 1988;85:492–496. doi: 10.1073/pnas.85.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bursaux E, Hilly M, Bluze A, Poyart C. Organic phosphates modulate anion self-exchange across the human erythrocyte membrane. Biochim Biophys Acta. 1984;777:253–260. doi: 10.1016/0005-2736(84)90427-9. [DOI] [PubMed] [Google Scholar]
- 48.Baggio B, Bordin L, Clari G, Gambaro G, Moret V. Functional correlation between the Ser/Thr-phosphorylation of band-3 and band-3-mediated transmembrane anion transport in human erythrocytes. Biochim Biophys Acta. 1993;1148:157–160. doi: 10.1016/0005-2736(93)90173-w. [DOI] [PubMed] [Google Scholar]
- 49.Condon MR, Feketova E, Machiedo GW, Deitch EA, Spolarics Z. Augmented erythrocyte band-3 phosphorylation in septic mice. Biochim Biophys Acta. 2007;1772:580–586. doi: 10.1016/j.bbadis.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kannan R, Labotka R, Low PS. Isolation and characterization of the hemichrome-stabilized membrane protein aggregates from sickle erythrocytes. Major site of autologous antibody binding. J Biol Chem. 1988;263:13766–13773. [PubMed] [Google Scholar]
- 51.Nagel RL, Krishnamoorthy R, Fattoum S, Elion J, Genard N, Romero J, Fabry ME. The erythrocyte effects of haemoglobin O(ARAB) Br J Haematol. 1999;107:516–521. doi: 10.1046/j.1365-2141.1999.01755.x. [DOI] [PubMed] [Google Scholar]
- 52.Olivieri O, Vitoux D, Galacteros F, Bachir D, Blouquit Y, Beuzard Y, Brugnara C. Hemoglobin variants and activity of the (K+Cl−) cotransport system in human erythrocytes. Blood. 1992;79:793–797. [PubMed] [Google Scholar]
- 53.Nagel RL. Disorders of hemoglobin: genetics, pathophysiology, and clinical management. 1. Cambridge University Press; 2000. [Google Scholar]
- 54.Clench J, Ferrell RE, Schull WJ. Effect of chronic altitude hypoxia on hematologic and glycolytic parameters. Am J Physiol. 1982;242:R447–451. doi: 10.1152/ajpregu.1982.242.5.R447. [DOI] [PubMed] [Google Scholar]
- 55.Ahmad B, Thieme H, Bloch W, Ferrari N, Raabe-Oetker A, Brixius K. Influence of acute and longtherm physical activity on erythrocyte flexibility in patients suffering from chronic obstructive pulmonary disease (COPD) Acta Physiologica. 2011;201:314. [Google Scholar]
- 56.Novak Z, Gyurkovits K. Examination of red blood cell deformability in cystic fibrosis. Acta Univ Carol Med (Praha) 1990;36:68–70. [PubMed] [Google Scholar]
- 57.Todoriko LD. [Changes in the morphofunctional state of the erythrocyte membranes in bronchial asthma in patients of different ages] Lik Sprava. 1998:51–54. [PubMed] [Google Scholar]
- 58.Romashchenko OV, Kamenev VF, Tret’iakov A, Alferov PK. [Erythrone system in aged patients with chronic cardiac insufficiency] Klin Med (Mosk) 2009;87:21–25. [PubMed] [Google Scholar]
- 59.Chick TW, Scotto P, Icenogle MV, Sikes CW, Doyle MP, Riedel CE, Wood SC, Loeppky JA. Effects of pentoxifylline on pulmonary hemodynamics during acute hypoxia in anesthetized dogs. Am Rev Respir Dis. 1988;137:1099–1103. doi: 10.1164/ajrccm/137.5.1099. [DOI] [PubMed] [Google Scholar]
- 60.Palareti G, Coccheri S, Poggi M, Tricarico MG, Magelli M, Cavazzuti F. Changes in the rheologic properties of blood after a high altitude expedition. Angiology. 1984;35:451–458. doi: 10.1177/000331978403500708. [DOI] [PubMed] [Google Scholar]
- 61.Porsche E, Stefanovich V. [The effect of pentoxifylline on the Ca2+-induced potassium efflux and on the ATPase-activity of erythrocytes (author’s transl)] Arzneimittelforschung. 1981;31:825–828. [PubMed] [Google Scholar]
- 62.Stuart J, Stone PC, Bilto YY, Keidan AJ. Oxpentifylline and cetiedil citrate improve deformability of dehydrated sickle cells. J Clin Pathol. 1987;40:1182–1186. doi: 10.1136/jcp.40.10.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.