Abstract
Context
Interest in the role of fibrates has intensified with the publication of the negative ACCORD trial with fenofibrate, especially since the evidence for clinical outcomes benefit for fibrates is heavily weighted on older fibrates, gemfibrozil and clofibrate.
Objective
This study seeks to examine trends in the current use of fibrates, and for fenofibrate, to illuminate the relationship between differences in the availability of proprietary versus generic formulations and use and economic implications in the United States (US) compared with Canada.
Design/Setting/Patients
Population-level, cohort study using IMS Health data in the United States and Canada of patients prescribed fibrates between 2002 and 2009.
Main Outcome Measure(s)
Fibrate prescribing and expenditures.
Results
From 2002–2009, fibrate prescriptions increased 117.1% in the US, by 12,000/month to 2.1 million prescriptions/month, yet only increased by 18.1% in Canada. (p<0.001) Fenofibrate use was relatively constant in Canada, while in the US, it increased by 159.3%, comprising 47.9% of total fibrate prescriptions in 2002 and 65.2% in 2009. The annual ratio of generic:brand fenofibrate use in the US from 2002 to 2008 ranged from 0:1 to 0.09:1, while the ratio in Canada steadily increased from 2005 to 2008 from 0.51:1 to 1.89:1. In the US, crude fenofibrate expenditures rose from $33.2 million/month in 2002 to $129.6 million/month in 2009, while those in Canada declined from $5.6 million/month to $5.1 million/month. Fibrate expenditures per 100,000 population were 3-fold higher in the US compared with Canada in 2009.
Conclusions
During the past decade, prescriptions for fibrates, particularly, fenofibrate, increased in the United States, while prescriptions for fibrates in Canada remained stable.
Introduction
Health care reform has generated interest in identifying strategies to decrease healthcare costs, without depriving patients of health benefits such as, greater use of evidence-based therapies, including generics.1–3 The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial recently showed that fenofibrate plus statins in patients with type 2 diabetes, did not reduce cardiovascular events more than statins alone.4 The only other fenofibrate study, Fenofibrate Intervention and Event Lowering in Diabetes (FIELD), also failed to show reduced cardiovascular morbidity and mortality.5 These negative studies raise questions about a medication with more than $1 billion in sales in the United States (US).6
Evidence that fibrates have clinical benefit is mixed, with most studies focusing on lipid effects.7,8 Fibrates primarily reduce triglycerides with only modest effects on low- and high-density lipoprotein.7,8 The main evidence for clinical outcomes benefit are placebo-controlled trials with the older fibrates, gemfibrozil, for which some safety concerns were raised, primarily when used with cerivastatin, and clofibrate, which is no longer available due to safety concerns.9–13 These trials exert substantial influence in the meta-analyses that show in aggregate, fibrates significantly reduce cardiac events, but not overall mortality.7,8 The relevance of this older evidence to contemporary practice is uncertain, particularly given that the only trial to assess fibrates in a population taking statins was negative.4 A post-ACCORD meta-analysis subgroup analysis found that individually, fenofibrate did not reduce coronary events versus placebo.8
Little is known about how fibrates are used in practice. Gemfibrozil and fenofibrate are available in the US and Canada, with bezafibrate only available in Canada, and Trilipix® (fenofibric acid), available only in the US.9,10 While generic fenofibrate has long been available in Canada, it has lagged behind in the US, creating market differences.14–15 (e-Figure 1) This study seeks to examine trends in the current use of fibrates, and for fenofibrate, to examine the association between differences in generic product availability and use and economic implications in the United States compared with Canada.
Methods
We conducted a population-level, observational cohort study using IMS Health US and Canada data from 2002 to 2009. This study was approved by the Institutional Review Board of Western University of Health Sciences. The source of prescription data was IMS Health’s CompuScript Audit® in Canada and the National Prescription Audit in the US, which measures through pharmacy audits the number of dispensed prescriptions, and their actual cost to the consumer (which includes product cost, mark-ups and pharmacist fees) in retail pharmacies in Canada and retail, mail order and long-term care pharmacies in the US.16 We had data on numbers and costs of prescriptions but we did not have information on patient or prescriber characteristics. The pharmacy outlet population is stratified by region, type (independent, chain, outlet, etc.) and size of outlet. Sample stores are selected from the reporting stores by applying criteria such as, prescription type and volume, consistency of reporting, and payment type and include approximately two-thirds of pharmacies. Data are collected electronically from the sample comprising drugstores and pharmacy outlets distributed proportionally within each stratum. After passing through various quality control checks and stability processes specific to the audits to ensure consistency and accuracy of the estimates, the collected data are projected to the population in each region and region totals are summed to provide a national estimate (US data rounded to the nearest 1,000 prescriptions nationally).17,18
The monthly number of prescriptions and expenditures for fibrate products in the US and Canada were the primary variables for analysis. We used descriptive statistics to characterize the number of prescriptions and costs of those prescriptions for single-entity and combination product fibrates. We standardized medication use and expenditures per 100,000 population using US and Canada 2001 census estimates.19,20 All expenditures are expressed in US dollars. In order to achieve comparable price differences between countries, Canadian dollar costs were converted to US dollar costs using yearly purchasing power parity values from 2002–2009.21
We calculated rates of use of fibrate prescriptions overall and for each individual fibrate (bezafibrate, fenofibrate, gemfibrozil, fenofibric acid [the active metabolite of fenofibrate]) by country and compared the rates of change from January 2002 through December 2009. Rates of use of fibrates were estimated and compared over time and by country by constructing an ordinary least squares linear regression model using monthly utilization data and time variables by country (R2=0.97 US; 0.09 Canada). The slopes for the rates of change were compared using t-tests. For comparison purposes, the rate of change in overall statin use over the same time period was calculated and compared in the same manner. In addition, using the same methods, the rate of change in fibrate use was compared with statin use within each country as a reference standard. The proportion of each individual fibrate prescription volume and cost compared with the total for the entire fibrate class in each country was calculated annually to determine the market share accounted for by each individual fibrate. The ratios of use of generic to brand fibrate products were compared by country overall, using Wilcoxon W, and by year, using Chi-square statistics. All analyses were performed using SPSS software, version 18.0.3 for Mac. A 2-sided p-value < 0.05 was considered statistically significant. The study was designed and written by the authors.
Results
Overall Fibrate Utilization
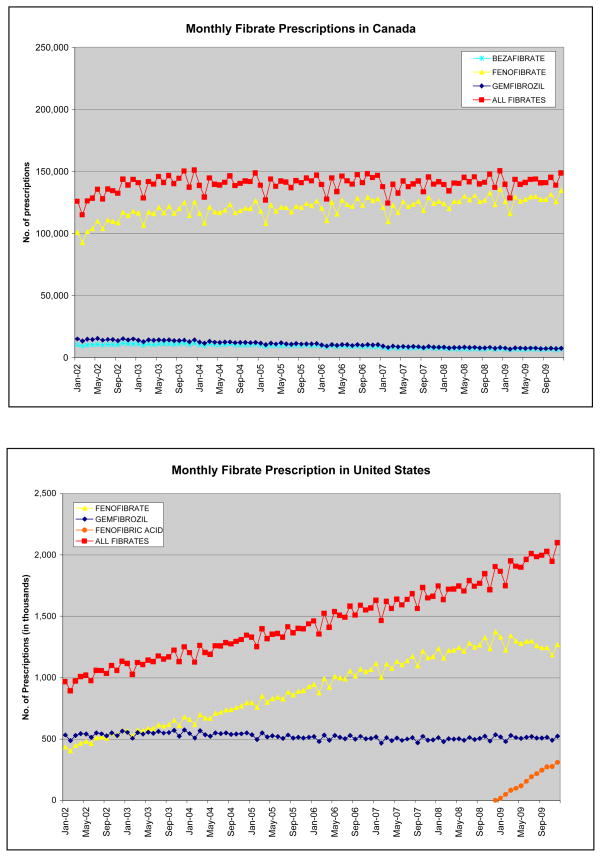
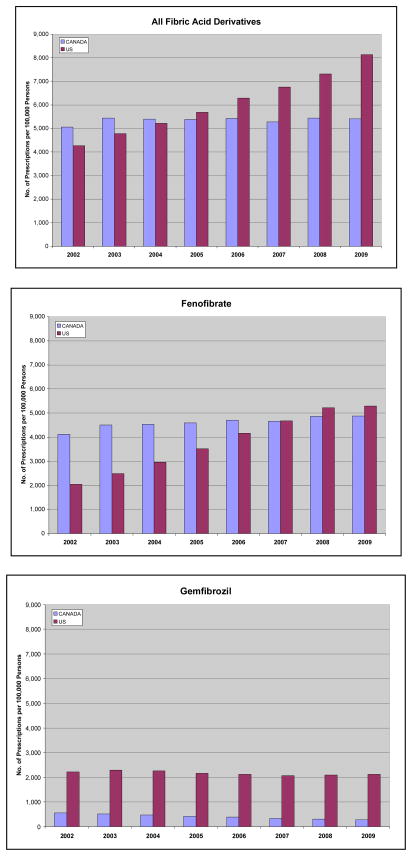
Fibrates accounted for 8.9% of all lipid lowering prescriptions in 2002 and 9.4% in 2009 in the United States, while in Canada, fibrate market share declined from 10.9% in 2002 to 5.3% in 2009. Between 2002 and 2009, fibrate use in the US increased by a mean of 12,000 prescriptions/month to reach 2,102,000 prescriptions in December 2009, an increase of 117.1%. In comparison, statin use increased 71.9% during this period (from 9,762,000 prescriptions per month in January 2002 to 16,781,000 prescriptions per month in December 2009). (p-value) In Canada, between 2002 and 2009, fibrate use increased to a lesser extent by a mean of 240 prescriptions/month to reach 148,849 prescriptions in December 2009, an increase of only 18.1%. (Figure 1) In comparison, statin use increased 164.1% during this period in Canada. (p-value) Fibrate prescriptions increased in both countries over the seven-year period, however, the rate of increase was substantially higher in the US compared with Canada. (p<0.001) Fibrate use overall was similar until 2006, when use in the US began to exceed that in Canada. (Figure 2) In 2002, there were 422 fibrate prescriptions/month/100,000 population dispensed in Canada and 356 in the US, and in December 2009, this increased to 474 and 730 prescriptions, respectively. In 2009, population-adjusted fibrate use in the US exceeded that in Canada by 50.4%. (Figure 2) Conversely, the rate of increase in population-adjusted statin use in the US was less than half that in Canada. (p<0.001)
Figure 1.
Figures 1A and 1B. Crude Number of Fibrate Prescriptions in the US and Canada per Month
NOTE: Y-axis for the United States is 10-fold greater than Canada, reflecting the approximate population differences between countries.
Source: IMS Health-US National Prescription Audit and IMS Health-Canada, Canadian CompuScript Audit®.
Figure 2.
Figures 2A, 2B, 2C. Standardized Annual Fibrate Prescriptions per 100,000 Population by Country
Source: IMS Health-US National Prescription Audit and IMS Health-Canada, Canadian CompuScript Audit®.
Individual Fibrate Product Utilization
During the study period, fenofibrate use in the US increased by a mean of 9,000 prescriptions/month to reach 1,268,000 prescriptions in December 2009, while gemfibrozil use slowly declined with only 524,000 prescriptions dispensed in December 2009. The relative use of individual fibrates in Canada changed minimally over the study period. Since Trilipix® was introduced in the US in December 2008, its use increased by a mean of 26,000 prescriptions/month to 310,000 prescriptions in December 2009. (Figure 1) Population-adjusted fenofibrate use increased in the US between 2002 and 2009. At baseline, there were 343 fenofibrate prescriptions/month/100,000 population dispensed in Canada and only 170 in the US, yet by December 2009, there were 429 and 440 prescriptions/month/100,000 population, respectively, making fenofibrate the predominant fibrate in both countries. The rate of gemfibrozil prescriptions/month/100,000 population dispensed in Canada and the US declined from 46 and 185, respectively at baseline, to 24 and 182 in December 2009, representing minimal decline in the US and greater in Canada. Figure 2 summarizes yearly prescription rates.
Individual Fibrate Product Marketshare
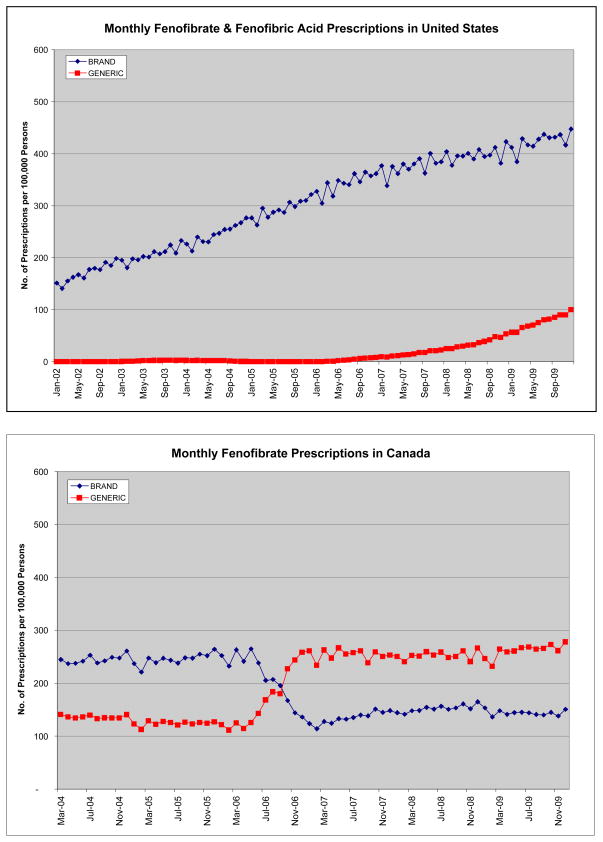
In Canada, fenofibrate use was relatively constant between 2002 and 2009, while in the US, fenofibrate use increased by 159.3%, comprising 47.9% of total fibrate prescriptions in 2002 and 65.2% in 2009. (Table 1; Figure 1) In 2009, fenofibrate and fenofibric acid products combined comprised 73.9% of the market share of fibrates in the US (fenofibric acid unavailable in Canada). While gemfibrozil comprised only 10.9% and 5.2% of fibrate use in Canada in 2002 and 2009, respectively, it comprised 52.1% and 26.1% of the US fibrate market. Bezafibrate (only in Canada), comprised 7.8% of the fibrate market in 2002, decreasing to 4.6% in 2009. The annual ratio of generic:brand fenofibrate in the US from 2002 to 2008 ranging from 0:1 to 0.09:1, demonstrated lower use of generic fenofibrate each year and overall than in Canada where the ratio steadily increased from 2005 to 2008 from 0.51:1 to 1.89:1 (p<0.001 each year; p=0.009 overall). (Figure 3)
Table 1.
Annual Prescription Volume and Expenditures of Fibrates Overall and by Individual Fibrate
| 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| thousands | Proportion of Total (%) |
thousands | Proportion of Total (%) |
thousands | Proportion of Total (%) |
thousands | Proportion of Total (%) |
thousands | Proportion of Total (%) |
thousands | Proportion of Total (%) |
thousands | Proportion of Total (%) |
thousands | Proportion of Total (%) |
|
| Volume of Prescriptions | ||||||||||||||||
| Canada | ||||||||||||||||
| Bezafibrate | 125 | 7.8% | 129 | 7.5% | 123 | 7.3% | 114 | 6.8% | 106 | 6.2% | 91 | 5.5% | 84 | 4.9% | 78 | 4.6% |
| Fenofibrate | 1,291 | 81.2% | 1,416 | 82.9% | 1,423 | 84.1% | 1,441 | 85.4% | 1,477 | 86.7% | 1,464 | 88.3% | 1,526 | 89.5% | 1,529 | 90.2% |
| Gemfibrozil | 173 | 10.9% | 164 | 9.6% | 146 | 8.6% | 132 | 7.8% | 120 | 7.1% | 103 | 6.2% | 95 | 5.6% | 88 | 5.2% |
| Total | 1,589 | 1,709 | 1,692 | 1,687 | 1,703 | 1,657 | 1,705 | 1,695 | ||||||||
| United States | ||||||||||||||||
| Fenofibrate | 5889 | 47.9% | 7,175 | 52.1% | 8,539 | 56.8% | 10,147 | 62.0% | 11,985 | 66.2% | 13,497 | 69.4% | 15,033 | 71.4% | 15,271 | 65.2% |
| Gemfibrozil | 6407 | 52.1% | 6,586 | 47.9% | 6,494 | 43.2% | 6,225 | 38.0% | 6,120 | 33.8% | 5,960 | 30.6% | 6,034 | 28.6% | 6,121 | 26.1% |
| Fenofibric acid | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 2,031 | 8.7% |
| Total | 12,296 | 13,761 | 15,033 | 16,372 | 18,105 | 19,457 | 21,067 | 23,423 | ||||||||
| Expenditures | ||||||||||||||||
| Canada | ||||||||||||||||
| Bezafibrate | $8,474 | 10.2% | $8,901 | 10.0% | $8,649 | 9.8% | $8,189 | 9.3% | $7,459 | 9.0% | $6,304 | 8.6% | $5,620 | 7.9% | $5,358 | 7.7% |
| Fenofibrate | $66,615 | 79.9% | $71,881 | 81.1% | $72,400 | 82.3% | $73,706 | 83.6% | $70,226 | 84.3% | $62,520 | 85.0% | $61,495 | 86.4% | $60,658 | 86.9% |
| Gemfibrozil | $8,230 | 9.9% | $7,893 | 8.9% | $6,916 | 7.9% | $6,232 | 7.1% | $5,620 | 6.7% | $4,767 | 6.5% | $4,097 | 5.8% | $3,822 | 5.5% |
| Total | $83,323 | $88,675 | $87,966 | $88,127 | $83,306 | $73,590 | $71,212 | $69,839 | ||||||||
| United States | ||||||||||||||||
| Fenofibrate | $398,815 | 64.5% | $557,866 | 70.0% | $735,817 | 75.5% | $894,477 | 80.8% | $1,088,418 | 85.2% | $1,279,653 | 87.4% | $1,471,662 | 88.8% | $1,555,005 | 78.7% |
| Gemfibrozil | $219,670 | 35.5% | $239,526 | 30.0% | $239,193 | 24.5% | $212,776 | 19.2% | $188,818 | 14.8% | $184,348 | 12.6% | $186,456 | 11.2% | $190,989 | 9.7% |
| Fenofibric acid | $0 | 0.0% | $1 | 0.0% | $230,740 | 11.7% | ||||||||||
| Total | $618,493 | $797,392 | $975,010 | $1,107,253 | $1,277,236 | $1,464,001 | $1,658,119 | $1,976,734 | ||||||||
Source: IMS Health-US National Prescription Audit and IS Health-Canada, Canadian CompuScript Audit®.
Figure 3.
Figures 3A and 3B. Number of Brand and Generic Fibrate Prescriptions in the US and Canada per Month
Source: IMS Health-US National Prescription Audit and IMS Health-Canada, Canadian CompuScript Audit®.
Overall and Individual Fibrate Expenditures
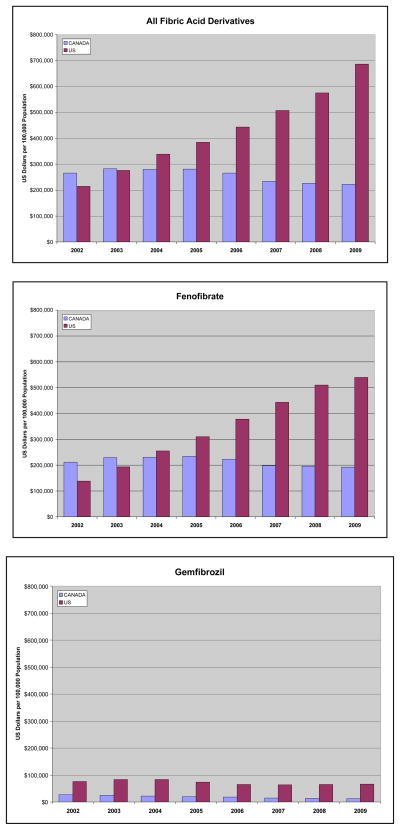
Between 2002 and 2009, the crude costs associated with fibrate use in the US increased from $51,541,000/month in 2002 to $164,728,000/month in 2009, with a notable rise in 2005, while costs in Canada decreased from $6,943,603/month to $5,819,921/month, most notably after 2006. An increase in fenofibrate costs in the US from $33,235,000/month in 2002 to $129,584,000/month in 2009 mirrored its increase in use, while stable use in Canada led to a slight decline from $5,551,247/month to $5,054,869/month, respectively. The decline in gemfibrozil use was paralleled by a decrease in costs to a low of $16,431,000/month in December 2009 in the US, and $321,698 in December 2009 in Canada. (Figure 4) The proportion of total fibrate costs accounted for by fenofibrate rose from 64.5% in 2002 to 78.7% in 2009, while in Canada, the proportion rose from 79.9% to 86.9%. (Table 1) Although fenofibrate accounts for only 65.2% of use in the US, it accounts for a disproportionate 78.7% of expenditures (p<0.001).
Figure 4.
Figures 4A, 4B, 4C. Standardized Annual Fibrate Prescription Expenditures per 100,000 Population by Country
Source: IMS Health-US National Prescription Audit and IMS Health-Canada, Canadian CompuScript Audit®.
Adjusted fibrate expenditures/100,000 population in 2009 were approximately three-fold higher in the US compared with Canada, despite only 50.4% more prescriptions. Despite similar numbers of population-standardized fenofibrate prescriptions, expenditures associated with this use was 2.5-fold higher in the US, with expenditures continuing to diverge through 2009. (Figure 4) For 2009, per-capita expenditure for fibrates was $6.86 in the US and $2.23 in Canada, for fenofibrate/fenofibric acid $6.20 and $1.93, and for gemfibrozil $0.66 and $0.12, respectively.
Discussion
Our study found that the use of fibrates steadily increased in the US in the last decade, but not in Canada, even as evidence emerged to question the benefit of newer fibrates in the contemporary statin era. Increased fibrate use in the US appears to be largely driven by a steady rise in fenofibrate use of nearly 200% over the study period, while in Canada, fenofibrate use remained stable. These rapidly rising rates are over double the increase in statin use in the US over the same period. This pattern is paradoxical to declines that might have been expected, since the only clinical outcomes evidence for fenofibrate during our study period was the FIELD trial, which failed to find a significant reduction in the primary endpoint of coronary events in a diabetes population.6 In fact, more robust outcomes evidence in reducing cardiac death and non-fatal MI supports the preferential use of gemfibrozil, though these studies preceded the statin era and enrolled patients with slightly worse lipid profiles.8,13,22 Prior reports have noted an increasing use of fenofibrate since 1999, over five years before publication of FIELD.23 Our study shows that the use of fenofibrate was increasing both before and after the FIELD study was published, suggesting that other factors besides clinical trial evidence are influencing fibrate prescribing.
While fenofibrate use rose in the US, gemfibrozil use declined. The increased use of fenofibrate in favor of gemfibrozil may be due to its greater perceived safety relative to gemfibrozil.11,12,24 However, fenofibrate use has been steadily increasing since 1999, preceding 2000, when the first pharmacokinetic study signaled a potential statin-gemfibrozil drug interaction, and certainly prior to 2001, when the gemfibrozil-cerivastatin drug interaction became apparent.25,26 Therefore, while this reasoning may account for some to switch or preferentially use fenofibrate over gemfibrozil, the rise in brand name fenofibrate use far exceeded declines in gemfibrozil use. Additionally, subsequent research has demonstrated that gemfibrozil could be used safely in patients receiving statins, such as the Veterans Affairs study that found a rhabdomyolysis rate with combined use was only 0.16%, well within expected rates.11,12,27
Our second major finding was that there was a strong preference observed for prescribing brand over generic fenofibrate products in the US, but not in Canada. The US pattern is unusual in that brand name formulations typically comprise only ~25–30% of product marketshare for medications 12 years post-product launch.28 Instead, brand name fenofibrate (mainly Tricor®) was the predominant fenofibrate used in the US, accounting for 90% of the fenofibrate market share until recently, while in Canada, the comparable Lipidil® brand declined from 66% to 35% of fenofibrate marketshare during the study. To illustrate this disparate pattern between countries, for every 100 brand name fenofibrate prescriptions dispensed in 2008, 166 generic fenofibrate prescriptions were dispensed in Canada, while in the US, only 9 generic fenofibrate prescriptions were dispensed.
Access differences to generic fenofibrate between the US and Canada likely contributed to vastly different patterns of fenofibrate use, and are associated with a great economic burden for US consumers and third-party payers.9 Although both countries had similar rates of population-adjusted fenofibrate use between 2007–2009, US fenofibrate expenditures exceeded those in Canada by nearly three-fold. Using 2008 rates of fenofibrate use, had the US market had open access to generic fenofibrate formulations, and prescribed with a generic:brand ratio similar to that in Canada where access was not limited, we would expect $364 million/year to be saved.
While Canada has benefited from access to generic fenofibrate for over a decade, creative patent protection actions with brand name fenofibrate products in the US appear to have instead allowed brand name fenofibrate products to dominate the market, potentially contributing to escalating fibrate drug costs.29,30 The preferential use of brand name fibrates continues with the latest product, Trilipix®, the active metabolite of fenofibrate, showing a rate of increase in utilization that far exceeds that even for fenofibrate, even though this specific formulation has yet to be evaluated in clinical outcomes studies.6 Trilipix’s® advantageous unique indication approves it for use with statins, while all other fibrates have warnings against combined use with statins.9 Given that this distinctive indication simplifies concomitant fibrate-statin therapy and may therefore facilitate the use of Trilipix® for clinicians, prompt evaluation of its efficacy in reducing cardiovascular morbidity and mortality when added to the current standard of lipid therapy, statins, along with evaluation of its safety is warranted.
During our study, new clinical outcomes evidence should have steered usage away from fenofibrate during the period where there was escalating use of fibrates, particularly fenofibrate. While clinicians may have been reluctant to initially accept the negative findings from the FIELD study in 2004, now in 2010, ACCORD, the only fibrate study to use a statin-treated comparison group, likewise found no clinical outcome benefit with fenofibrate plus a statin compared with a statin alone, reiterating the negative findings from FIELD.4 At a time when a “less-is-more” approach is being embraced by the medical community, this ever-increasing pattern of brand name medication use without evidence of clinical outcomes benefit warrants attention and close scrutiny in order to ensure medication use is optimized for clinical outcomes benefit, while avoiding unwarranted costs.2,31 Current US guidelines recommend that fibrates, without regard to type, should only be considered for reducing very high TG to prevent pancreatitis, for treatment of dysbetalipoproteinemia, and as supplemental therapy to statins for non-HDL cholesterol in diabetics.32,33 The Canadian guidelines, 2006 revision, now more cautiously reserves fibrates primarily for severe hypertriglyceridemia. Continued caution is warranted in guideline recommendations for fibrates as we await evidence of clinical outcomes benefits with fibrates.
Our study has some limitations. IMS Health uses data collected from audits of prescriptions dispensed to describe general trends in drug utilization. These data do not provide exact drug utilization by individual patients or providers to determine the appropriateness of drug use. We did not have access to state-level data, patient or prescriber characteristics or clinical data, such as medical conditions, or lipid profile to determine whether fibrate prescribing was clinically appropriate. Although increased fibrate use was demonstrated, its relationship to patient outcomes could not be evaluated.
Conclusion
Fibrates are used commonly in the US and Canada, with use rising steadily in the US over the last decade, despite negative fibrate trials in patients with diabetes published in the statin era, while use in Canada remained stable. Fenofibrate dominates the market, despite it having the least supportive clinical outcomes evidence. While this growth, in the setting of a strong preference for brand over generic fenofibrate in the US has been associated with escalating medication costs, improvement in clinical outcomes is uncertain.
Supplementary Material
Acknowledgments
We gratefully acknowledge IMS Health-US and IMS Health-Canada for providing the data required for the analyses from the National Prescription Audit and the CompuScript Audit®, respectively. The authors acknowledge Janice Adelman, M.Sc., M.A., Ph.D. of Claremont Graduate University, for assistance (compensated) with data analysis.
Funding sources: This study was funded in part by the College of Pharmacy of Western University of Health Sciences, Pomona, California and in part by a Canadian Institutes of Health Research team grant in cardiovascular outcomes research to the Canadian Cardiovascular Outcomes Research Team, neither of which was involved in the design and conduct of the study; collection, management, analysis or interpretation of the data; and preparation, review or approval of the manuscript. Dr. Tu is supported by a Canada Research Chair in Health Services Research, and by a Career Investigator award of the Heart and Stroke Foundation of Ontario, Toronto, Ontario. The Institute for Clinical Evaluative Sciences is supported by an operating grant from the Ontario Ministry of Health and Long-Term Care, Toronto, Ontario. Dr. Ross is currently supported by the National Institute on Aging (K08 AG032886) and by the American Federation of Aging Research through the Paul B. Beeson Career Development Award Program. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs, the Canadian Institutes of Health Research or the Ontario Ministry of Health and Long-Term Care. Neither the Department of Veterans Affairs nor the Hartford Foundation had any role in the design or conduct of the study; collection, management, analysis or interpretation of the data; preparation, review or approval of the manuscript. Dr. Ko is supported by a Clinician-Scientist award from the Heart and Stroke Foundation of Ontario.
Footnotes
Disclosures
Dr. Krumholz is a member of an advisory board for UnitedHealthCare and is under contract to develop performance measures for the Centers for Medicare and Medicaid Services. The remaining authors have no disclosures to report.
Author access to data: Dr. Jackevicius had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Authors’ Contributions:
Study concept and design: Jackevicius, Krumholz, Tu, Ko, Ross
Acquisition of data: Jackevicius, Krumholz
Analysis and interpretation of data: Jackevicius, Krumholz, Carreon, Tu, Ko, Ross
Drafting of the manuscript: Jackevicius
Critical revision of the manuscript for important intellectualcontent: Jackevicius, Krumholz, Tu, Ross, Ko, Carreon
Statistical analysis: Carreon, Jackevicius
Obtained funding: Jackevicius, Krumholz
Supervision: Jackevicius, Krumholz
References
- 1.Brody H. Medicine’s ethical responsibility for health care reform – the top five list. N Engl J Med. 2010;362:283–5. doi: 10.1056/NEJMp0911423. [DOI] [PubMed] [Google Scholar]
- 2.Redberg RF. Less is more. Arch Intern Med. 2010;170:584. doi: 10.1001/archinternmed.2010.48. [DOI] [PubMed] [Google Scholar]
- 3.Fischer MA, Avorn J. Economic consequences of underuse of generic drugs: evidence from Medicaid and implications for prescription drug benefit plans. Health Serv Res. 2003;38:1051–63. doi: 10.1111/1475-6773.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010 doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849–1861. doi: 10.1016/S0140-6736(05)67667-2. [Erratum, Lancet 2006;368:1415, 1420.] [DOI] [PubMed] [Google Scholar]
- 6.Abbott Sues Lupin, AMD, Apple, Time Warner, Zazzle: Intellectual Property. Bloomberg.com; [Accessed April 20, 2010]. http://www.bloomberg.com/apps/news?pid=newsarchive&sid=a3qi6DC_WPeA. [Google Scholar]
- 7.Abourbih S, Filion KB, Joseph L, et al. Effect of fibrates on lipid profiles and cardiovascular outcomes: a systematic review. Am J Med. 2009;122:962.e1–962.e8. doi: 10.1016/j.amjmed.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 8.Jun M, Foote C, Lv J, et al. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet. 2010 doi: 10.1016/S0140-6736(10)60656-3. [DOI] [PubMed] [Google Scholar]
- 9. [Accessed March 27, 2010];FDA Approved Drug Products. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Search_Drug_Name.
- 10.Health Canada Notice of Compliance. 2010 Mar 27; http://www.hc-sc.gc.ca/dhp-mps/prodpharma/notices-avis/index_e.html.
- 11.Graham DJ, Staffa JA, Shatin D, et al. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA. 2004;292:2585–90. doi: 10.1001/jama.292.21.2585. [DOI] [PubMed] [Google Scholar]
- 12.Jones PH, Davidson MH. Reporting rate of rhabdomyolysis with fenofibrate + statin versus gemfibrozil + any statin. Am J Cardiol. 2005;95:120–122. doi: 10.1016/j.amjcard.2004.08.076. [DOI] [PubMed] [Google Scholar]
- 13.Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. N Engl J Med. 1999;341:410–418. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- 14.Attorney General Cuomo Announces Settlement of Antitrust Suit Against Drug Manufacturers. Office of the Attorney General State of New York; [Accessed March 25, 2010]. http://www.ag.ny.gov/media_center/2010/jan/jan7a_10.html. [Google Scholar]
- 15.TriCor Case May Illuminate Patent Limits. [Accessed March 25, 2010];The Wall Street Journal. http://online.wsj.com/article/SB121236509655436509.html?mod=2_1566_topbox.
- 16.Data and Information Resources. IMS Data Assets. IMS Health Incorporated; [Accessed January 27, 2011]. http://www.imshealth.com/portal/site/imshealth/menuitem.a953aef4d73d1ecd88f611019418c22a/?vgnextoid=63417ec23ff77110VgnVCM10000071812ca2RCRD&vgnextfmt=default. [Google Scholar]
- 17.Jackevicius CA, Tu JV, Ross JS, Ko DT, Krumholz HM. Use of ezetimibe in the United States and Canada. N Engl J Med. 2008;358:1819–28. doi: 10.1056/NEJMsa0801461. [DOI] [PubMed] [Google Scholar]
- 18.Jackevicius CA, Cox J, Carreon D, Tu JV, Rinfret S, So D, Johansen H, Kalavrouziotis D, Demers V, Humphries K, Pilote L. Long-term trends in cardiovascular medication utilization & expenditures: 1996–2006. CMAJ. 2009;181:E19–28. doi: 10.1503/cmaj.081913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. [Accessed April 3, 2010];Statistics Canada Census. http://www12.statcan.ca/english/census01/home/Index.cfm.
- 20.U.S. Census Bureau. [Accessed April 3, 2010]; http://factfinder.census.gov/servlet/DTTable?_bm=y&-geo_id=01000US&-ds_name=PEP_2009_EST&-mt_name=PEP_2009_EST_G2009_T001.
- 21.Vachris MA, Thomas J. International price comparisons based on purchasing power parity. Month Labor Rev. 1999 Oct;:3–12. [Google Scholar]
- 22.Frick MH, Elo O, Haapa K, et al. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med. 1987;317:1237–1245. doi: 10.1056/NEJM198711123172001. [DOI] [PubMed] [Google Scholar]
- 23.Alsheikh-Ali AA, Kuvin JT, Karas RH. Risk of adverse events with fibrates. Am J Cardiol. 2004;94:935–8. doi: 10.1016/j.amjcard.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 24.Omar MA, Wilson JP. FDA adverse event reports on statin-associated rhabdomyolysis. Ann Pharmacother. 2002;36:288–95. doi: 10.1345/aph.1A289. [DOI] [PubMed] [Google Scholar]
- 25.Staffa JA, Chang J, Green L. Cerivastatin and reports of fatal rhabdomyolysis. N Engl J Med. 2002;346:539–540. doi: 10.1056/NEJM200202143460721. [DOI] [PubMed] [Google Scholar]
- 26.Backman JT, Kyrklund C, Kivisto KT, Wang JS, Neuvonen PJ. Plasma concentrations of active simvastatin acid are increased by gemfibrozil. Clin Pharmacol Ther. 2000;68:122–9. doi: 10.1067/mcp.2000.108507. [DOI] [PubMed] [Google Scholar]
- 27.Statin-Fibrate Report: Focus on Safety. VHA Pharmacy Benefits Management; [Accessed April 11, 2010]. www.pbm.va.gov/Safety%20Reports/87ry38statin-fibrate-Final.pdf. [Google Scholar]
- 28.Danzon PM, Furukawa MF. International prices and availability of pharmaceuticals in 2005. Health Affairs. 2008;27:221–33. doi: 10.1377/hlthaff.27.1.221. [DOI] [PubMed] [Google Scholar]
- 29.TriCor Settlement Agreement. Office of the Attorney General State of California; [Accessed March 24, 2010]. http://ag.ca.gov/cms_attachments/press/pdfs/n1844_tricor_settlement.pdf. [Google Scholar]
- 30.Antitrust Case Against Abbott Moves Forward: Were TriCor Formulation Changes Illegal? [Accessed March 24, 2010];Orange Book Blog. http://www.orangebookblog.com/2006/05/antitrust_case_.html.
- 31.IOM (Institute of Medicine) Evaluation of biomarkers and surrogate endpoints in chronic disease. Washington, DC: The National Academies Press; 2010. [PubMed] [Google Scholar]
- 32.Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 33.Grundy SM, Cleeman JI, Bairey-Merz CN, et al. for the Coordinating Committee of the National Cholesterol Education Program. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–39. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.