Abstract
1. The hypothesis that selective predation on larvae of the invasive Aedes albopictus (Skuse) could account for its stable coexistence with the native mosquito species and inferior competitor Ochlerotatus triseriatus (Say) in Florida treeholes and container systems was tested experimentally.
2. Functional responses of the two dipteran predators Toxorhynchites rutilus (Coquillett) and Corethrella appendiculata (Grabham) were evaluated separately for A. albopictus and O. triseriatus prey. Both predators exhibited type II functional responses and consistently consumed more of the invasive species. Handling time of T. rutilus feeding upon O. triseriatus was significantly longer than when preying upon the invasive species.
3. When either predator species was offered varying ratios of the two prey species, A. albopictus was consumed preferentially. The absence of a prey ratio effect on preference indicated that switching probably does not occur.
4. The higher maximum feeding rate upon, and preference for, A. albopictus suggests that differential predation may foster coexistence of the invasive and native mosquito prey species in Florida.
Keywords: Aedes albopictus, coexistence, Corethrella appendiculata, invasive species, native species, Ochlerotatus triseriatus, predation, Toxorhynchites rutilus, treeholes, vulnerability
Introduction
Successful invasive species commonly disrupt ecological communities by displacing native species (Sandlund et al., 1999). Such invasive species may be characterised by rapid growth, short lifespan, high fecundity, the ability to utilise a broad range of habitats, association with human activity, and few if any natural enemies in their new habitat (Morton, 1996). Understanding how invaders alter community structure may facilitate prediction of their long-term consequences (Shea & Chesson, 2002).
Aedes albopictus (Skuse) is an invasive mosquito species from Asia that has been broadly dispersed via used tyres and has spread rapidly in the past two decades in the U.S.A. (O'Meara et al., 1995) as well as in southern Europe, West Africa, and South America (Lounibos, 2002). Larvae of this species inhabit treeholes and artificial containers (e.g. tyres) and feed by filtering or browsing upon microbes (Hawley, 1988). The successful spread of A. albopictus appears to be linked to its high growth rate and ability to displace other species in this habitat (Barrera, 1996). A. albopictus may also gain early access to resources by inhibiting egg hatching of other mosquito species (Edgerly et al., 1993). There is strong evidence that A. albopictus has competitively displaced a resident mosquito species, Aedes aegypti (L.), from artificial containers throughout much of the southeastern U.S.A. (O'Meara et al., 1995; Juliano, 1998)
In forested areas of the eastern U.S.A., A. albopictus has invaded treeholes and containers occupied by the native mosquito Ochlerotatus triseriatus (Say), whose larvae also acquire resources by browsing and filtering microbes (Jenkins & Carpenter, 1946). Laboratory studies have shown that A. albopictus outcompetes O. triseriatus for limiting larval resources (Barrera, 1996). On the basis of microcosm experiments, Livdahl and Willey (1991) predicted that A. albopictus would competitively exclude O. triseriatus from artificial container habitats. However, despite the strong negative effects of A. albopictus on O. triseriatus in laboratory experiments, there is no evidence of competitive displacement between these species in nature (Lounibos et al., 2001).
Predation is an important factor structuring aquatic communities and may stabilise communities through density-dependent predation (Holling, 1965; Hassell, 1978) and switching (Murdoch & Oaten, 1975). Switching occurs when the predator chooses the most abundant prey type more often than it would be if chosen at random (Murdoch & Oaten, 1975). Switching can lead to stable coexistence between two prey species by consumption of the more abundant, having a positive effect on the less abundant species; but may only do so in the presence of density-dependent factors. Alternately, when the predator exhibits a strong preference for one species, the prey that can withstand the highest level of predation should persist (Bonsall & Hassell, 1999).
Toxorhynchites rutilus (Coquillett) and Corethrella appendiculata (Grabham) are the two common dipteran predators of mosquito larvae in peninsular Florida treeholes (Lounibos, 1983; Bradshaw & Holzapfel, 1984). The larger, the culicid T. rutilus, is a generalist predator feeding on anything its own size or smaller, including conspecifics (Campos & Lounibos, 2000). The corethrellid C. appendiculata is a facultative predator, feeding on microfauna as a small larva and on larger prey, such as first- and second-instar mosquito larvae, in its third and fourth instars (Grabham, 1906; Lounibos, 1985). The predators primarily capture prey by ambushing them (Grabham, 1906; Steffan & Evenhuis, 1981); however, T. rutilus may also actively search for prey (Linley & Darling, 1993). Diets of the two predators overlap when T. rutilus are in early and C. appendiculata in late instars (Lounibos, 1985). Both predator species decrease O. triseriatus abundance in treeholes (Bradshaw & Holzapfel, 1983; Lounibos, 1983, 1985), but little research has been done to examine how predation affects the invasion success of A. albopictus in this habitat.
The functional response of a predator describes the number of prey eaten per predator across varying densities of prey (Holling, 1965). Calculations of attack constants and handling times from functional response experiments can be used to understand the mechanisms behind single predator–single prey systems, but they may not accurately predict events in systems where multiple prey species are present. Preference for a particular prey species can be predicted from estimates of the attack constant and handling time taken from functional response experiments using a single prey species (Cock, 1978). The first objective of this research was to determine the nature of the functional responses of T. rutilus and C. appendiculata to individual prey species and to estimate the associated parameters. Comparisons of parameters allowed determination of whether the predator's response to each prey species differed, and the functional response curves predicted for each prey species the maximum number of prey that could be consumed in a 24-h period. If predators play a role in allowing A. albopictus and O. triseriatus to coexist, both predators should have a higher maximum feeding rate on the invasive species, and A. albopictus should be preferred when O. triseriatus and A. albopictus are offered to predators in the same microcosm.
Materials and methods
Organisms and experiments
Predator larvae and eggs were obtained from laboratory colonies of both species from Florida and maintained in an insectary at 25±(SD) °C, LD 14:10 h and 70% RH. Eggs of C. appendiculata and T. rutilus were obtained from mated wild caught adults. Larvae of C. appendiculata were reared to fourth instars on a diet consisting of cultured nematodes (L. P. Lounibos, unpubl. data). Eggs of Florida A. albopictus and O. triseriatus were obtained from F1 colonies of these species at the Florida Medical Entomology Laboratory (Lounibos et al., 2001). One day before the start of the experiment, eggs of both prey species were hatched in deoxygenated water.
To determine the functional response and the maximum number of individual prey consumed, each predator was offered 12, 40, 80, 120, 200, or 250 first-instar A. albopictus or O. triseriatus in 400-ml beakers filled with dechlorinated tap water. Toxorhynchites rutilus was added as a first instar (less than 8 h old), and C. appendiculata was added as a fourth-instar larva, which had moulted 1–2 days earlier and was deprived of food 1 day prior to the start of the experiment. The treatments were randomly assigned to positions on a single shelf of the insectary, and the predators were allowed to consume prey for 24 h. After 24 h, the predators were removed and the remaining prey were counted. Due to constraints on numbers of prey and predators available, replications were run at different periods under the same controlled conditions. More replicates were run at lower than at higher densities (Juliano, 2001).
In a separate experiment to examine preference, first-instar prey at a fixed density of 100 per 400 ml were offered to each predator at varying ratios of the two prey species. The predators were of the same instar as above. Corethrella appendiculata were offered prey at ratios of A. albopictus : O. triseriatus of 0:100, 20:80, 40:60, 50:50, 60:40, 80:20, and 100:0, which were each replicated four times. Toxorhynchites rutilus were offered prey at ratios of A. albopictus : O. triseriatus of 0:100, 10:90, 30:70, 50:50, 70:30, 90:10, and 100:0, each replicated six times. After 24 h, predators were removed and remaining prey were identified to species and counted under a dissecting microscope.
Statistical analyses
The shape of the functional response curve was determined by logistic regression of the proportion of prey eaten as a function of the number of prey available (Trexler et al., 1988). Since cubic models are of a high enough order to describe most curves (Juliano, 2001), the following polynomial function was fit:
| (1) |
with the procedure CATMOD (SAS Institute Inc, 1989), where Ne is the number of prey consumed, N0 is the number of prey available, and Ne/N0 is the probability that the prey will be eaten by a predator. The P-values are parameters to be estimated using maximum likelihood methods. A type II functional response occurs when the linear term (or slope of Ne/N0 vs. N0 near N0=0) is negative and a type III functional response occurs when the linear term is positive (Juliano, 2001). The attack constants and handling times for each predator were estimated using iterative nonlinear least squares regression in the procedure NLIN (SAS Institute Inc., 1989) to solve the implicit function given by the random predator equation (Rogers, 1972; Juliano, 2001).
To determine if the functional response parameters were significantly different between prey species for each predator, nonlinear least squares regression was used on the implicit function given by the random predator equation (Rogers, 1972; Juliano, 2001). Each prey species was assigned an indicator variable and compared using the equation:
| (2) |
where j is the indicator variable with a value of 0 for O. triseriatus and a value of 1 for A. albopictus. T xis the amount of time the experiment was run, Th is the handling time, a the attack constant, and N0 and Ne are as previously defined. Da and DTh are the differences in parameter estimates for the two prey species. Parameters were deemed to be significantly different when the 95% confidence interval for the difference in parameters did not include zero. Significance was further tested with a t-test comparing the differences in parameter estimates to zero (Juliano, 2001).
Prey preference in the two-prey experiment was determined using Manly's α (Manly, 1974), modified by Chesson (1983) for prey depletion:
| (3) |
where N is the initial number and C is the number consumed of A. albopictus (A) and O. triseriatus (T). The predicted preference (α) for each predator was determined using attack constants from the functional response experiments using a multiplicative model:
| (4) |
where is the predicted preference for A. albopictus, and aa and at are attack constants for A. albopictus and O. triseriatus, respectively, estimated from the functional response. This equation can be used even when prey are not being replaced (Chesson, 1983). Resulting α values were compared among prey ratios by anova to determine if preference changes with prey ratios. Since α values did not vary with density, they were pooled and tested against the predicted α value with a t-test to determine if there was a significant preference for either prey species.
Results and discussion
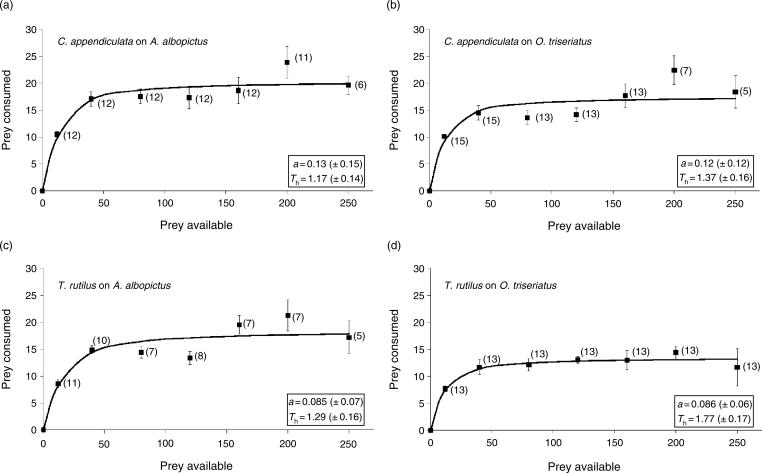
Logistic regressions showed that the linear term was negative for all predator–prey treatments, indicating type II functional responses (Fig. 1). These results were confirmed by plotting observed mean proportions consumed vs. predicted proportions consumed (not shown). However, at the highest density of 250 prey, the number of A. albopictus and O. triseriatus consumed by both T. rutilus and C. appendiculata decreased from the 200 prey density (Fig. 1), indicating that predator behaviour at very high prey densities may be influenced by a secondary factor(s).
Fig. 1.
Functional response of (a) Corethrella appendiculata to Aedes albopictus, (b) C. appendiculata to Ochlerotatus triseriatus, (c) Toxorhynchites rutilus to A. albopictus, and (d) T. rutilus to O. triseriatus. Each point represents the mean number of prey consumed over a 24-h period, with one SE above and below each point. a and Th are the estimated attack rates and handling times, respectively (±95% CI). The solid line is predicted from the random predator model. Number of replicates are indicated in parentheses.
There were no significant differences among attack constants or handling times between the prey species when exposed to C. appendiculata. There were no significant differences in attack constants between prey species exposed to T. rutilus, but handling time was significantly greater for O. triseriatus than for A. albopictus (t122 = 3.87, P < 0.05). The average maximum feeding rate for C. appendiculata was ≈24 prey per 24 h on A. albopictus and 18 prey per 24 h on O. triseriatus (Fig. 1a,b). The average maximum feeding rate for T. rutilus was 21 prey per 24 h on A. albopictus and 14 prey per 24 h on O. triseriatus (Fig. 1c,d).
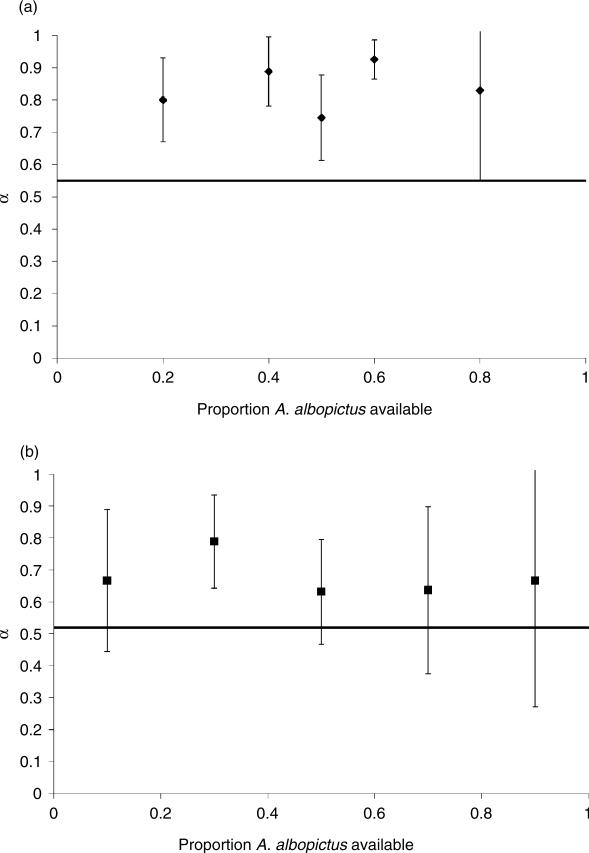
anova detected no significant variation in Manly's α among ratios of prey species with C. appendiculata (F4,21 = 0.98, P > 0.4). Predicted preference of C. appendicu lata for A. albopictus was slight, with an α value of 0.55. When pooled results were compared with an α level of 0.52 for no preference, C. appendiculata consistently consumed fewer O. triseriatus than predicted, significantly preferring A. albopictus overall (t21 = 9.24, P < 0.001) (Fig. 2a). anova detected no significant variation in Manly's α among ratios of prey species with T. rutilus (F4,28 = 0.34, P > 0.8). Predicted preference of T. rutilus for A. albopictus was slight with an α value of 0.52. When pooled results were compared with an α level of 0.51 for no preference, T. rutilus significantly preferred A. albopictus to O. triseriatus overall (t28 = 3.87, P < 0.001) (Fig. 2b).
Fig. 2.
Preference of (a) Corethrella appendiculata for Aedes albopictus and (b) Toxorhynchites rutilus for A. albopictus indicated by Manly's alpha (α) (±SD) vs. the proportion of A. albopictus available. Solid lines indicate no preference for either prey species predicted from the functional response. α=0.52 for C. appendiculata and α=0.51 for T. rutilus.
The results of this study suggest that two important predators in Florida container habitats may adversely affect production of the invasive mosquito A. albopictus through preferential consumption. The preference for A. albopictus by C. appendiculata was stronger than that by T. rutilus, suggesting that the former, abundant predator in south Florida treeholes (Lounibos, 1983) may be more important for fostering coexistence between these two prey species under the size structure studied. A number of studies have shown that predation as a whole can reduce the effects of competition (Chambers, 1985). When predation is more intense on the superior competitor, the inferior competitor may coexist through a keystone predator effect (Paine, 1966). Here, evidence is provided suggesting that this may be the case in Florida treeholes and containers. This may be especially important in container habitats, where A. albopictus is predicted to exclude O. triseriatus in the absence of predators. However, in treehole habitats the prey species may be able to coexist (Livdahl & Willey, 1991).
Functional response curves allow for prediction of predation intensity and predator behaviour over a range of conditions and act as a baseline for predicting stability of predator–prey interactions (Cock, 1978). In the current experiment T. rutilus and C. appendiculata exhibited a type II functional response to changes in prey density, resulting in negative density dependence of predator-induced mortality (Holling, 1965). Type II functional responses are typical of invertebrate predators (Hassell, 1978), have been found in other studies on Toxorhynchites and Corethrella larvae (Livdahl, 1979; Lounibos, 1983), and are less likely to result in stable predator–prey relationships than are type III responses (Hassell, 1978).
Based on attack constants and handling times, the functional response of the predator to its prey should predict how the predator behaves when multiple prey species are present (Cock, 1978). The lack of any differences between the prey species in handling time, attack constant, or maximum feeding rate when exposed to C. appendiculata suggests that this predator reacts similarly when exposed alone to the prey species. This is not surprising from the predator's view since C. appendiculata is a generalist and captures its prey primarily by ambush (Grabham, 1906). However, when two prey species were exposed together, C. appendiculata consistently consumed more A. albopictus (and fewer O. triseriatus) than expected based on single species data. This suggests that prey behaviour may affect feeding preference by this predator and that the presence of O. triseriatus may negatively affect A. albopictus survival in the presence of C. appendiculata. The second predator, T. rutilus took significantly longer to handle O. triseriatus, resulting in a prediction of slight preference for A. albopictus. However, the interspecific difference in attack constants was not significant for either predator species. The results indicate that first-instar T. rutilus may consume a larger number of A. albopictus, but will reach its maximum feeding rate with fewer O. triseriatus larvae. A similar but not significant trend was found for C. appendiculata, indicating that both predators may consume the invasive species more easily than the native prey species.
The addition of a second prey species can have a number of direct and indirect effects on both the predator and prey populations. Preference for the invasive species may be important for allowing the two species to coexist. Although both predators preferred A. albopictus to O. triseriatus under certain conditions, A. albopictus has a higher growth and development rate (Barrera, 1996; Lounibos et al., 2001), which may enable it to escape predation by size-selective predators better than O. triseriatus (Lounibos et al., 2001). Apparent mutualism occurs between prey species when the presence of one lowers predation rates on the other (Holt & Lawton, 1994). Thus, greater predation intensity on A. albopictus may also relax predation on O. triseriatus, enabling this species to increase in numbers. Prey that share a common predator should have positive effects on each other's density when the predator switches, shows a strongly saturating functional response, or when the per capita death rate of the predator increases with predator density (Abrams & Matsuda, 1996). However, more available prey resulting from the addition of A. albopictus may lead to negative indirect effects among the two prey species by increasing the abundance of predators (Holt, 1977). Thus, multigenerational studies are needed to determine whether A. albopictus has an overall positive or negative effect on O. triseriatus in the context of shared predators.
Acknowledgements
Thanks goes to R. Escher, J. Butler, and S. Juliano for providing mosquitoes, insectary space, and statistical assistance, respectively. Previous drafts of the manuscript were improved by comments from S. Juliano, C. Lord, G. O'Meara, and S. Yanoviak. Research was supported in part by NIH grant AI-44793. This is Florida Agricultural Experiment Station Journal Series No. R-10457.
References
- Abrams PA, Matsuda H. Positive indirect effects between prey species that share predators. Ecology. 1996;77:610–616. [Google Scholar]
- Barrera R. Competition and resistance to starvation in larvae of container-inhabiting Aedes mosquitoes. Ecological Entomology. 1996;21:117–127. [Google Scholar]
- Bonsall MB, Hassell MP. Parasitoid-mediated effects: apparent competition and the persistence of host-parasitoid assemblages. Researches on Population Ecology. 1999;41:59–68. [Google Scholar]
- Bradshaw WE, Holzapfel CM. Predator-mediated, non-equilibrium coexistence of tree-hole mosquitoes in southeastern North America. Oecologia. 1983;57:239–256. doi: 10.1007/BF00379586. [DOI] [PubMed] [Google Scholar]
- Bradshaw WE, Holzapfel CM. Seasonal development of tree-hole mosquitoes (Diptera, Culicidae) and Chaoborids in relation to weather and predation. Journal of Medical Entomology. 1984;21:366–378. [Google Scholar]
- Campos RE, Lounibos LP. Natural prey and digestion times of Toxorhynchites rutilus (Diptera: Culicidae) in southern Florida. Annals of the Entomological Society of America. 2000;93:1280–1287. [Google Scholar]
- Chambers RC. Competition and predation among larvae of three species of treehole-breeding mosquitoes. In: Lounibos LP, Rey JR, Frank JH, editors. Ecology of Mosquitoes: Proceedings of a Workshop. Florida Medical Entomology Laboratory; Vero Beach, Florida: 1985. pp. 25–53. [Google Scholar]
- Chesson J. The estimation and analysis of preference and its relationship to foraging models. Ecology. 1983;64:1297–1304. [Google Scholar]
- Cock MJW. Assessment of preference. Journal of Animal Ecology. 1978;47:805–816. [Google Scholar]
- Edgerly JS, Willey MS, Livdahl TD. The community ecology of Aedes egg hatching – implications for a mosquito invasion. Ecological Entomology. 1993;18:123–128. [Google Scholar]
- Grabham M. A new Corethrella from Jamaica. Entomological News. 1906;17:343–345. [Google Scholar]
- Hassell MP. Sigmoid functional responses and population stability. Theoretical Population Biology. 1978;9:75–98. doi: 10.1016/0040-5809(78)90004-7. [DOI] [PubMed] [Google Scholar]
- Hawley WA. The biology of Aedes albopictus. Journal of the American Mosquito Control Association. 1988;4(Suppl):1–39. [PubMed] [Google Scholar]
- Holling CS. The functional response of predators to prey density and its role in mimicry and population regulation. Memoirs of the Entomological Society of Canada. 1965;45:3–60. [Google Scholar]
- Holt RD. Predation, apparent competition, and structure of prey communities. Theoretical Population Biology. 1977;12:197–229. doi: 10.1016/0040-5809(77)90042-9. [DOI] [PubMed] [Google Scholar]
- Holt RD, Lawton JH. The ecological consequences of shared natural enemies. Annual Review of Ecology and Systematics. 1994;25:495–520. [Google Scholar]
- Jenkins DW, Carpenter SJ. Ecology of the treehole breeding mosquitoes of nearctic North America. Ecological Monographs. 1946;16:33–47. [Google Scholar]
- Juliano SA. Species introduction and replacement among mosquitoes: interspecific resource competition or apparent competition? Ecology. 1998;79:255–268. [Google Scholar]
- Juliano SA. Nonlinear curve fitting: predation and functional response curves. In: Scheiner SM, Gurevitch J, editors. Design and Analysis of Ecological Experiments. Oxford University Press; New York: 2001. pp. 178–196. [Google Scholar]
- Linley JR, Darling K. Search behaviour associated with egg cannibalism in Toxorhynchites amboinensis and Toxorhynchites rutilus rutilus (Diptera, Culicidae) Journal of Medical Entomology. 1993;30:561–570. doi: 10.1093/jmedent/30.3.561. [DOI] [PubMed] [Google Scholar]
- Livdahl TP. Evolution of handling time – functional-response of a predator to the density of sympatric and allopatric strains of prey. Evolution. 1979;33:765–768. doi: 10.1111/j.1558-5646.1979.tb04728.x. [DOI] [PubMed] [Google Scholar]
- Livdahl TP, Willey MS. Prospects for an invasion – competition between Aedes albopictus and native Aedes triseriatus. Science. 1991;253:189–191. doi: 10.1126/science.1853204. [DOI] [PubMed] [Google Scholar]
- Lounibos LP. The mosquito community of treeholes in subtropical Florida. In: Frank JH, Lounibos LP, editors. Phytotelmata: Terrestrial Plants as Hosts for Aquatic Insect Communities. Plexus; Medford, New Jersey: 1983. pp. 223–246. [Google Scholar]
- Lounibos LP. Interactions influencing production of treehole mosquitoes in south Florida. In: Lounibos LP, Rey JR, Frank JH, editors. Ecology of Mosquitoes: Proceedings of a Workshop. Florida Medical Entomology Laboratory; Vero Beach, Florida: 1985. pp. 65–77. [Google Scholar]
- Lounibos LP. Invasions by insect vectors of human disease. Annual Review of Entomology. 2002;47:233–266. doi: 10.1146/annurev.ento.47.091201.145206. [DOI] [PubMed] [Google Scholar]
- Lounibos LP, O'Meara GF, Escher RL, Nishimura N, Cutwa M, Nelson T, et al. Testing predictions of displacement of native Aedes by the invasive Asian Tiger Mosquito Aedes albopictus in Florida, U.S.A. Biological Invasions. 2001;3:151–166. [Google Scholar]
- Manly BFJ. Model for certain types of selection experiments. Biometrics. 1974;30:281–294. [Google Scholar]
- Morton B. The aquatic nuisance species: a global perspective and review. In: D'itri F, editor. Zebra Mussels and Other Aquatic Nuisance Species. Ann Arbor Press; Ann Arbor, Michigan: 1996. pp. 1–54. [Google Scholar]
- Murdoch WW, Oaten A. Predation and population stability. Advances in Ecological Research. 1975;9:1–132. [Google Scholar]
- O'Meara GF, Evans LF, Gettman AD, Cuda JP. Spread of Aedes albopictus and decline of Aedes aegypti (Diptera, Culicidae) in Florida. Journal of Medical Entomology. 1995;32:554–562. doi: 10.1093/jmedent/32.4.554. [DOI] [PubMed] [Google Scholar]
- Paine RT. Food web complexity and species diversity. American Naturalist. 1966;100:65–75. [Google Scholar]
- Rogers D. Random search and insect population models. Journal of Animal Ecology. 1972;41:369–383. [Google Scholar]
- Sandlund OT, Schei PJ, Viken A, editors. Invasive Species and Biodiversity Management. Kluwer Academic; Boston, Massachusetts: 1999. [Google Scholar]
- SAS Institute Inc . SAS Institute Inc. Cary; North Carolina: 1989. SAS/STAT User's Guide. [Google Scholar]
- Shea K, Chesson P. Community ecology theory as a framework for biological invasions. Trends in Ecology and Evolution. 2002;17:170–176. [Google Scholar]
- Steffan WA, Evenhuis NL. Biology of Toxorhynchites. Annual Review of Entomology. 1981;26:159–181. [Google Scholar]
- Trexler JC, McCulloch CE, Travis J. How can the functional-response best be determined. Oecologia. 1988;76:206–214. doi: 10.1007/BF00379954. [DOI] [PubMed] [Google Scholar]