Abstract
Purpose
We aimed to ascertain the degree of association between bladder cancer and human papillomavirus (HPV) infection.
Materials and Methods
We performed a meta-analysis of observational studies with cases and controls with publication dates up to January 2011. The PubMed electronic database was searched by using the key words "bladder cancer and virus." Twenty-one articles were selected that met the required methodological criteria. We implemented an internal quality control system to verify the selected search method. We analyzed the pooled effect of all the studies and also analyzed the techniques used as follows: 1) studies with DNA-based techniques, among which we found studies with polymerase chain reaction (PCR)-based techniques and 2) studies with non-PCR-based techniques, and studies with non-DNA-based techniques.
Results
Taking into account the 21 studies that were included in the meta-analysis, we obtained a heterogeneity chi-squared value of Qexp=26.45 (p=0.383). The pooled odds ratio (OR) was 2.13 (95% confidence interval [CI], 1.54 to 2.95), which points to a significant effect between HPV and bladder cancer. Twenty studies assessed the presence of DNA. The overall effect showed a significant relationship between virus presence and bladder cancer, with a pooled OR of 2.19 (95% CI, 1.40 to 3.43). Of the other six studies, four examined the virus's capsid antigen and two detected antibodies in serum by Western blot. The estimated pooled OR in this group was 2.11 (95% CI, 1.27 to 3.51), which confirmed the relationship between the presence of virus and cancer.
Conclusions
The pooled OR value showed a moderate relationship between viral infection and bladder tumors.
Keywords: Human papillomavirus, Infection, Meta-analysis, Urinary bladder neoplasms, Viruses
INTRODUCTION
Bladder carcinoma is the most common of the malignant neoplasias of the urinary tract and is characterized by wide variability in prognosis. Carcinomas of transitional cells account for over 90% of bladder tumors [1]. The majority of patients tend to relapse, but a group of patients progress toward muscle-invasive disease [2]. To date, the mechanisms associated with the initiation and progression of these tumors, along with possible risk factors, are not well known [3].
Many studies have tried to assess the carcinogenic risk of viruses such as human papillomavirus (HPV), only to find conflicting results. The papillomaviruses are a group of small, double-stranded DNA viruses that infect the stratified epithelium of the skin and mucosa [4]. Over 120 different types of HPV have been described, only some of which implant their DNA into the host cell's genome. HPVs have been classified as "low risk" (types 6 and 11) and "high risk" (types 16 and 18) on the basis of their implantation properties and their tendencies to associate with benign processes or invasive carcinomas [1].
It has been shown that infection with HPV is implicated in the pathogenesis of several intraepithelial lesions and cancers, such as anal, cervical, and oral cancer [5]. However, the association of HPV with the development of tumors of the urinary tract continues to be controversial, given that in most cases, the evaluation of the results was difficult because appropriate control groups were lacking [4].
In relation to bladder carcinoma, until now, few studies have found an association between HPV infection and an increased risk for the development of neoplasias. This possible relationship is based on the epithelial tropism of HPV and the anatomical proximity of the urethra (which is considered a reservoir for the virus) and the bladder [1,6].
For this reason, it is open for debate whether the association between chronic HPV infection and bladder carcinoma is a coincidence or whether the relationship is causal. To address this, our study identified and analyzed internationally published studies (published before January 2011) that analyzed the relationship between bladder carcinoma and HPV and that described defined materials and methods. We conducted a meta-analysis of all of the results presented in these studies to analyze the currently available data on the relationship between this virus and bladder cancer.
MATERIALS AND METHODS
We conducted a search of the PubMed database by using the keywords "bladder cancer and virus." From this search, we identified 763 studies published before January 2011. We then selected a total of 22 studies [4,7-27] that had well-established groups of cases and controls; that were published in English, French, or Spanish; and that analyzed the relationship between HPV and bladder cancer. Of these studies, two were eliminated, in which the number of positive cases was zero in both studied groups (which would not allow for the calculation of any effect measurement between virus presence and bladder cancer) [26,27]. Additionally, we added results associating HPV and bladder carcinoma that were found in a doctoral thesis [28].
We conducted an internal quality check system to verify the selected search method, which consisted of tracing the bibliography of each one of the selected studies. Using this method, we were able to confirm that no study was omitted.
Given the diversity of the studies, before the meta-analysis, the studies were classified on the basis of the laboratory test used.
The heterogeneity of the studies was compared by using the Cochran chi-squared test (heterogeneity chi-squared-Qexp) and the consistency index (I2). The odds ratio (OR) was calculated for each study with a 95% confidence interval (95% CI). The mean of the pooled OR was calculated by using the random effects model by the DerSimonian-Laird method. We considered the effect significant when the CI for the OR did not contain the value 1.
Publication bias was examined by using Begg's and Egger's tests.
A forest plot was used to graphically represent the estimated ORs of all studies, including the pooled effects. The analyses were conducted by using Metadisc software (Informer Technologies Inc.).
RESULTS
We analyzed the pooled effect of all the studies and also analyzed the techniques used as follows: 1) studies with DNA-based techniques, among which we found studies with polymerase chain reaction (PCR)-based techniques and studies with non-PCR-based techniques, and 2) studies with non-DNA-based techniques.
1. Pooled analysis of all studies
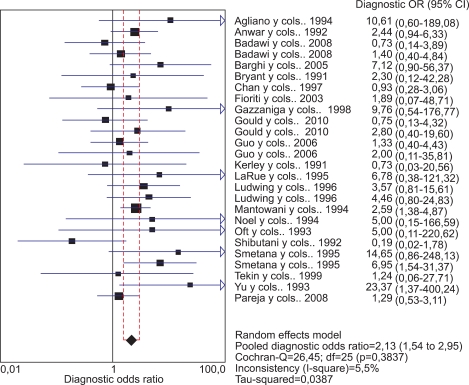
Taking into account the 21 studies included in the pooled meta-analysis, we obtained a heterogeneity chi-squared value of Qexp=26.45, with 25 degrees of freedom (p=0.383), which allowed us to confirm that the studies were sufficiently homogeneous compared with each other. Additionally, the inconsistency index I2 for the 21 studies had a value of 5.7%, which confirmed that the percentage of heterogeneity was very low. The pooled OR was 2.13 (95% CI, 1.54 to 2.95), which demonstrated a significant effect between HPV presence and bladder cancer (Fig. 1).
FIG. 1.
Meta-analysis of all included studies.
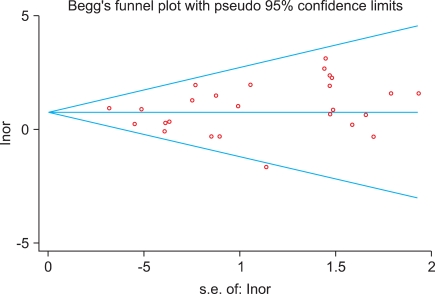
The result of the Begg's test was not significant (p=0.508), allowing us to conclude that there were no indications of publication bias, which was confirmed graphically by the Funnel Plot (Fig. 2).
FIG. 2.
Funnel Plot of all included studies.
2. Studies that used DNA-based techniques
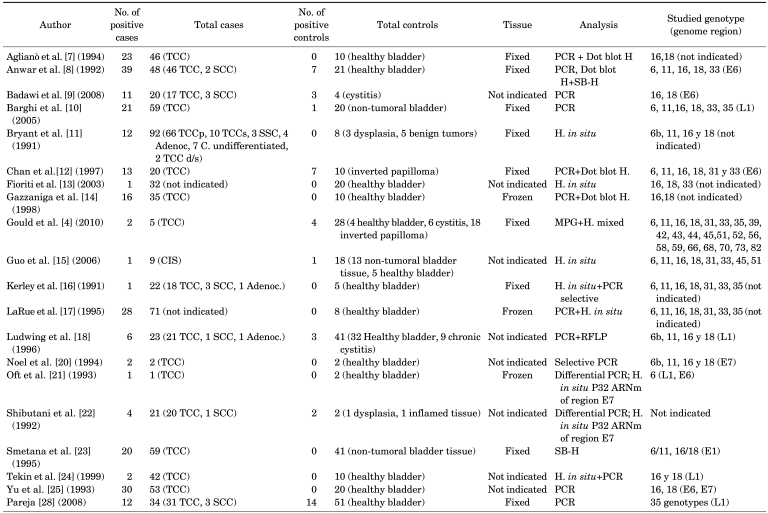
Table 1 lists the authors, the total numbers of cases and controls with the numbers of positives found in both groups, and the methods used. In the cases, 79.4% were transitional cell bladder tumors; in the controls, the samples were predominantly healthy bladder tissue (63.7%). PCR was used in the majority of the cases (80%). The amplified regions varied and tended to specify the detected viral genotype (the majority were type 16 or 18). Only three samples (15%) used frozen tissue biopsies.
TABLE 1.
Characteristics of human papillomavirus DNA-based studies included in the meta-analysis
TCC, transitional cell carcinoma; PCR, polymerase chain reaction; SCC, squamous cell carcinoma; Adenoc., adenocarcinoma; SB-H, Southern blot H; H, hybridization; RFLP, restriction fragment length polymorphism; MPG, multiplex HPV genotyping; d/s, deep/superficial; CIS, squamous cell carcinoma in situ.
The prevalence of HPV infection in the cases ranged from 3.12 to 100%. In the controls, the prevalence ranged from 0 to 100%.
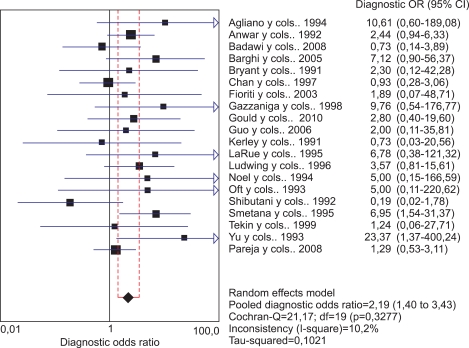
We included a total of 20 studies that used DNA-based techniques for determination of infection. These studies met the hypothesis of homogeneity, with a Qexp=21.17 with 19 degrees of freedom (p=0.3277) and an I2=10.2%, which allowed us to assume comparability between the studies. The pooled effect showed a significant relationship between viral presence and bladder cancer, with a pooled OR of 2.19 (95% CI, 1.40 to 3.43). Fig. 3 shows the OR and 95% CI values for each of the studies.
FIG. 3.
Meta-analysis of the studies that used DNA-based techniques.
There was no evidence of publication bias by Begg's test (p=1).
1) Studies that used PCR-based techniques
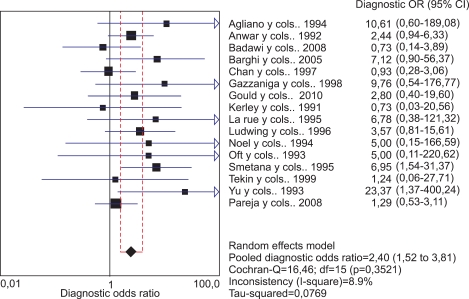
The 16 studies that used PCR-based techniques also met the homogeneity criteria with a Qexp=16.46, with 15 degrees of freedom (p=0.352) and an I2=8.9%. The pooled OR also confirmed a significant relationship between viral presence and bladder cancer, with a pooled OR of 2.40 (95% CI, 1.51 to 3.81) (Fig. 4).
FIG. 4.
Meta-analysis of studies that used PCR-based techniques.
Begg's test did not show evidence of publication bias for this subgroup (p=0.558).
2) Studies that used non-PCR-based techniques
The subgroup of studies that used non-PCR-based techniques was significantly smaller than the others, including only four studies. Despite the low number, this group also met the homogeneity hypothesis with a Qexp=2.78, with 3 degrees of freedom (p=0.426) and an I2=0.0%. In this subgroup, the pooled OR was 0.84 (95% CI, 0.21 to 3.31). Statistical significance was not reached, which was probably related to the small number of studies included (Fig. 5).
FIG. 5.
Meta-analysis of studies that used non-PCR-based techniques.
This subgroup also did not show evidence of publication bias by Begg's test (p=0.734).
3. Studies that used non-DNA-based techniques
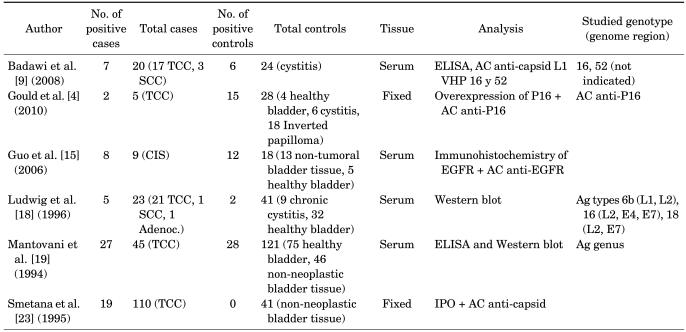
Table 2 lists the authors, the total numbers of cases and controls with the numbers of positives found in both groups, the materials used for the studies (carcinoma biopsies of transitional cells in 93.4% of the cases and samples of healthy bladder tissue in 42.5% of controls), and the methods used (four studies assessed the viral capsid antigen, and two evaluated serum antibodies by use of Western blot).
TABLE 2.
Characteristics of studies based on viral antigen and antibody detection included in the meta-analysis
TCC, transitional cell carcinoma; SCC, squamous cell carcinoma; Adenoc., adenocarcinoma; IPO, immunoperoxidase; AC, antibody; Ag, antigen; ELISA, enzyme-linked immunosorbent assay; EGFR, immunohistochemistry for epithelial growth factor receptor; CIS, squamous cell carcinoma in situ.
The prevalence of HPV infection in these cases ranged from 17.3 to 88.8%. In the controls, the prevalence ranged from 0 to 66.6%.
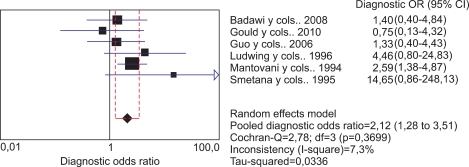
Despite the fact that the subgroup of studies using non-DNA-based techniques was small, these six studies could be considered homogeneous with a value of Qexp=5.39, with 5 degrees of freedom (p=0.370) and a low I2 of 7.3%. Although the number of studies was not very large, the estimated pooled OR was statistically significant at 2.11 (95% CI, 1.27 to 3.51), which confirms the relationship between viral presence and cancer in this group (Fig. 6).
FIG. 6.
Meta-analysis of studies that used non-DNA-based techniques.
This subgroup did not show evidence of publication bias by Begg's test (p=0.707).
DISCUSSION
Bladder transitional cell carcinoma is one of the most common neoplasias in developed countries [10]. It is a clinical entity of which certain aspects are unknown, such as the existence of a genetic factor that determines its predisposition, despite the existence of various involved oncogenes, or the identity of the main risk factor involved in its genesis. These unknowns prevent us from implementing an effective prevention campaign. In this study, we evaluated HPV, which has been mentioned in the literature as a possible etiological agent of genital tumors, although its exact effect on bladder carcinoma is still vague, and numerous hypotheses exist regarding its role in this regard.
The integration of HPV frequently occurs in the vicinity of known proto-oncogenes in human epithelial cells, although it is still unknown whether this occurrence favors the progression of pre-cancerous lesions towards cancer. The viral circular double-stranded DNA must be broken for the oncogenic phenomenon of integration to take place. It has been shown that this breakage is specifically produced in the viral genome region encoding the E2 gene, which then loses its functional capacity. In the most oncogenic HPVs (types 16 and 18), the E2 gene seems to act mainly as a repressor of the transcription of genes E6 and E7, whose fundamental activities are to promote cellular proliferation and transformation by producing centrosome amplification and chromosomal delay (i.e., lag) during mitosis, which leads to chromosomal instability [10]. This genomic instability is thought to be an essential part of the conversion of a normal cell to a cancerous one.
The HPV E6 and E7 genes, especially in high-risk HPVs (types 16 and 18), acquire their transforming activities as a result of encoding E6 and E7 oncoproteins, which specifically interact with proteins regulating cell proliferation. It has been shown that oncoprotein E7 interacts with the cellular protein p105 Rb, which is encoded by the retinoblastoma gene Rb1. The interaction of the HPV E7 oncoprotein with the regulator protein p105-Rb presumably inactivates p105-Rb, with similar consequences as those that occur in tumors encoding retinoblastoma gene alterations. In contrast, the association of E6 with p53 may give rise to an effect similar to what happens after mutation of the p53 gene. It is thought that this constitutes one of the oncogenic pathways of HPV [29].
Some authors have reported that the sensitivity of HPV detection greatly depends on several technical factors, such as tissue fixation, DNA preparation, and amplification conditions [19].
Over the years, microbiological tests used for the detection of infection have changed. PCR and in situ hybridization are techniques with high sensitivities and specificities for viral genome detection [1]; these techniques have been used most commonly in studies. This, however, should not detract from the value of other techniques for which specificities and sensitivities have been reported.
Li et al. [30] presented the outcomes of a meta-analysis of articles published from January 1989 until August 2010 in which they showed an association between HPV and bladder cancer. The authors discussed important issues that were not analyzed in previous meta-analyses, such as the geographic variation in risk of bladder cancer with HPV infection; the most common HPV types identified, which are similar to those identified in other tumors, mainly cervical tumors; and the sensitivity of detection of infection as a function of the method used.
In the present meta-analysis, in addition to the techniques based on DNA detection (PCR-based methods and non-PCR-methods) noted by Li et al. [30], we included the analysis of seven studies that used virus detection techniques that were not based on DNA (i.e., the detection of viral capsid antigen and antibodies in serum by Western blotting). In this case, the joint point estimate of the prevalence of infection through the detection of antigen or antibody was 32.4%.
We can observe that the association between HPV and bladder cancer varies with geographical location. Elevated percentages of HPV detection have been documented in bladder tumors in Southeast Asia, reaching up to a reported 81% in Hong Kong [31]. Lower percentages have been detected in Europe and North America, not exceeding 39% [1]. It is possible that these differences are related to problems in diagnostic techniques, such as not using a wide enough range of specific primers for PCR, or having samples containing DNA from less-common types of HPV, leading to false-negatives [1].
Another issue to consider is that of justifying the association, given that the virus could be present in a lesion before it appears as an inductor to be acquired during sexual intercourse. This does not rule out that the tumor may be secondarily colonized as part of the mucosal microbiota [6].
In our study, the Qexp values obtained from the analysis of studies using non-DNA-based and DNA-based techniques, viral genome PCR detection methods, and non-PCR detection methods showed homogeneity of data and a small dispersion of results. Of the 21 selected studies, we obtained a pooled OR of 2.13, allowing us to conclude that there is a clear and moderate association between virus exposure and the presence of bladder cancer.
CONCLUSIONS
As of January 2011, there were only 21 studies with both case and control groups that analyzed the relationship between HPV and bladder cancer. The methods used varied, but PCR use predominated. The studies demonstrated the presence of infection in the majority of cases, although an important dispersion was observed. The pooled OR value showed a moderate relationship between infection by the virus and the tumor. There is a lack in the literature of a sufficiently large number of cases and samples that are compared with controls and that use a combination of microbiological techniques in the same subjects and samples to obtain a definitive answer to this question. For this reason, the relationship between the virus and this tumor should continue to be evaluated through pathogenic studies of the disease.
Footnotes
The authors have nothing to disclose.
References
- 1.Ben Selma W, Ziadi S, Ben Gacem R, Amara K, Ksiaa F, Hachana M, et al. Investigation of human papillomavirus in bladder cancer in a series of Tunisian patients. Pathol Res Pract. 2010;206:740–743. doi: 10.1016/j.prp.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Moonen PM, Bakkers JM, Kiemeney LA, Schalken JA, Melchers WJ, Witjes JA. Human papilloma virus DNA and p53 mutation analysis on bladder washes in relation to clinical outcome of bladder cancer. Eur Urol. 2007;52:464–468. doi: 10.1016/j.eururo.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 3.Erill N, Colomer A, Verdú M, Román R, Condom E, Hannaoui N, et al. Genetic and immunophenotype analyses of TP53 in bladder cancer: TP53 alterations are associated with tumor progression. Diagn Mol Pathol. 2004;13:217–223. doi: 10.1097/01.pdm.0000137098.03878.00. [DOI] [PubMed] [Google Scholar]
- 4.Gould VE, Schmitt M, Vinokurova S, Reddy VB, Bitterman P, Alonso A, et al. Human papillomavirus and p16 expression in inverted papillomas of the urinary bladder. Cancer Lett. 2010;292:171–175. doi: 10.1016/j.canlet.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 5.Spano JP, Marcelin AG, Carcelin G. HPV and cancer. Bull Cancer. 2005;92:59–64. [PubMed] [Google Scholar]
- 6.Gutiérrez J, Jiménez A, de Dios Luna J, Soto MJ, Sorlózano A. Meta-analysis of studies analyzing the relationship between bladder cancer and infection by human papillomavirus. J Urol. 2006;176(6 Pt 1):2474–2481. doi: 10.1016/j.juro.2006.07.157. [DOI] [PubMed] [Google Scholar]
- 7.Aglianò AM, Gradilone A, Gazzaniga P, Napolitano M, Vercillo R, Albonici L, et al. High frequency of human papillomavirus detection in urinary bladder cancer. Urol Int. 1994;53:125–129. doi: 10.1159/000282652. [DOI] [PubMed] [Google Scholar]
- 8.Anwar K, Naiki H, Nakakuki K, Inuzuka M. High frequency of human papillomavirus infection in carcinoma of the urinary bladder. Cancer. 1992;70:1967–1973. doi: 10.1002/1097-0142(19921001)70:7<1967::aid-cncr2820700726>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 9.Badawi H, Ahmed H, Ismail A, Diab M, Moubarak M, Badawy A, et al. Role of human papillomavirus types 16, 18, and 52 in recurrent cystitis and urinary bladder cancer among Egyptian patients. Medscape J Med. 2008;10:232. [PMC free article] [PubMed] [Google Scholar]
- 10.Barghi MR, Hajimohammadmehdiarbab A, Moghaddam SM, Kazemi B. Correlation between human papillomavirus infection and bladder transitional cell carcinoma. BMC Infect Dis. 2005;5:102. doi: 10.1186/1471-2334-5-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bryant P, Davies P, Wilson D. Detection of human papillomavirus DNA in cancer of the urinary bladder by in situ hybridisation. Br J Urol. 1991;68:49–52. doi: 10.1111/j.1464-410x.1991.tb15256.x. [DOI] [PubMed] [Google Scholar]
- 12.Chan KW, Wong KY, Srivastava G. Prevalence of six types of human papillomavirus in inverted papilloma and papillary transitional cell carcinoma of the bladder: an evaluation by polymerase chain reaction. J Clin Pathol. 1997;50:1018–1021. doi: 10.1136/jcp.50.12.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fioriti D, Pietropaolo V, Dal Forno S, Laurenti C, Chiarini F, Degener AM. Urothelial bladder carcinoma and viral infections: different association with human polyomaviruses and papillomaviruses. Int J Immunopathol Pharmacol. 2003;16:283–288. doi: 10.1177/039463200301600315. [DOI] [PubMed] [Google Scholar]
- 14.Gazzaniga P, Vercillo R, Gradilone A, Silvestri I, Gandini O, Napolitano M, et al. Prevalence of papillomavirus, Epstein-Barr virus, cytomegalovirus, and herpes simplex virus type 2 in urinary bladder cancer. J Med Virol. 1998;55:262–267. doi: 10.1002/(sici)1096-9071(199808)55:4<262::aid-jmv2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 15.Guo CC, Fine SW, Epstein JI. Noninvasive squamous lesions in the urinary bladder: a clinicopathologic analysis of 29 cases. Am J Surg Pathol. 2006;30:883–891. doi: 10.1097/01.pas.0000213283.20166.5a. [DOI] [PubMed] [Google Scholar]
- 16.Kerley SW, Persons DL, Fishback JL. Human papillomavirus and carcinoma of the urinary bladder. Mod Pathol. 1991;4:316–319. [PubMed] [Google Scholar]
- 17.LaRue H, Simoneau M, Fradet Y. Human papillomavirus in transitional cell carcinoma of the urinary bladder. Clin Cancer Res. 1995;1:435–440. [PubMed] [Google Scholar]
- 18.Ludwig M, Köchel HG, Fischer C, Ringert RH, Weidner W. Human papillomavirus in tissue of bladder and bladder carcinoma specimens. A preliminary study. Eur Urol. 1996;30:96–102. doi: 10.1159/000474152. [DOI] [PubMed] [Google Scholar]
- 19.Mantovani G, Cermelli C, Malagoli M, Provvisionato A, Ferrari P, Castagnetti M, et al. IgG antibodies to papillomavirus genus-antigens in adult men with cancer of the urinary bladder. New Microbiol. 1994;17:1–8. [PubMed] [Google Scholar]
- 20.Noel JC, Peny MO, Mat O, Antoine M, Firket C, Detremmerie O, et al. Human papillomavirus type 16 associated with multifocal transitional cell carcinomas of the bladder in two transplanted patients. Transpl Int. 1994;7:340–343. doi: 10.1007/BF00336709. [DOI] [PubMed] [Google Scholar]
- 21.Oft M, Böhm S, Wilczynski SP, Iftner T. Expression of the different viral mRNAs of human papilloma virus 6 in a squamous-cell carcinoma of the bladder and the cervix. Int J Cancer. 1993;53:924–931. doi: 10.1002/ijc.2910530610. [DOI] [PubMed] [Google Scholar]
- 22.Shibutani YF, Schoenberg MP, Carpiniello VL, Malloy TR. Human papillomavirus associated with bladder cancer. Urology. 1992;40:15–17. doi: 10.1016/0090-4295(92)90429-z. [DOI] [PubMed] [Google Scholar]
- 23.Smetana Z, Keller T, Leventon-Kriss S, Huszar M, Lindner A, Mitrani-Rosenbaum S, et al. Presence of human papilloma virus in transitional cell carcinoma in Jewish population in Israel. Cell Mol Biol (Noisy-le-grand) 1995;41:1017–1023. [PubMed] [Google Scholar]
- 24.Tekin MI, Tuncer S, Aki FT, Bilen CY, Aygün C, Ozen H. Human papillomavirus associated with bladder carcinoma? Analysis by polymerase chain reaction. Int J Urol. 1999;6:184–186. doi: 10.1046/j.1442-2042.1999.06435.x. [DOI] [PubMed] [Google Scholar]
- 25.Yu ST, Wu MM, Li LM. Prevalence of human papillomaviruses 16 and 18 in transitional cell carcinoma of bladder. Chin Med J (Engl) 1993;106:494–496. [PubMed] [Google Scholar]
- 26.Yavuzer D, Karadayi N, Salepci T, Baloglu H, Bilici A, Sakirahmet D. Role of human papillomavirus in the development of urothelial carcinoma. Med Oncol. 2011;28:919–923. doi: 10.1007/s12032-010-9540-1. [DOI] [PubMed] [Google Scholar]
- 27.Knowles MA. Human papillomavirus sequences are not detectable by Southern blotting or general primer-mediated polymerase chain reaction in transitional cell tumours of the bladder. Urol Res. 1992;20:297–301. doi: 10.1007/BF00300263. [DOI] [PubMed] [Google Scholar]
- 28.Pareja Vilchez M. Detection of the human papillomavirus in samples of bladder cancer by a DNA hybridization test. Clinical Relations [tesis doctoral] Granada: Granada Univ.; 2008. [Google Scholar]
- 29.Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 30.Li N, Yang L, Zhang Y, Zhao P, Zheng T, Dai M. Human papillomavirus infection and bladder cancer risk: a meta-analysis. J Infect Dis. 2011;204:217–223. doi: 10.1093/infdis/jir248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furihata M, Inoue K, Ohtsuki Y, Hashimoto H, Terao N, Fujita Y. High-risk human papillomavirus infections and overexpression of p53 protein as prognostic indicators in transitional cell carcinoma of the urinary bladder. Cancer Res. 1993;53:4823–4827. [PubMed] [Google Scholar]