Abstract
The production of vocalizations is intimately linked to the respiratory system. Despite our understanding of neural circuits that generate normal respiratory patterns, very little is understood regarding how these ponto-medullary circuits become engaged during vocal production. Songbirds offer a potentially powerful model system for addressing this relationship. Songs dramatically alter the respiratory pattern in ways that are often highly predictable and songbirds have a specialized telencephalic vocal motor circuit that provides massive innervation to a brainstem respiratory network that shares many similarities with its mammalian counterpart. In this review, we highlight interactions between the song motor circuit and the respiratory system, describing how both systems likely interact to produce the complex respiratory patterns that are observed during vocalization. We also discuss how the respiratory system, through its bilateral bottom-up projections to thalamus, might play a key role in sending precisely timed signals that synchronize premotor activity in both hemispheres.
Keywords: vocal production, respiration, songbirds
Introduction
Control of respiration is an autonomous function of the vertebrate brain. While the rhythm generating circuits are located in the ponto-medullary regions of the brainstem, respiration needs to be integrated with other physiological and behavioral functions through carefully orchestrated interactions between numerous different brain circuits and the respiratory system. Song production in songbirds is a particularly interesting example of such interactions because it requires coordination between respiratory circuits and the telencephalic1 vocal motor areas that control production of this learned vocal behavior (Wild, 2004; Schmidt and Ashmore, 2008). The neural mechanisms of this integration are still largely unknown, but initial work suggests that the respiratory system interacts with these vocal motor cortical circuits both as their driver and target.
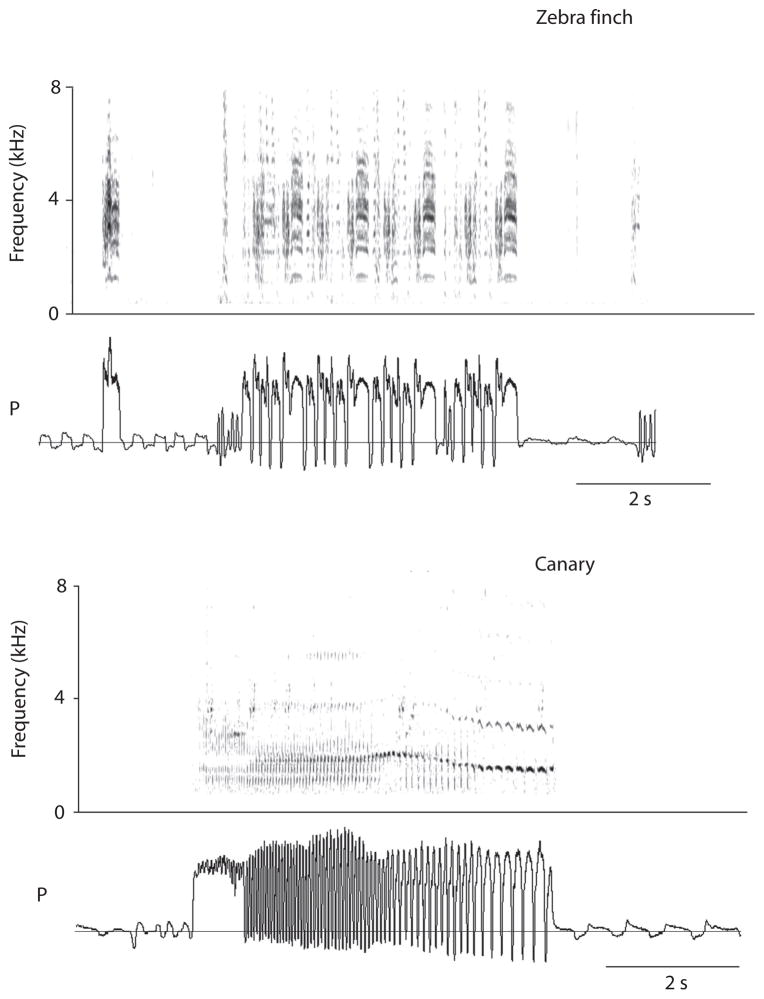
Song production involves the coordinated activity of multiple motor systems (respiration, vocal organ and upper vocal tract structures) (e.g., Suthers et al., 1999; Suthers and Zollinger, 2008; Goller and Cooper 2008). Respiratory patterns underlying song production are characterized by stereotyped sequences of expiratory and inspiratory pulses that determine many aspects of the often complex temporal pattern of sound and silence within a song. In some species, sound pulses and silent periods are repeated (phrases) and different phrases are strung together into songs, which then contain multiple different respiratory rhythms (Figure 1). Because respiratory patterns underlie this complex temporal structure of song, song motor control areas must interact with respiratory pattern generators to produce these rhythms.
Figure 1. Modulation of the respiratory rhythm in two different songbirds species.
Amplitude and temporal pattern of respiratory pressure (P, horizontal line indicates ambient pressure) during vocalization (illustrated as spectrogram in top panel) change markedly from that during normal, silent respiration. Top: Zebra finch song consists of a repetition of a motif (M) composed of 4 (in this example) distinct pressure pulses (top), which is repeated four times in the depicted song bout. A distance call (C) precedes the song bout. Bottom: Canary song is generated with a series of pressure pulses that give rise to different trills. Each sound of the various trills corresponds to an air sac pressure peak, either during a sustained expiratory pressure pulse (first pulse of song) or during rapid alterations between expiration and inspiration (mini-breaths). Song is a series of different trill patterns, each generated by a series of stereotyped respiratory pulses. The respiratory pattern of song therefore contains a sequence of different respiratory rhythms.
Sound production in songbirds is driven mostly, but not exclusively, by expiratory airflow (an active process in birds) through the vocal organ, the syrinx, which in songbirds is a bipartite structure. The mechanical properties of each half of this muscular vocal organ can be independently controlled, allowing it to operate as two separate sound sources. In many songbirds, individual syllables generated during song can therefore be produced either from the left side, the right side or both sides simultaneously (e.g., Suthers and Zollinger, 2008). A remarkable feature of many songbirds is their ability to rapidly switch between contributions from the left and right half of the syrinx.
One important characteristic of the interaction between telencephalic vocal motor centers and the respiratory system in avian song control is that the respiratory system, rather than simply being driven by these higher-order areas in a top-down manner, also likely plays a direct active role in driving motor activity in these areas by way of its “thalamo-cortical” projections (Schmidt and Ashmore, 2008). The bottom-up role has the respiratory control centers acting as protagonist in the generation of key timing signals that drive the generation of vocal motor sequences. Additionally, because of the bilateral nature of these projections and because birds lack a corpus callosum, these signals might also be involved in the coordination of vocal motor activity between hemispheres (Schmidt, 2008).
Brainstem respiratory networks in mammals and birds share many similarities, both in their overall anatomical organization and neuron types. The well defined interaction between specialized vocal motor circuits in the forebrain that control song production and the respiratory system, coupled with the precise manner with which song can be studied in birds, make the avian song system an outstanding model for studying how the respiratory system interacts with the telencephalon both as a target and driver of these vocal motor circuits.
The respiratory system is linked to a specialized vocal control circuit in songbirds
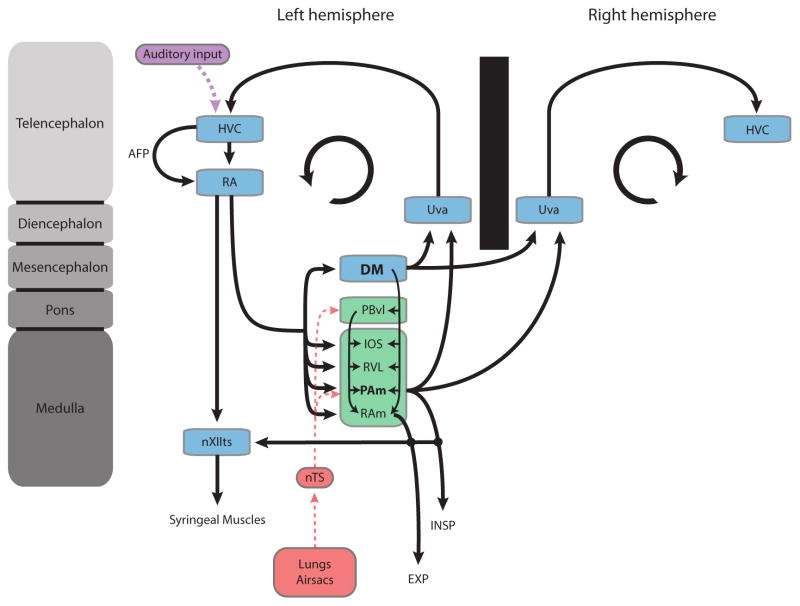
The specialized circuit of interconnected brain nuclei that control vocal production in songbirds is known as the “song system” (Nottebohm et al., 1976; Schmidt, 2010). The motor component of this circuit is made up of nuclei HVC (used as proper name) and RA (robust nucleus of the arcopallium). Although there is no known structure homologous to RA in mammals, the expression of genes in RA that are also selective for layer 5 neurons of mammalian motor cortex suggests that RA is functionally equivalent to the output layer of motor cortex (Dugas-Ford, 2009). RA innervates the motor neurons of the hypoglossal nucleus that drive the syrinx. It also sends a dense network of projections to respiratory nuclei in the medulla (Figure 2).
Figure 2. Avian song system and its link to the respiratory system.
Schematic illustration of the avian song system (shown in blue) and its anatomical relationship with ponto-medullary respiratory areas (shown in green) which receive direct sensory input from putative mechano- and chemosensitive receptors in the lungs and air sacs (red) via parts of the nTS. The song motor system also receives auditory input (purple) by way of nucleus HVC. The inspiratory nucleus PAm (likely homologue to mammalian rVRG) and DM (likely homologue to mammalian lateral PAG) receive a strong projection from RA and project bilaterally back to thalamus (Uva) which itself projects directly back to HVC. The circular arrow in each hemisphere symbolizes the recurrent nature of the song motor circuit. For simplicity many of song system nuclei are not shown in the right hemisphere. Note also the direct connections between the respiratory areas (PAm & RAm) and nXIIts, which contains the motor neurons that innervate the syrinx. All respiratory areas are defined in the text. The one structure that does not receive direct projections from RA but is intimately tied with the structures of the respiratory brainstem is PBvl (nucleus parabrachialis ventrolateralis), which is the likely avian homologue to the Kölliker-fuse nucleus. AFP: anterior forebrain pathway.
Two of the primary respiratory targets of RA are nuclei PAm (nucleus paraambigualis) and RAm (nucleus retroambigualis) in the ventrolateral medulla. Anatomically, these nuclei are thought to be homologous to the caudal (RAm) and rostral (PAm) portions of the ventral respiratory group and innervate motor neurons in the spinal cord that respectively drive expiration and inspiration (Wild, 2008). An additional major target of RA is the midbrain nucleus DM (dorsomedial nucleus of the intercollicular complex). Based on comparative and hodological arguments (Wild, 1997), DM has been suggested to be very much akin to the lateral and ventrolateral regions of periaqueductal gray (PAG), which in mammals seems to coordinate some of the respiratory changes that are associated with vocalizations (Holstege, 1989; Subramanian et al., 2008). DM plays a central role in producing innate vocalizations (Potash, 1970; Vicario and Simpson, 1995) and based on its dense interconnectivity with the respiratory brainstem is likely intimately involved in respiratory control during vocal production (Wild, 2008). In addition to these primary respiratory targets, RA also projects to RVL (ventrolateral nucleus of the rostral medulla) and the IOS (nucleus infra-olivarus superior) which might be avian homologues of the Bötzinger complex (BötC ) and the retrofacial nucleus, however, a strict comparison with BötC is complicated because birds do not possess a functional diaphragm or a phrenic nucleus. The pontine nucleus parabrachialis ventrolateralis (PBvl) does not receive a direct input from RA but is nevertheless likely to play an important role in vocal respiratory control. Based on its anatomical location and connectivity, it is a likely homologue of the mammalian Kölliker-Fuse complex, which is necessary in determining respiratory timing, both in terms of the inspiration to expiration switch (Dutschmann et al., 2004) but also in the coordination and learning of breathing patterns during vocalization (Smotherman et al. 2006; Dutschmann et al., 2009).
The avian song motor circuit receives robust inputs from higher order auditory areas. During vocal learning, auditory feedback signals relayed through these pathways play a major role in shaping many of the properties of HVC and RA (Mooney, 2009). Critical to this process is a specialized basal ganglia-thalamo-cortical loop that receives a major input from HVC and terminates in RA, the final common output of the song motor system (Fee and Scharff, 2010). The song motor circuit also receives afferent feedback information from pressure and chemosensitive receptors in the air sacs and lungs. These are relayed into the song motor system through afferent connections from a part of the nucleus of the solitary tract (nTS) to both PAm and PBvl (Wild, 2004b). The exact role of these inputs on song production and learning remains poorly understood but preliminary findings involving unilateral transections of the vagus nerve suggest that these inputs are necessary for certain aspects of vocal production (Mendez et al. 2010)
Top-down drive of respiratory network
Because respiratory patterns of song are distinctly different in amplitude and temporal structure from those of silent respiration, a major question of song motor control is how HVC and RA exert control over the respiratory pattern generating circuits. Investigation of this question is facilitated by an easily quantified behavioral output (song) whose temporal structure reflects the underlying respiratory patterns. Potential mechanisms for forebrain control of respiration during song are bracketed by two opposing hypotheses.
The integration hypothesis postulates that motor instruction signals from HVC and RA interact with the respiratory circuits to give rise to the stereotyped sequences of song. Such an integrative model was used to explain the respiratory patterns of song in the canary (Serinus canaria) (Trevisan et al., 2006). A simple non-linear network (composed of excitatory and inhibitory neurons, presumably located in the respiratory pre-motor nuclei RAm and PAm), could explain the stereotyped respiratory patterns of all syllable types observed in canaries. Most importantly, the input into this non-linear network (from HVC and RA) does not have to contain all temporal information that emerges in the respiratory output. Simple linear changes in amplitude and frequency of the driving instructions can produce qualitatively different respiratory patterns underlying different syllables. If such an integrative mechanism is used in song motor control, it provides a simple mechanism for motor diversity. Forebrain control of respiration for large repertoires of different song syllables would therefore only require small changes in the instructive signal, rather than precise information containing all temporal aspects. As these changes in input are processed by the respiratory control circuits, distinctly different respiratory patterns emerge from the non-linear properties.
Another hypothesis postulates that all temporal information contained in the behavioral output, the song, originates from higher-order motor instructions originating in the telencephalon. Sparse firing patterns of RA-projecting HVC neurons in the zebra finch (Taeniopygia guttata), are considered, at every time scale, to be instructing neurons in RA to relay this temporal information to the respiratory control circuits (Hahnloser et al., 2002). Support for this hypothesis was derived from experiments in which the temperature of either HVC or RA was experimentally manipulated, and cooling of the former caused a stretching of the song, whereas the same effect was not achieved by cooling of RA (Long & Fee, 2008). This “music box model” suggested a mechanism in which respiratory circuits simply follow, rather than interact with, instructions from the cortical song control centers (Fee et al., 2004; Schmidt and Ashmore, 2008).
Experimental testing of these hypotheses is difficult during song production. However, by stimulating HVC and RA in awake birds and monitoring respiratory output, the basic processing of forebrain input by the respiratory network can be investigated. With this approach, the respiratory pattern showed entrainment in the predicted patterns of various stimulation rhythms, and respiratory output reflected the combined input to the left and right song control areas. Interestingly, effects of the same stimulation regimes on respiratory patterns were different in awake and anesthetized animals, suggesting a strong role of inhibitory processes (Mendez et al., submitted). Although this experimental paradigm of stimulating telencephalic areas during quiet respiration cannot mimic activation during song production, it is likely that this illustrated integration of RA input by the respiratory network also plays a role during respiratory control of song.
Bottom-up drive and hemispheric coordination by respiratory network
During singing, each half of the syrinx is controlled by 6 separate muscles. These are innervated exclusively by motor neurons in the ipsilateral hypoglossal nucleus, which, in the zebra finch, is almost exclusively innervated by the ipsilateral RA (Suthers and Zollinger, 2008). Muscles on each syringeal half therefore receive motor commands generated by ipsilateral song control nuclei. The exquisite coordination between each syringeal half during the production of many syllables implies a tight coordination between the motor commands generated in each hemisphere. Support for this idea comes from simultaneous recordings from left and right HVC where the onset of premotor activity for each syllable in the song is perfectly synchronized between sides as if premotor activity in each HVC were synchronized by a common timing signal (Schmidt, 2003). Because songbirds lack direct connections between song control nuclei in each hemisphere, this synchronizing signal must come from a common source projecting to both the right and left HVC.
Unilateral lesions of the small thalamic relay nucleus uvaeformis (Uva), which sends a direct ipsilateral projection to HVC, causes a desynchronization of premotor activity in the left and right HVC (Coleman and Vu, 2000). It also prevents birds from producing normal syllable sequences (Coleman and Vu, 2005). Based on recordings in singing birds, neurons in Uva appear to produce precisely timed bursts of action potentials at the onset of each syllable, suggesting that these action potentials might serve as the neural correlate of the timing signals that drive HVC activity during key transitions, such as syllable onset, in the song (Williams and Vicario, 1993; Aronov and Fee, 2007). Because PAm (indirectly) and DM (directly) are connected to each other across the midline and send a bilateral projection to Uva, both of these structures are anatomically well situated for synchronizing hemispheres during vocal production (Wild, 2004a). This anatomical argument is supported by physiological evidence showing that activation of PAm (or DM) can drive activity in both ipsilateral and contralateral HVC (or RA) in an Uva-dependent manner (i.e. lesions of Uva abolish stimulus evoked responses) (Ashmore et al., 2008). Therefore stimulation of PAm, a nucleus which drives the motor neurons of inspiration and receives massive inputs from the song motor nucleus RA, is also able to activate, with very short latencies, the same song motor areas (HVC and RA) from which it receives the song motor commands. PAm is therefore a critical component of a recurrent loop for song production and may play a key role in generating the timing signals for hemispheric synchronization during singing.
Neurons in PAm share similarities with mammalian rVRG with a few interesting exceptions
Given its proposed dual role in driving inspiratory behavior and in synchronizing premotor activity in both hemispheres, we were interested in characterizing the types of neurons found in PAm and comparing them to those found in the mammalian rVRG (McLean et al. In preparation). We therefore recorded extracellular units in anesthetized adult zebra finches in combination with air sac pressure to monitor respiratory activity and assigned neurons to a category based on their discharge patterns (augmenting or decrementing) and the phase of their response with respect to the respiratory cycle. In mammals, the respiratory rhythm consists of three phases (Richter, 1982) and at least six different types of respiratory neurons have been described throughout the ventral respiratory column and the majority of these cell types have been postulated to be necessary for the generation of this respiratory rhythm (Smith et al. 2007).
We found that the firing patterns of most neurons, out of a total of 130, matched one of six categories of neuron that have previously been described in the mammalian respiratory brainstem (Bianchi et al., 1995). The majority of these cells were inspiratory related neurons (~48%) as previously reported (Reinke and Wild, 1998; Ashmore et al., 2008), however we also found expiratory related neurons (~15%) and phase-spanning neurons (~22%). In addition to these major classes of neurons, we recorded from neurons whose firing pattern was modulated with the respiratory rhythm but firing patterns did not match any of the categories described above (~10%). Finally, we also recorded from neurons (~5%) that did not show any phase locking to the respiratory rhythm. This last type of neuron, which often could be recorded at the same electrode tip as respiratory neurons, showed phasic patterns of bursting that were identical to those described previously (Ashmore et al., 2008). When recorded simultaneously with the contralateral RA, activity in these “non-respiratory bursting” (NRB) neurons was highly correlated with the bursting pattern recorded in RA of anesthetized birds (Ashmore et al., 2008). While we do not know the projection pattern of this class of neuron, their correlated activity with RA suggests they might form part of a subpopulation of neurons in PAm that, during singing, sends the precisely timed signals that synchronize premotor activity in both hemispheres.
Conclusion
Respiratory control circuits play an important role in song production. On the one hand, this observation is trivial, because the respiratory pattern of song production must accommodate the physiological needs of gas exchange. On the other hand, the specific mechanisms by which the respiratory network integrates descending “cortical” motor commands and sensory input from the periphery to produce the complex respiratory patterns associated with singing is of great interest. The discussed evidence, while illustrating the potential of the songbird system for studying interactions between the forebrain and respiratory circuits, indicates the need for future research.
Although we can make a strong case for interactive connectivity, it is largely unclear how song motor control areas direct the respiratory control network. Here we propose that intrinsic properties of the respiratory network interact with telencephalic vocal motor instructions to generate the respiratory diversity that gives rise to temporal structure of the acoustic signals (songs). This model suggests that temporal complexity and diversity are the product of intrinsic properties of the respiratory circuitry and the higher-order inputs that impinge upon it during singing.
We also propose that the respiratory control network serves an important function by providing input to the forebrain song control centers. Information from the respiratory centers is necessary to inform the song control network about the physiological state of respiratory homeostasis (feedback) and provide phase information. This latter role indicates that the respiratory network is a suitable component of the song control circuit for providing synchronizing information to the left and right HVC at the onset of and during the song sequence. The specific mechanisms of how feedback and timing information are sent to and processed in HVC are exciting research areas with the possibility for providing insight into cortical motor control in general.
Footnotes
Although avian brains do not contain a neocortex, which is a term that defines the layered outer cortex of mammals, birds have a highly developed dorsal telencephalon (pallium) whose organization is in many ways functionally similar to the mammalian cerebral cortex (Butler et al. 2011; Wang et al. 2010).
Contributor Information
Marc F. Schmidt, Department of Biology, Neuroscience Graduate Group, University of Pennsylvania
Judith McLean, Department of Biology, University of Pennsylvania.
Franz Goller, Department of Biology, University of Utah.
References
- Alonso LM, Alliende JA, Goller F, Mindlin GB. Low-dimensional dynamical model for the diversity of pressure patterns used in canary song. Physical Review E. 2009;79:041929. doi: 10.1103/PhysRevE.79.041929. [DOI] [PubMed] [Google Scholar]
- Aronov D, Fee MS. 2007 Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience; 2007. Recordings of antidromically identified projection neurons in motor thalamic nucleus uvaeformis (Uva) of the songbird Program No. 430.5/LLL1; p. 2007. Online. [Google Scholar]
- Ashmore RC, Renk JA, Schmidt MF. Bottom-up activation of the vocal motor forebrain by the respiratory brainstem. J Neurosci. 2008;28:2613–2623. doi: 10.1523/JNEUROSCI.4547-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi AL, Denavit-Saubie M, Champagnat J. Central Control of Breathing in Mammals: neuronal Circuitry, Membrane properties, and Neurotransmitters. Physiological Reviews. 1995;75:1–45. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- Butler AB, Reiner A, Karten HJ. Evolution of the amniote pallium and the origins of mammalian neocortex. Ann NY Acad Sci. 2011;1225:14–27. doi: 10.1111/j.1749-6632.2011.06006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman MJ, Vu ET. Neural activity in HVc of adult zebra finches during the song recovery following unilateral lesions of nucleus uvaeformis. Soc Neurosci Abstract. 2000;26:2031. [Google Scholar]
- Coleman MJ, VUET recovery of impaired songs following unilateral but not bilateral lesions of nucleus uvaeformis of adult zebra finches. J Neurobiol. 2005;63:70 – 89. doi: 10.1002/neu.20122. [DOI] [PubMed] [Google Scholar]
- Dugas-Ford JB. PhD Thesis. University of Chicago; 2009. A comparative molecular study of the amniote dorsal telencephalon. [Google Scholar]
- Dutschmann M, Mörschel M, Kron M, Herbert H. Development of adaptive behaviour of the respiratory network: implications for the pontine Kölliker-Fuse nucleus. Respiratory Physiology & Neurobiology. 2004;143:155–165. doi: 10.1016/j.resp.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Mörschel M, Rybak IA, Dick TE. Learning to breathe: control of the inspiratory-expiratory phase transition shifts from sensory- to central-dominated during postnatal development in rats. J Physiol. 2009;587:4931–4948. doi: 10.1113/jphysiol.2009.174599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fee MS, Scharff C. The songbird as a model for the generation and learning of complex sequential behaviors. ILAR. 2010;51:362 – 377. doi: 10.1093/ilar.51.4.362. [DOI] [PubMed] [Google Scholar]
- Fee MS, Kozhevnikov AA, Hahnloser RH. Neural mechanisms of vocal sequence generation in the songbird. Ann N Y Acad Sci. 2004;1016:153–70. doi: 10.1196/annals.1298.022. [DOI] [PubMed] [Google Scholar]
- Goller F, Cooper BG. Peripheral sensorimotor mechanisms and the control of song. In: Zeigler HP, Marler P, editors. The Neuroscience of Birdsong. Cambridge University Press; 2008. pp. 99–114. [Google Scholar]
- Hahnloser RH, Kozhevnikov AA, Fee MS. An ultra-sparse code underlies the generation of neural sequences in a songbird. Nature. 2002;419:65–70. doi: 10.1038/nature00974. [DOI] [PubMed] [Google Scholar]
- Holstege G. Anatomical study of the final common pathway for vocalization in the cat. J Comp Neurol. 1989;284:242–52. doi: 10.1002/cne.902840208. [DOI] [PubMed] [Google Scholar]
- Long MA, Fee MS. Using temperature to analyze temporal dynamics in the songbird motor pathway. Nature. 2008;456:189–194. doi: 10.1038/nature07448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez JM, Dall’Asén AG, Goller F. Disrupting vagal feedback affects birdsong motor control. J Exp Biol. 2010;213:4193–4204. doi: 10.1242/jeb.045369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez JM, Mindlin GB, Goller F. Interaction between telencephalic signals and respiratory dynamics in songbirds. J Neurophysiol. doi: 10.1152/jn.00646.2011. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney R. Neural mechanisms for learned birdsong. Learning & Memory. 2009;16:655–669. doi: 10.1101/lm.1065209. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary. Serinus canaria. J Comp Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- Potash LM. Vocalizations elicited by electrical brain stimulation in Coturnix coturnix japonica. Behaviour. 1970;3:149 – 167. doi: 10.1163/156853970x00286. [DOI] [PubMed] [Google Scholar]
- Reinke H, Wild JM. Distribution and connections of inspiratory premotor neurons in songbirds and budgerigar. J Comp Neurol. 1998;391:147 – 163. [PubMed] [Google Scholar]
- Schmidt MF. Pattern of interhemispheric synchronization in HVc during singing correlates with key transitions in the song pattern. J Neurophysiol. 2003;90:3931 – 3949. doi: 10.1152/jn.00003.2003. [DOI] [PubMed] [Google Scholar]
- Schmidt MF. Using Both Sides of Your Brain: The Case for Rapid Interhemispheric Switching. PLoS Biology. 2008;6:2089–2093. doi: 10.1371/journal.pbio.0060269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MF. ENCYCLOPEDIA OF LIFE SCIENCES. Chichester John Wiley & Sons, Ltd; 2009. Neural Control of Birdsong. [Google Scholar]
- Schmidt MF, Ashmore R. Integrating breathing and singing: forebrain and brainstem mechanisms. In: Zeigler HP, Marler P, editors. Neuroscience of Birdsong. Cambridge: Cambridge University Press; 2008. pp. 115–135. [Google Scholar]
- Smith JC, Abdala AP, Koizumi H, Rybak IA, Paton JF. Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J Neurophysiol. 2007;98:3370 – 3387. doi: 10.1152/jn.00985.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotherman M, Kobayasi K, Ma J, Zhang S, Metzner W. A mechanism for vocal-respiratory coupling in the mammalian parabrachial nucleus. J Neurosci. 2006;26:4860–4869. doi: 10.1523/JNEUROSCI.4607-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian HH, Balnave RJ, Holstege G. The midbrain periaqueductal gray control of respiration. J Neurosci. 2008;28:12274–12283. doi: 10.1523/JNEUROSCI.4168-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthers RA, Zollinger SA. From brain to song: the vocal organ and vocal tract. In: Zeigler HP, Marler P, editors. Neuroscience of Birdsong. Cambridge: Cambridge Univ. Press; 2008. pp. 78–98. [Google Scholar]
- Suthers RA, Goller F, Pytte C. The neuromuscular control of birdsong. Philos Trans R Soc Lond B Biol Sci. 1999;354:927–39. doi: 10.1098/rstb.1999.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevisan MA, Goller F, Mindlin GB. Nonlinear model predicts diverse respiratory patterns of birdsong. Phys Rev Lett. 2006;96:058103. doi: 10.1103/PhysRevLett.96.058103. [DOI] [PubMed] [Google Scholar]
- Vicario DS, Simpson HB. Electrical stimulation in forebrain elicits learned vocal patterns in songbirds. J Neurophysiol. 1995;73:2602 – 2607. doi: 10.1152/jn.1995.73.6.2602. [DOI] [PubMed] [Google Scholar]
- Wang Y, Brzozowska-Prechtl A, Karten HJ. Laminar and columnar auditory cortex in avian brain. PNAS 2010. 2010;107(28):12676–12681. doi: 10.1073/pnas.1006645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild JM. Projections of the dorsomedial nucleus of the intercollicular complex (DM) in relation to respiratory-vocal nuclei in the brainstem of pigeaon (Comlumba livia) and zebra finch (Taeniopygia guttata) J Comp Neurol. 1997;377:392–413. doi: 10.1002/(sici)1096-9861(19970120)377:3<392::aid-cne7>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Wild JM. Functional neuroanatomy of the sensorimotor control of singing. Ann NY Acad Sci. 2004a;1016:438–462. doi: 10.1196/annals.1298.016. [DOI] [PubMed] [Google Scholar]
- Wild JM. Pulmonary and tracheosyringeal afferent inputs to the avian song system. Seventh Congress of the International Society for Neuroethology; Nyborg, Denmark. 2004b. [Google Scholar]
- Wild JM. Birdsong: anatomical foundations and central mechanisms of sensorimotor integration. In: Zeigler HP, Marler P, editors. Neuroscience of Birdsong. Cambridge: Cambridge Univ. Press; 2008. pp. 136–152. [Google Scholar]
- Williams H, Vicario DS. Temporal patterning of song production: participation of nucleus uvaeformis of the thalamus. J Neurobiol. 1993;24:903 – 912. doi: 10.1002/neu.480240704. [DOI] [PubMed] [Google Scholar]