Abstract
Mesenteric fibromatosis poses a diagnostic and therapeutic challenge. This paper presents a 35-year-old female complaining of vague abdominal pain of 2 mo duration. Her computed tomography scan and magnetic resonance imaging revealed a pelvi-abdominal heterogenous mass with significant displacement of the small bowel and urinary bladder. She underwent surgical excision of the mass with resection and anastomosis of the involved loop of the small intestine. Histological examination confirmed mesenteric fibromatosis without infiltration of the bowel. The patient remained well during the 6 mo follow-up.
Keywords: Desmoid tumors, Mesenteric fibromatosis, Radiotherapy
INTRODUCTION
Fibromatosis are a rare heterogenous group of conditions which includes mesenteric fibromatosis[1]. Mesenteric fibromatosis are benign proliferation of mesenteric fibrous tissue that tends to recur without distant metastasis[2]. These are locally aggressive tumors of the mesentery with a high propensity for bowel involvement[3]. Data concerning these tumors as a distinct pathological entity are lacking[4]. The real etiology of mesenteric fibromatosis is unknown. Most reported cases have been in association with Gardner’s Syndrome, previous trauma and prolonged intake of estrogen[5], but mesenteric fibromatosis can occur as a primary condition in the absence of any predisposing condition. The tumor presents either due to its mass effect or due to obstruction of the surrounding structures, including ureters and small bowel.
The present report outlines a case of mesenteric fibromatosis presenting with generalized and vague abdominal pain which was managed in the Department of Surgery in Imam Abdelrehman Bin Faisal Hospital, Dammam, Kingdom of Saudi Arabia.
CASE REPORT
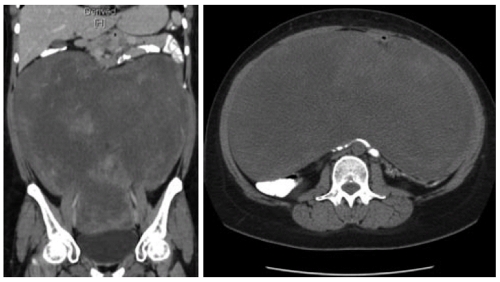
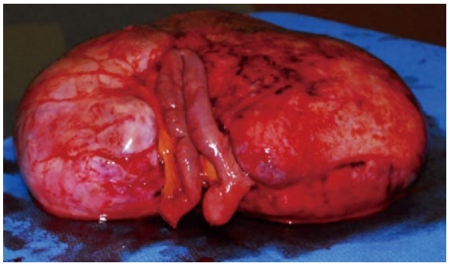
A 35-year old lady presented with history of vague abdominal pain of two months duration. She had no past history of colonic polyps, abdominal trauma or surgical therapy for any other disease. Her general physical examination was unremarkable. Abdominal examination revealed a mobile, non-tender, firm and globular mass encompassing the entire abdomen. All her base-line blood investigations were normal. A contrast enhanced computed tomography (CT) scan showed a huge pelvi-abdominal mass (10 cm × 28 cm × 27cm) extending from the upper surface of the urinary bladder to the under surface of the liver, leading to upward displacement of the small bowel and mesentery (Figure 1). magnetic resonance imaging of the abdomen and pelvis demonstrated an intraperitoneal mass with iso to low signals on T1-weighted images and heterogenous bright and low signals on T2-weighted images, consistent with the fibrous and myxoid nature of the lesion (Figure 2). The patient was prepared for surgery and, under general anesthesia, bilateral ureteric stents were placed for clear identification of the ureters during excision of the mass. A laparotomy was performed which revealed a massive growth occupying the abdomen attached to the mid-ileal region of the bowel. The mass was excised with a part of the adherent bowel using linear GIA stapler anastomosis (Figure 3). The specimen weighed 2.5 kg. The patient had an uneventful post operative recovery. The histopathology of the specimen demonstrated a tumor composed of stellate and spindle cells arranged in a storiform pattern (Figure 4). The cells were cytologically bland with variable areas of dense fibrous tissue and scattered keloid type collagen fibers. The tumor was immunoreactive to Vimentin (Monoclonal M-clone V9) but equivocal to Beta-Catentin. Final histological diagnosis of mesenteric fibromatosis was established with no infiltration of the bowel. The patient was free of recurrence during the 6 mo follow-up period, as observed by a three monthly ultrasound scan and physical examination.
Figure 1.
Contrast enhanced computed tomography scan showing a huge inhomogenous mass occupying almost whole of the abdomen and pelvis with displacement of urinary bladder and bowel loops. A: sagittal; B: axial sections.
Figure 2.
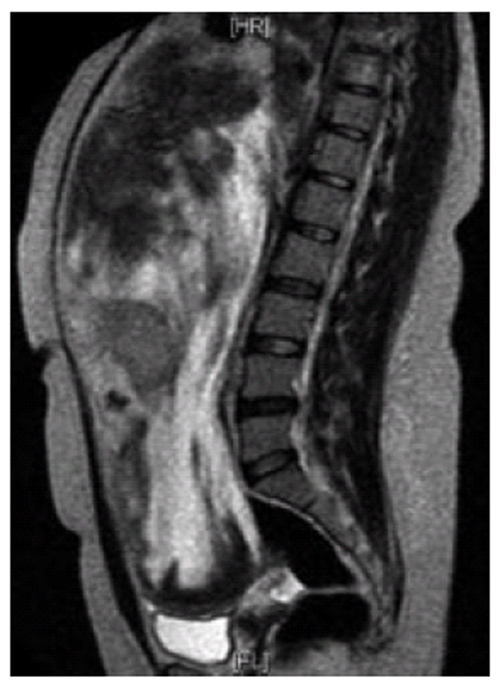
Magnetic resonance imaging abdomen and pelvis. T2 weighted image (sagittal section) showing a huge intraperitoneal well defined mass displacing the liver and bowels cranially and bladder caudally with heterogenous bright and and low signals.
Figure 3.
Resected specimen alongwith attached segment of the bowel.
Figure 4.
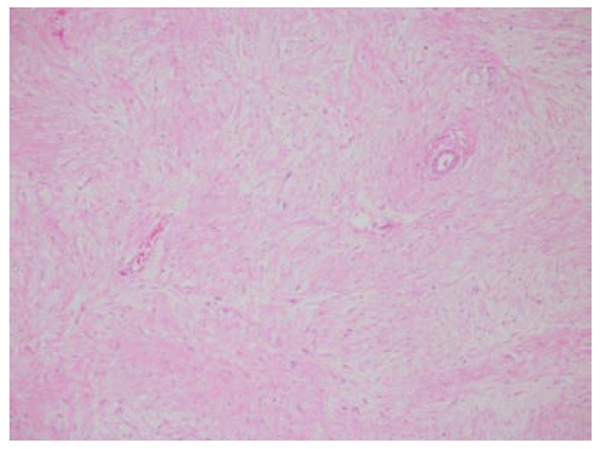
Stellate and spindle cells arranged in a storiform pattern.
DISCUSSION
The concept of fibromatosis as a group of similar lesions was first coined by Stout in 1961[6]. The fibromatosis can affect both superficial and deep parts of the body. Superficial fibromatosis involves the face and neck (fibromatosis coli), palms (Duputyren’s contracture), feet (Ledderhose’s disease), penis (Peyronie’s disease), shoulder, thigh, buttock and trunk[7]. The deep variant involves the abdominal wall, mesentery, retroperitoneum, mediastinum and abdominal cavity[8,9]. Fibromatosis are rare, accounting for 0.03% of all tumors[10].
Mesenteric fibromatosis are most frequently found in young adults with a peak incidence at 30 years. The sex incidence varies, with Yannopoulos and Stout[11] reporting an equal frequency in the males and females while Dong-Heup et al[12] documented a female predominance. The majority of patients with mesenteric fibromatosis remain clinically asymptomatic, with little or no focal symptoms until later in their course, at which stage they complain of abdominal pain and discomfort, constipation, vomiting, weight loss[7] and organ compression symptoms, such as small bowel obstruction and hydronephrosis[7]. Koh et al[13] divided the clinical course of mesenteric fibromatosis into five categories: (1) spontaneous regression; (2) stable; (3) variable growth; (4) progressive growth; and (5) aggressive growth. Of these categories, 75% have been reported to show a progressive growth pattern, necessitating the need for a vigilant work up and early surgical intervention.
The etiopathogenesis of mesenteric fibromatosis is unclear; various factors have been implicated by different researchers. Trauma, especially operative trauma, may contribute to the formation of these lesions[14]. Growth of these tumors is slowest in young girls and reaches a peak at menopause, suggesting that estrogen may act as a growth factor. Furthermore, estradiol receptors have been demonstrated in these tumors in amounts higher than in control tissue[8]. Genetic predisposition also plays a role and patients with Gardner’s Syndrome have a much greater potential of developing mesenteric fibromatosis compared with the normal population. An infectious etiology is also suggested by an association of human herpes virus and retroperitoneal fibromatosis in the macaque monkey[15].
A preoperative differential diagnosis of mesenteric fibromatosis may include intestinal carcinoma, carcinoid tumor, lymphoma and retroperitoneal fibrosis. As there is no classical symptomatology related to mesenteric fibromatosis, the diagnosis is confirmed only after the histological analysis of the tumor. Imaging remains the mainstay of preoperative investigations to establish a working diagnosis of mesenteric fibromatosis. Plain radiographs may show a soft tissue mass. Barium studies may reveal compression and angulation of the distal duodenum, separation of the small bowel loops and, rarely, mucosal tethering[8]. The sonographic features of mesenteric fibromatosis are non specific and chiefly dependent on collagen and fibroblast content and intralesional vascularity of the tumor[10]. Due to the tendency of aggressive mesenteric fibromatosis to invade adjacent structures, CT scan is considered the first-line imaging modality for identifying, characterizing and staging fibromatosis. On CT, these tumors appear as a soft tissue mass displacing/involving surrounding viscera, usually appearing as encasement of bowel loops[16]. Although the mass may appear well-circumscribed, it often has irregular margins reflecting its infiltrative nature. On magnetic resonance imaging, these tumors appear hypointense on T1-weighted images, due to their major fibrous composition. On T-2 weighted images, they show variable signal intensity with a hyperintense signal from those that had demonstrated marked growth on follow-up imaging[17].
Most mesenteric fibromatosis measure 5-10 cm in the greatest dimension, although larger lesions up to 24 cm have been reported[18]. However, to the best of our knowledge, the tumor described here is the first ever case of mesenteric fibromatosis that attained this enormous size. Microscopically, mesenteric fibromatosis is characterized by a spatially homogenous proliferation of wavy spindle cells without atypia, associated with collagen among dilated vessels. The mitotic count is relatively low with no evidence of necrosis and nuclear dedifferention[19]. Immunohistochemistry demonstrates tumor cells being positive for vimentin and smooth muscle actin[20]. The histological differential diagnosis includes reactive fibrosis and fibrosarcoma[21]. The absence of pleomorphism, significant mitotic activity or atypical mitoses assists to differentiate fibromatosis from fibrosarcoma. There is no evidence of inflammation, fat necrosis and vascular invasion, as seen in retroperitoneal fibrosis.
Wide field surgical excision is the first-line treatment for most mesenteric fibromatosis[1]. As noted in our case, the majority of these lesions require resection of the attached segment of the bowel[22]. Radiotherapy may be used before surgery in cases of recurrence and inoperable lesions to shrink the tumor and make it operable. Adjuvant radiation therapy reduces recurrence of mesenteric fibromatosis to 20%-40%, compared to 40%-70% with resection only[23]. In cases where surgery and radiotherapy are not rewarded by the desired success, systemic therapy with pharmacological agents (antiproliferative and cytotoxic drugs) can be employed, including estrogen receptor antagonist tamoxifen, nonsteroidal anti-inflammatory drugs agent sulindac and chemotherapy with dactinomycin, vincristine and cyclophosphamide, singly or in combination, with varying success[24]. Tonelli et al[14] reported that the daily use of raloxofene decreased mesenteric fibromatosis size without significant side effects. Teik-Ying and co-researchers[25] demonstrated the resolution of hydronephrosis due to massive mesenteric fibromatosis by using cyclo-oxygenase 2 inhibitors. Despite all these sporadic reports, surgical excision is the gold standard primary treatment for mesenteric fibromatosis.
To conclude, mesenteric fibromatoses present with bizarre clinical features and demonstrate a wide spectrum of imaging and histological details. Treating physicians should have a high index of suspicion while managing a patient with an abdominal mass.
ACKNOWLEDGMENTS
The authors thank Dr. Nadia Al-Hawashim, FRCPA, FIAC, IFCAP, Chairman Pathology and Lab Medicine, Eastern Region, Consultant Pathologist, National Guard Hospital Al Ahsa, Saudi Arabia.
Footnotes
Peer reviewer: Francis Seow-Choen, MBBS, FRCS, FAMS, Seow-Choen Colorectal Centre Pte Ltd., 3 Mt Elizabeth #09-10, Mt Elizabeth Medical Centre, Singapore 228510, Singapore
S- Editor Wang JL L- Editor Roemmele A E- Editor Xiong L
References
- 1.Yang CH, Sheen-Chen SM, Lu CC, Ko SF, Eng HL. Computed tomographic presentation of mesenteric fibromatosis. Dig Dis Sci. 2005;50:348–350. doi: 10.1007/s10620-005-1609-x. [DOI] [PubMed] [Google Scholar]
- 2.Enzinger FM, Weiss SW. Fibromatosis. In: Enzinger FM, Weiss SW, editors. Soft tissue tumor. St. Louis, MO: C.V. Mosby Co.;; 1995. pp. 201–229. [Google Scholar]
- 3.Lahat G, Nachmany I, Itzkowitz E, Abu-Abeid S, Barazovsky E, Merimsky O, Klauzner J. Surgery for sporadic abdominal desmoid tumor: is low/no recurrence an achievable goal? Isr Med Assoc J. 2009;11:398–402. [PubMed] [Google Scholar]
- 4.McCormack D, Kesha K, Tittle SL, Saldinger PF. Mesenteric fibromatosis mimicking a gastrointestinal stromal tumor. Conn Med. 2010;74:197–200. [PubMed] [Google Scholar]
- 5.Karakousis CP, Berjian RA, Lopez R, Rao U. Mesenteric fibromatosis in Gardner's syndrome. Arch Surg. 1978;113:998–1000. doi: 10.1001/archsurg.1978.01370200092018. [DOI] [PubMed] [Google Scholar]
- 6.Stout AP. The fibromatosis. Clin Orthop. 1961;19:11–18. [Google Scholar]
- 7.Faria SC, Iyer RB, Rashid A, Ellis L, Whitman GJ. Desmoid tumor of the small bowel and the mesentery. AJR Am J Roentgenol. 2004;183:118. doi: 10.2214/ajr.183.1.1830118. [DOI] [PubMed] [Google Scholar]
- 8.Lath C, Khanna PC, Gadewar SB, Agrawal D. Inoperable aggressive mesenteric fibromatosis with ureteric fistula. Case report and literature review. Eur J Radiol. 2006;59:117–121. doi: 10.1016/j.ejrad.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 9.Kabra V, Chaturvedi P, Pathak KA, deSouza LJ. Mesenteric fibromatosis: a report of three cases and literature review. Indian J Cancer. 2001;38:133–136. [PubMed] [Google Scholar]
- 10.Guglielmi G, Cifaratti A, Scalzo G, Magarelli N. Imaging of superficial and deep fibromatosis. Radiol Med. 2009;114:1292–1307. doi: 10.1007/s11547-009-0458-7. [DOI] [PubMed] [Google Scholar]
- 11.Yannopoulos K, Stout AP. Primary solid tumors of the mesentery. Cancer. 1963;16:914–927. doi: 10.1002/1097-0142(196307)16:7<914::aid-cncr2820160708>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 12.Dong-Heup K, Kim DH, Goldsmith HS, Quan SH, Huvos AG. Intra-abdominal desmoid tumor. Cancer. 1971;27:1041–1045. doi: 10.1002/1097-0142(197105)27:5<1041::aid-cncr2820270506>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 13.Koh PK, Loi C, Cao X, Cheah PY, Ho KS, Ooi BS, Tang CL, Eu KW. Mesenteric desmoid tumors in Singapore familial adenomatous polyposis patients: clinical course and genetic profile in a predominantly Chinese population. Dis Colon Rectum. 2007;50:75–82. doi: 10.1007/s10350-006-0759-z. [DOI] [PubMed] [Google Scholar]
- 14.Tonelli F, Ficari F, Valanzano R, Brandi ML. Treatment of desmoids and mesenteric fibromatosis in familial adenomatous polyposis with raloxifene. Tumori. 2003;89:391–396. doi: 10.1177/030089160308900408. [DOI] [PubMed] [Google Scholar]
- 15.Sherman NE, Romsdahl M, Evans H, Zagars G, Oswald MJ. Desmoid tumors: a 20-year radiotherapy experience. Int J Radiat Oncol Biol Phys. 1990;19:37–40. doi: 10.1016/0360-3016(90)90131-3. [DOI] [PubMed] [Google Scholar]
- 16.Brooks AP, Reznek RH, Nugent K, Farmer KC, Thomson JP, Phillips RK. CT appearances of desmoid tumours in familial adenomatous polyposis: further observations. Clin Radiol. 1994;49:601–607. doi: 10.1016/s0009-9260(05)81875-6. [DOI] [PubMed] [Google Scholar]
- 17.Healy JC, Reznek RH, Clark SK, Phillips RK, Armstrong P. MR appearances of desmoid tumors in familial adenomatous polyposis. AJR Am J Roentgenol. 1997;169:465–472. doi: 10.2214/ajr.169.2.9242755. [DOI] [PubMed] [Google Scholar]
- 18.Kulaylat MN, Karakousis CP, Keaney CM, McCorvey D, Bem J, Ambrus Sr JL. Desmoid tumour: a pleomorphic lesion. Eur J Surg Oncol. 1999;25:487–497. doi: 10.1053/ejso.1999.0684. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez JA, Guarda LA, Rosai J. Mesenteric fibromatosis with involvement of the gastrointestinal tract. A GIST simulator: a study of 25 cases. Am J Clin Pathol. 2004;121:93–98. doi: 10.1309/59VA-H0KV-F53W-B633. [DOI] [PubMed] [Google Scholar]
- 20.Rakha EA, Kandil MA, El-Santawe MG. Gigantic recurrent abdominal desmoid tumour: a case report. Hernia. 2007;11:193–197. doi: 10.1007/s10029-006-0165-4. [DOI] [PubMed] [Google Scholar]
- 21.Hailemariam S, Jaeger P, Goebel N, Grant JW. Mesenteric fibromatosis with ureteric stenosis. Postgrad Med J. 1988;64:79–81. doi: 10.1136/pgmj.64.747.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith AJ, Lewis JJ, Merchant NB, Leung DH, Woodruff JM, Brennan MF. Surgical management of intra-abdominal desmoid tumours. Br J Surg. 2000;87:608–613. doi: 10.1046/j.1365-2168.2000.01400.x. [DOI] [PubMed] [Google Scholar]
- 23.Khorsand J, Karakousis CP. Desmoid tumors and their management. Am J Surg. 1985;149:215–218. doi: 10.1016/s0002-9610(85)80067-2. [DOI] [PubMed] [Google Scholar]
- 24.Yantiss RK, Spiro IJ, Compton CC, Rosenberg AE. Gastrointestinal stromal tumor versus intra-abdominal fibromatosis of the bowel wall: a clinically important differential diagnosis. Am J Surg Pathol. 2000;24:947–957. doi: 10.1097/00000478-200007000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Ng TY, Yang MD, Chen YF, Chang CH. Resolution of hydronephrosis due to massive mesenteric fibromatosis using cyclo-oxygenase 2 inhibitors. Urology. 2007;70:591.e3–591.e4. doi: 10.1016/j.urology.2007.07.025. [DOI] [PubMed] [Google Scholar]