Abstract
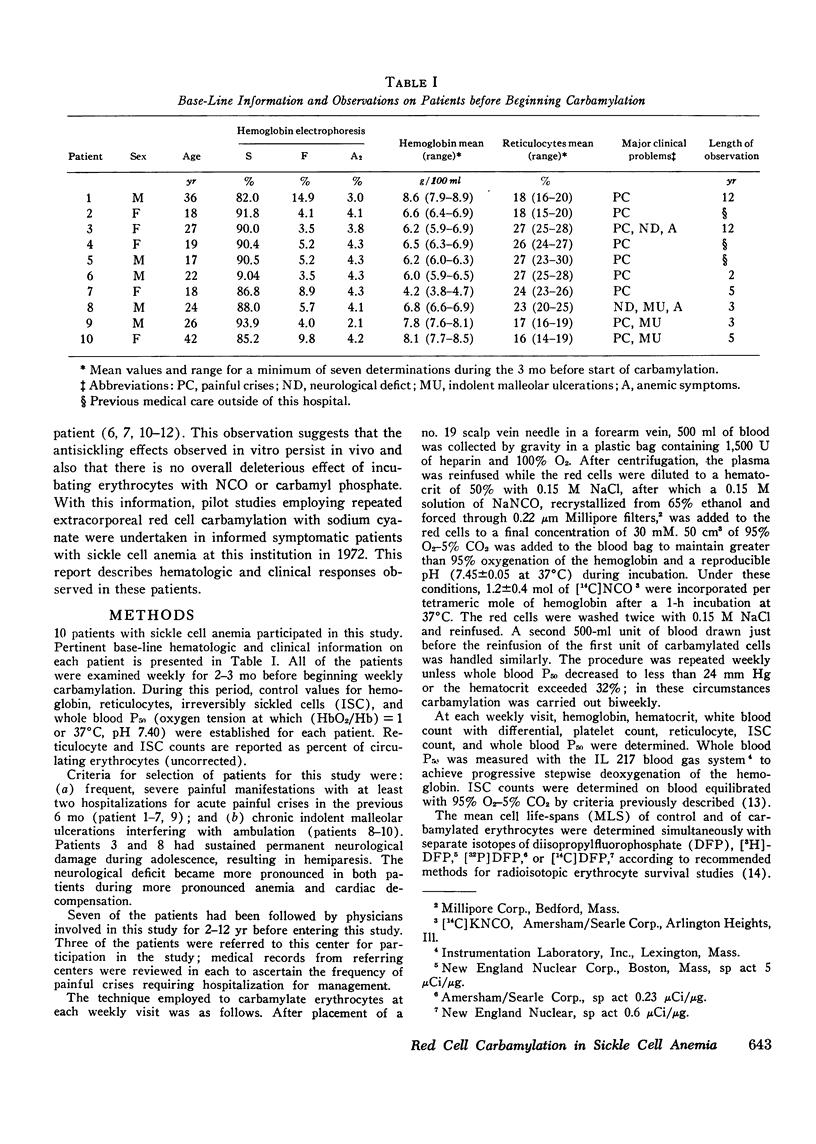
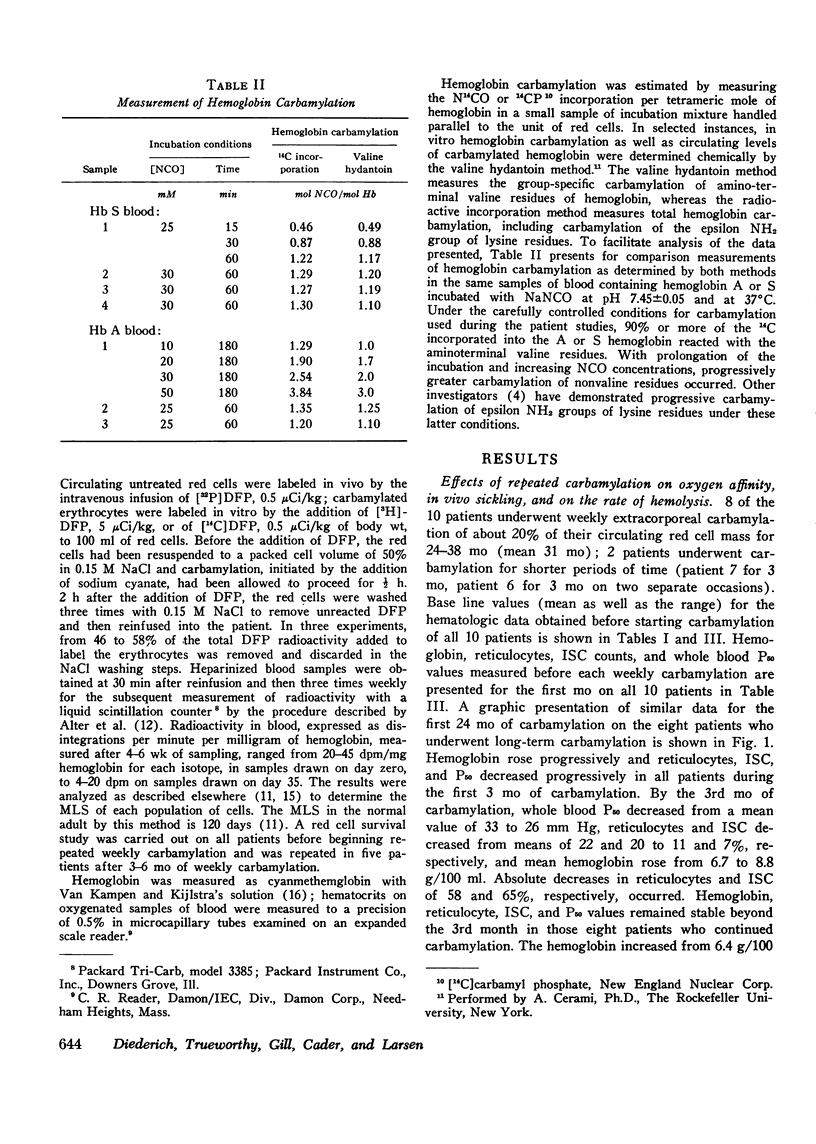
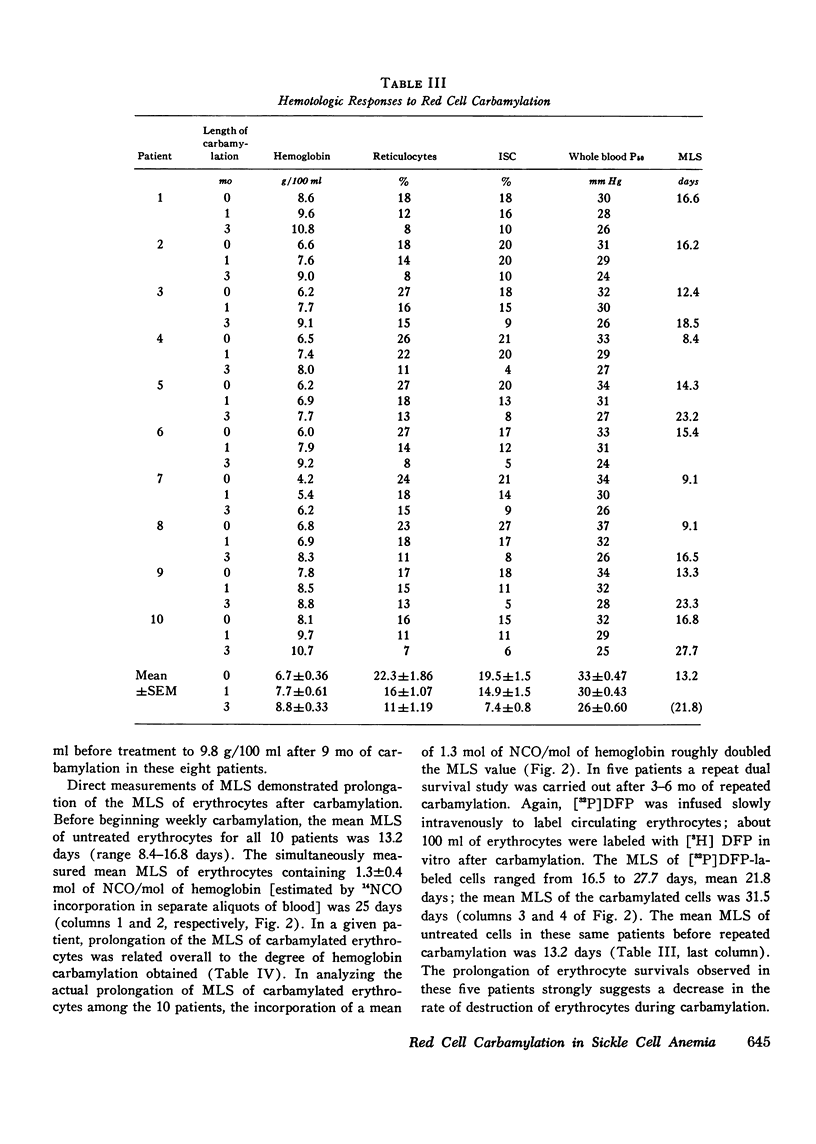
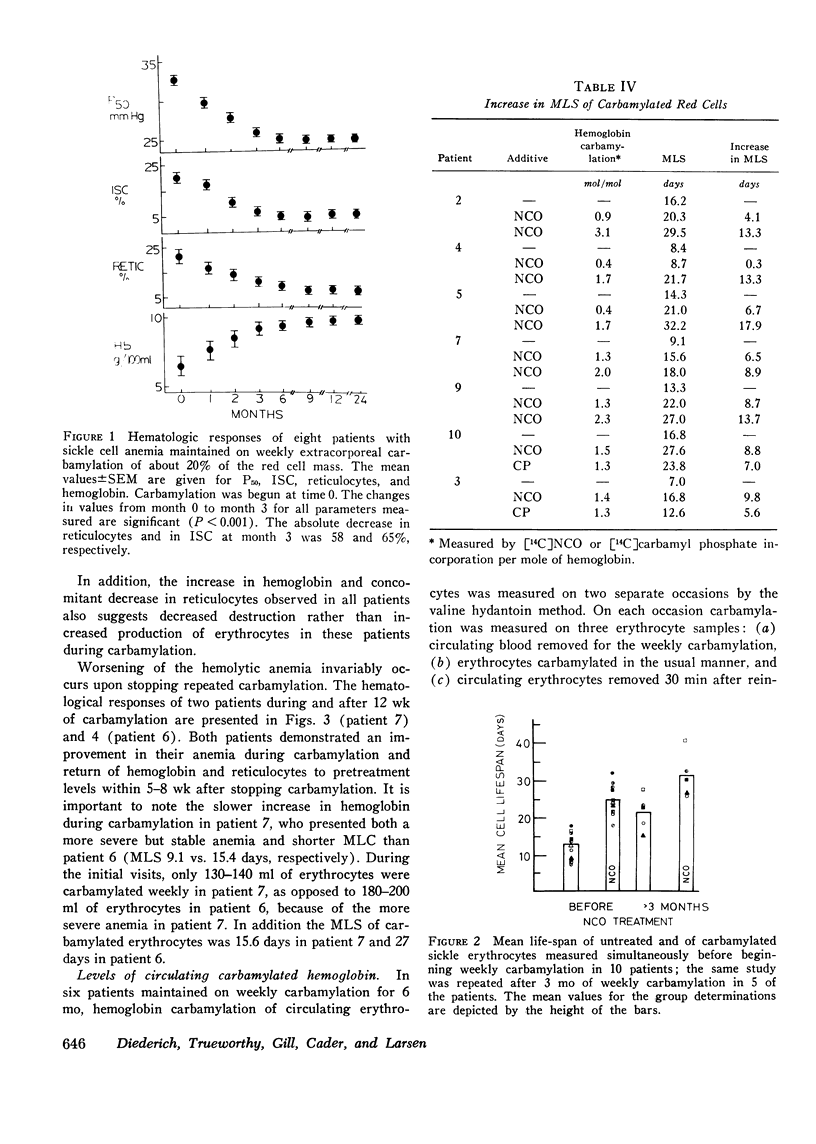
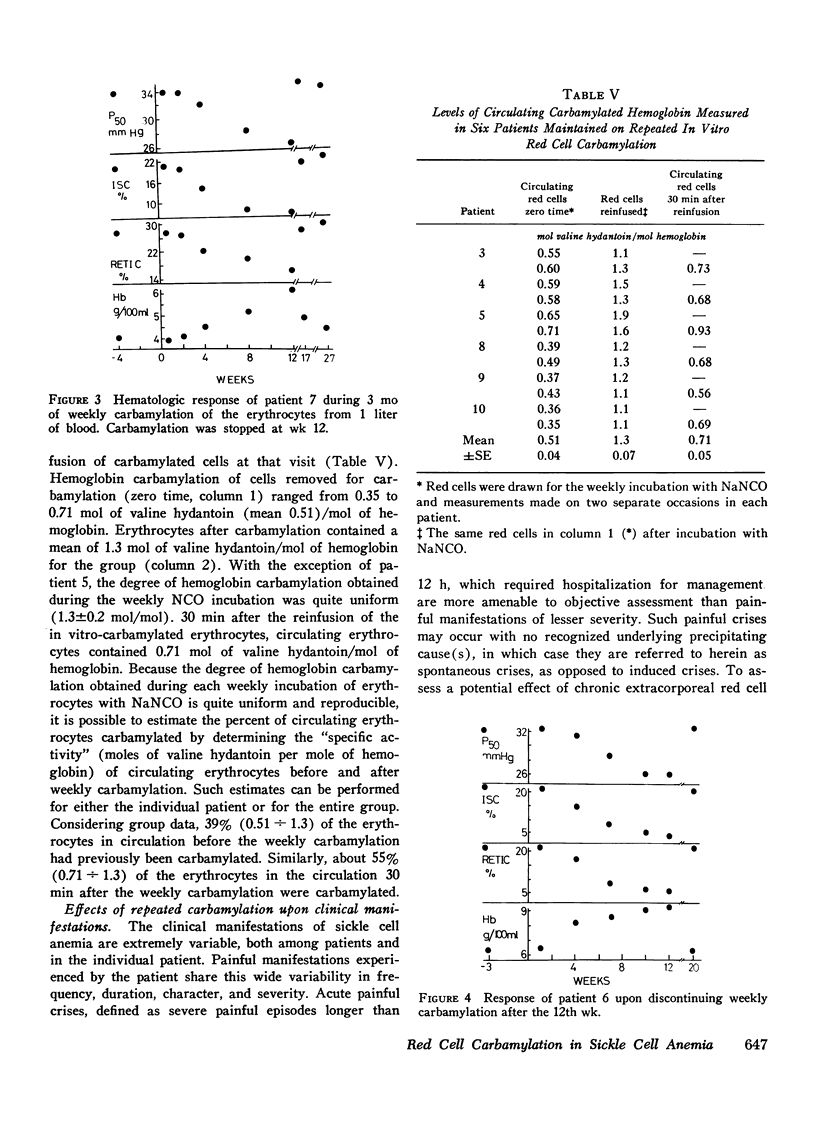
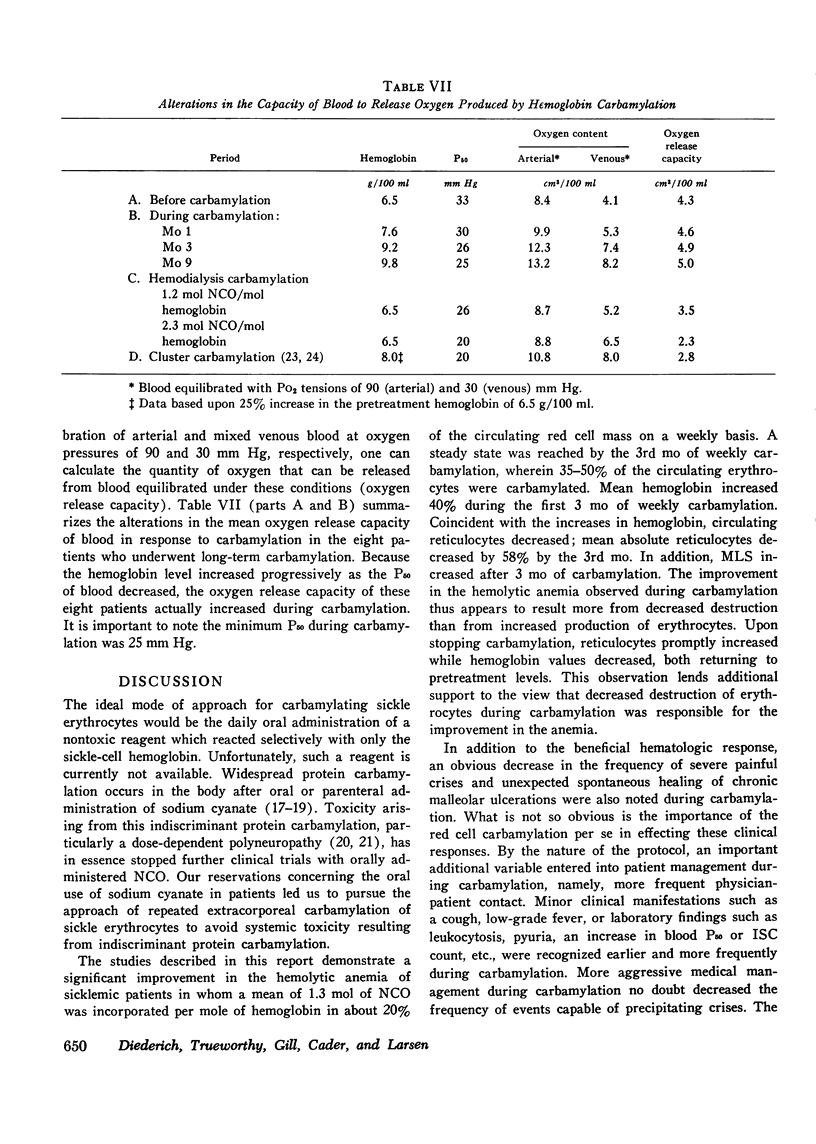
In eight patients with sickle cell anemia, weekly extracorporeal carbamylation of about 20% of the circulating red cell mass was carried out for 2 yr or longer. At each visit, a mean of 1.3+/-0.2 mol of cyanate were incorporated per mole of hemoglobin in the carbamylated erythrocytes. Within 3 mo, a stable level of about 35-50% of the circulating erythrocytes was carbamylated. This quantity and degree of hemoglobin carbamylation produced a decrease in mean whole blood P50 from 33 to 26 mm Hg. During the first 3 mo of carbamylation, the mean hemoglobin increased from 6.4 to 9.1 g/100 ml, while mean absolute reticulocytes decreased by 58% and circulating irreversibly sickled erythrocytes decreased by 65%. The mean red cell life span increased from 13 days before treatment to 21.6 days after 3 mo of carbamylation. Beyond the 3rd mo of carbamylation, blood P50, hemoglobin, and reticulocytes remained quite stable. No toxic effects of extracorporeal carbamylation of erythrocytes were noted. The capacity of blood to release oxygen at 30 mm Hg PO2 increased from 4.3 to 5.0 cm3/100 ml blood during carbamylation. The overall frequency of severe painful crises decreased by about 80% during carbamylation. Before carbamylation, 34% of the crises were induced by a concomitant illness, usually an infection. During carbamylation, the incidence of induced crises decreased 50% while spontaneous crises virtually disappeared. The marked improvements in hematologic parameters and the decreased frequency of severe painful crises observed during this study offer sufficient promise to warrant further exploration, hopefully using more efficient techniques, of the clinical efficacy of extracorporeal erythrocyte carbamylation in sickle cell anemia.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alter B. P., Kan Y. W., Nathan D. G. Reticulocyte survival in sickle cell anemia: effect of cyanate. Blood. 1972 Nov;40(5):733–739. [PubMed] [Google Scholar]
- Bertles J. F., Milner P. F. Irreversibly sickled erythrocytes: a consequence of the heterogeneous distribution of hemoglobin types in sickle-cell anemia. J Clin Invest. 1968 Aug;47(8):1731–1741. doi: 10.1172/JCI105863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerami A., Allen T. A., Graziano J. H., DeFuria F. G., Manning J. M., Gillette P. N. Pharmacology of cyanate. I. General effects on experimental animals. J Pharmacol Exp Ther. 1973 Jun;185(3):653–666. [PubMed] [Google Scholar]
- Cerami A. Cyanate as an inhibitor of red-cell sickling. N Engl J Med. 1972 Oct 19;287(16):807–812. doi: 10.1056/NEJM197210192871606. [DOI] [PubMed] [Google Scholar]
- Cerami A., Manning J. M. Potassium cyanate as an inhibitor of the sickling of erythrocytes in vitro. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1180–1183. doi: 10.1073/pnas.68.6.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crist R. D., Grisolia S., Bettis C. J., Grisolia J. Carbamoylation of proteins following administration to rats of carbamoyl phosphate and cyanate and effects on memory. Eur J Biochem. 1973 Jan 3;32(1):109–116. doi: 10.1111/j.1432-1033.1973.tb02585.x. [DOI] [PubMed] [Google Scholar]
- De Furia F. G., Miller D. R., Cerami A., Manning J. M. The effects of cyanate in vitro on red blood cell metabolism and function in sickle cell anemia. J Clin Invest. 1972 Mar;51(3):566–574. doi: 10.1172/JCI106845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diederich D. Relationship between the oxygen affinity and in vitro sickling propensity of carbamylated sickle erythrocytes. Biochem Biophys Res Commun. 1972 Feb 16;46(3):1255–1261. doi: 10.1016/s0006-291x(72)80110-4. [DOI] [PubMed] [Google Scholar]
- GARBY L. Analysis of red-cell survival curves in clinical practice and the use of di-iso-propylfluorophosphonate (DF32P) as a label for red cells in man. Br J Haematol. 1962 Jan;8:15–27. doi: 10.1111/j.1365-2141.1962.tb06490.x. [DOI] [PubMed] [Google Scholar]
- Gillette P. N., Manning J. M., Cerami A. Increased survival of sickle-cell erythrocytes after treatment in vitro with sodium cyanate. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2791–2793. doi: 10.1073/pnas.68.11.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen M., Nathan D. G., Bunn H. F. The reaction of cyanate with the alpha and beta subunits in hemoglobin. Effects of oxygenation, phosphates, and carbon dioxide. J Biol Chem. 1973 Dec 10;248(23):8057–8063. [PubMed] [Google Scholar]
- Kilmartin J. V., Fogg J., Luzzana M., Rossi-Bernardi L. Role of the alpha-amino groups of the alpha and beta chains of human hemoglobin in oxygen-linked binding of carbon dioxide. J Biol Chem. 1973 Oct 25;248(20):7039–7043. [PubMed] [Google Scholar]
- Kilmartin J. V., Rossi-Bernardi L. Inhibition of CO2 combination and reduction of the Bohr effect in haemoglobin chemically modified at its alpha-amino groups. Nature. 1969 Jun 28;222(5200):1243–1246. doi: 10.1038/2221243a0. [DOI] [PubMed] [Google Scholar]
- Kraus L. M., Kraus A. P. Carbamyl phosphate mediated inhibition of the sickling of erythrocytes in vitro. Biochem Biophys Res Commun. 1971 Sep 17;44(6):1381–1387. [PubMed] [Google Scholar]
- May A., Bellingham A. J., Huehns E. R., Beaven G. H. Effect of cyanate on sickling. Lancet. 1972 Mar 25;1(7752):658–661. doi: 10.1016/s0140-6736(72)90462-x. [DOI] [PubMed] [Google Scholar]
- Milner P. F., Charache S. Life span of carbamylated red cells in sickle cell anemia. J Clin Invest. 1973 Dec;52(12):3161–3171. doi: 10.1172/JCI107516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigen A. M., Njikam N., Lee C. K., Manning J. M. Studies on the mechanism of action of cyanate in sickle cell disease. Oxygen affinity and gelling properties of hemoglobin S carbamylated on specific chains. J Biol Chem. 1974 Oct 25;249(20):6611–6616. [PubMed] [Google Scholar]
- Peterson C. M., Tsairis P., Onishi A., Lu Y. S., Grady R. Sodium cyanate induced polyneuropathy in patients with sickle-cell disease. Ann Intern Med. 1974 Aug;81(2):152–158. doi: 10.7326/0003-4819-81-2-152. [DOI] [PubMed] [Google Scholar]
- van KAMPEN E., ZIJLSTRA W. G. Standardization of hemoglobinometry. II. The hemiglobincyanide method. Clin Chim Acta. 1961 Jul;6:538–544. doi: 10.1016/0009-8981(61)90145-0. [DOI] [PubMed] [Google Scholar]



