Abstract
To find the prevalence and causes of thrombocytopenia during pregnancy. An analytical prospective observational study was conducted in Department of Obstetrics & Gynecology, CSMMU, Lucknow. 1079 antenatal women screened for thrombocytopenia and investigated for cause and management strategies and fetomaternal outcome were recorded. Prevalence of thrombocytopenia was 8.8%. Gestational thrombocytopenia was seen in 64.2%, obstetric in 22.1% and medical in 13.68% cases. Mean platelet count in controls was lower with a significant fall (P < 0.001) in the platelet count as pregnancy advanced. Hypertensive and hepatic disorders were the most common obstetric causes of thrombocytopenia. Mode of delivery was not affected by thrombocytopenia. Maternal morbidity and mortality was seen only in medical and obstetric thrombocytopenia. The low platelet counts and declining trend with increasing gestational age predispose Indian women to risk of thrombocytopenia and a routine platelet count is suggested.
Keywords: Platelet count, Gestational thrombocytopenia, Obstetric thrombocytopenia, DIC, Medical thrombocytopenia, Pregnancy with ITP
Keywords: Medicine & Public Health, Oncology, Human Genetics, Blood Transfusion Medicine, Hematology
Introduction
Thrombocytopenia, defined as platelet count less than 150,000 μl−1 [1, 2] is a common hematological disorder. It is second only to anemia as the most common hematological abnormality in pregnancy [3]. Thrombocytopenia has been more commonly diagnosed in pregnant women in the last 20 years. It usually results in bleeding into mucus membranes presenting as petechiae, echymoses, epistaxis, gingival bleeding etc. However, bruising, hematuria, gastrointestinal bleeding and rarely intracranial hemorrhage can also occur. Thrombocytopenia in pregnant women may result from the effects of several diverse processes, which may be either physiological or pathological. Magann et al. [4] divided thrombocytopenia according to severity into mild (≥100,000 to <150,000 μl−1), moderate (≥50,000 to <100,000 μl−1) and severe (<50,000 μl−1) thrombocytopenia.
The majority of thrombocytopenic pregnant women is healthy, has no history of thrombocytopenia, and is incidentally diagnosed by blood testing. This condition, called incidental or gestational thrombocytopenia (GT), usually has no influence on pregnancy, labor & delivery or on the newborn. However, in a significant proportion of cases it may have great clinical impact. There may not be a risk of severe hemorrhage in GT, but pre eclampsia, HELLP syndrome and ITP (Immune Thrombocytopenic Purpura) expose mother and child to potentially life threatening complications. Other rare causes of thrombocytopenia like Thrombotic thrombocytopenic purpura (TTP), Hemolytic uremic syndrome (HUS), disseminated intravascular coagulopathy (DIC) and vonWillebrand disease IIB (vWD IIB) are also associated with severe complications. An accurate etiological diagnosis is essential to ensure optimal therapeutic management. Thrombocytopenia is thus divided according to etiology into gestational, medical (ITP, hypersplenism, hepatic disorders, etc.) and obstetric (hypertensive disorders, DIC, multifetal gestation etc.) thrombocytopenia.
Thrombocytopenia during pregnancy is an underexplored condition in Indian women, so the study was planned to find out the prevalence and causative factors of thrombocytopenia during pregnancy and to review management strategies for best fetomaternal outcome.
Materials and Methods
In this study, 1,079 pregnant women were recruited from Department of Obstetrics and Gynecology, CSM Medical University, Lucknow after approval from institutional Ethical Clearance Committee from September 2007 to August 2008. Written informed consent was taken from all women recruited. Antenatal women were enrolled in the study at first visit, irrespective of gestational age. All women had platelet count estimation at the time of enrollment. Platelet count assessment was done through automated blood count analyzer with routine antenatal hematological evaluation of the patient. Women with normal platelet counts were taken as controls and women with low platelet counts were taken as cases.
The detailed work up of all cases of thrombocytopenia was done to ascertain the cause of thrombocytopenia. History of petechiae, bruising, drug usage, viral infection, thrombocytopenia in previous pregnancy was taken. General, systemic and obstetric examination was done to find any signs of thrombocytopenia. All women were subjected to blood test for Hb, TLC, DLC, GBP, bleeding time, clotting time, KFT, LFT, HBsAg & HIV. Women with fever were tested for Dengue IgM. Coagulation tests (PT, APTT, FDP and fibrinogen) were done in those with signs or symptoms of DIC.
Women with normal platelet count before 28 weeks had a repeat platelet count in third trimester to detect gestational thrombocytopenia. All the thrombocytopenic cases were followed up throughout the antenatal period till delivery to record any complications that developed due to low platelet counts. Platelet counts were repeated once in each trimester and in the postpartum period at 1 & 6 weeks. Babies of all cases were tested for thrombocytopenia and were followed up for any complications.
Standard statistical methods, ANOVA, student’s “t”-test, were used to find the association between different causes and severity of thrombocytopenia with hemorrhagic complications.
Results
Out of 1,079 antenatal women studied, 1,038 (96.2%) were enrolled after 28 weeks. Only 36 controls and five cases were enrolled before 28 weeks. Out of 36 controls, 20 had repeat platelet count in third trimester. 16 controls did not have third trimester platelet count because eight of them delivered preterm and other eight were lost to follow up. All five thrombocytopenia cases enrolled in early pregnancy were followed up till delivery.
Out of 1,079 antenatal cases studied, 95 were found thrombocytopenic, giving a prevalence of 8.8%. There were 74.7% cases of mild thrombocytopenia, 17.9% of moderate thrombocytopenia and 7.4% with severe thrombocytopenia. There was no significant difference in the distribution of cases and controls according to age (P = 0.923), religion (P = 0.947) and parity (P = 0.068). The mean platelet count in controls was 182,000 ± 0.302 μl−1 while that in cases was 103,200 ± 0.279−1, showing a statistically significant difference in between the two groups (P < 0.001). The mean platelet counts in gestational, obstetric and medical thrombocytopenia were 113,000, 105,000 and 53,850 μl−1, respectively. Women with medical thrombocytopenia had significantly (P < 0.001) lower mean platelet count as compared to other causes of thrombocytopenia.
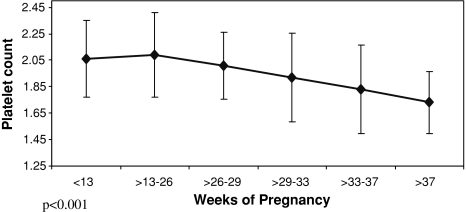
There was a statistically significant fall (P < 0.001) in mean platelet count of controls followed from first trimester (212000 μl−1) to third trimester (171,000 μl−1), however, none of them developed frank thrombocytopenia. A statistically significant decrease in platelet count (P < 0.001) was also seen among controls with increasing period of gestation (Fig. 1). However, no such change in mean platelet count was seen with increasing period of gestation among thrombocytopenia cases.
Fig. 1.
Change in platelet count with increasing period of gestation in controls
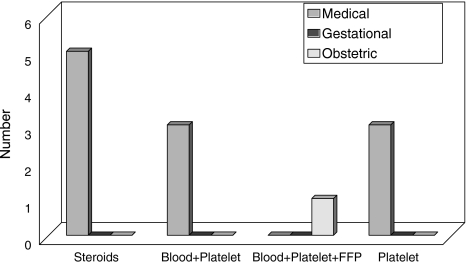
The distribution of 95 cases of thrombocytopenia according to identified cause is shown in Table 1. Risk factors associated with thrombocytopenia were present in significantly higher proportion of cases as compared to controls as shown in Table 2. PIH was the most common (24.2%) risk factor but 22% of patients with hepatic disease developed thrombocytopenia as compared to only 12% of patients with PIH. Out of 13 cases of medical thrombocytopenia, 8 were treated with steroids, blood transfusion and platelet transfusions. These eight included ITP, megaloblastic anemia and malaria cases. One case of DIC (obstetric thrombocytopenia) was also managed with transfusion of blood, platelets and FFP but no medical or surgical intervention was required in any case of GT as shown in Fig. 2.
Table 1.
Distribution of thrombocytopenia cases according to etiology
| S. no. | Cause | No. of cases | % |
|---|---|---|---|
| 1. | Gestational | 61 | 64.21 |
| 2. | Obstetric | 21 | 22.11 |
| (a) Hypertensive disorders | 20 | 21.05 | |
| PET | 19 | 20.0 | |
| Eclampsia | 1 | 1.05 | |
| (b) DIC | 1 | 1.05 | |
| 3. | Medical | 13 | 13.68 |
| (a) Hypersplenism | 2 | 2.11 | |
| (b) Hepatic diseases | 3 | 3.17 | |
| (c) Malarial | 2 | 2.11 | |
| (d) Megaloblastic anemia | 1 | 1.05 | |
| (e) ITP | 5 | 5.26 |
Table 2.
Association of various risk factors in controls and cases
| Total patients (N = 1079) | No. of controls (n = 984) | No. of cases with thrombocytopenia (n = 95) | χ2 | P | ||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | |||||
| 1. | Hypertensive disorders | 181 | 158 | 16.06 | 23 | 24.21 | 4.126 | 0.042 |
| 2. | Hepatic disorders | 31 | 24 | 2.44 | 7 | 7.37 | 7.544 | 0.006 |
| 3. | IUD | 18 | 16 | 1.63 | 2 | 2.11 | 0.934 | 0.334 |
| 4. | Abruptio | 12 | 10 | 1.02 | 2 | 2.11 | 0.121 | 0.728 |
| 5. | Viral diseases | 11 | 9 | 0.91 | 2 | 2.11 | 6.088 | 0.014 |
| 6. | Hypersplenism | 5 | 3 | 0.30 | 2 | 2.11 | 1.217 | 0.270 |
Fig. 2.
Comparision of treatment used in different types of thrombocytopenia in pregnancy
91 cases delivered during the study. 68.1% delivered at term whereas 31.9% delivered preterm. 61.54% had normal vaginal delivery, 36.26% had CS and 2.2% had instrumental delivery. All the cesarean sections were performed for obstetric/medical causes and none for thrombocytopenia.
Incidence of PPH was 9.89% among cases. PPH was seen in 30% of medical, 15% of obstetric and only 4.92% of gestational thrombocytopenia. Incidence was significantly higher in medical thrombocytopenia (P = 0.008). Three cases of obstetric and two of medical thrombocytopenia died during the study giving a mortality rate of 5.26%. Significantly higher mortality (P = 0.009) was seen in these cases as compared to GT that showed nil mortality.
96.67% of delivered cases had normal platelet counts within 2 weeks of delivery. Thrombocytopenia persisted in 4 (3.33%) cases; three with medical thrombocytopenia (1 ITP, 1 megaloblastic anemia and 1 hypersplenism) and one with obstetric cause (HELLP syndrome). Out of the 91 newborns, platelet count assessment could be done in 75 (81.4%). All had normal platelet counts at birth except the one born to mother with ITP. Neonatal thrombocytopenia of 65,000 cumm−1 returned to normal on day eight. None of the babies had any bleeding complications.
Discussion
Thrombocytopenia is a common problem during pregnancy, often under diagnosed and mismanaged. In the present study, incidence of thrombocytopenia during pregnancy was 8.8%. Burrows [2], found thrombocytopenia in 6% and Sainio et al. [5] reported a 7.3% prevalence of thrombocytopenia in a population based surveillance study. Thus, the prevalence of thrombocytopenia in Indian population is similar to world literature (5–12%). The present study found no influence of age and religion on prevalence of thrombocytopenia in pregnancy similar to Mathews et al. [6]. 96.3% of controls and 95.7% of cases were enrolled in third trimester since most antenatal women in our population seek advice in late pregnancy or when a complication arises. Cases enrolled in first trimester (4.21%) were significantly higher (P < 0.001) than controls (0.9%) because diagnosed cases of ITP were referred early in pregnancy.
The mean platelet count of 180,000 μl−1 among controls of this study is significantly lower than that reported (213,000 μl−1) for healthy antenatal women [7]. Among the cases also, the platelet counts (90,000–130,000 μl−1) were lower than the reported 116,000–149,000 μl−1. Karim et al. [8] documented that severe thrombocytopenia is rare, occurs in less than 0.1% of pregnancies. In the present study, 0.648% of women had severe thrombocytopenia which is comparatively higher. Thus, Indian women have lower platelet counts during pregnancy with or without thrombocytopenia. Among the associated risk factors, hepatic disorders were of concern because 22% of women with hepatic disorders developed thrombocytopenia.
The mean platelet count of controls showed a statistically significant fall (P < 0.001) with progression of gestation. Fay et al. [9] found that the platelet count fell continuously as pregnancy progressed, this fall being statistically significant from 32 weeks’ gestation onward. They suggested that it makes pregnancy a compensated state of subclinical coagulopathy. Verdy et al. [10] concluded that the platelet counts fall by about 10% during an uncomplicated pregnancy, with the decline being greatest in the last trimester leading to moderate and asymptomatic thrombocytopenia in about 8% women just before delivery. Ajzenburg et al. [11] assumed GT to be secondary to increased platelet consumption within the placental circulation and/or normal inhibition of megakaryocytopoiesis. Nadine Shehata et al. [1] found that GT of pregnancy is characterized by mild thrombocytopenia (platelet count 70,000–150,000 μl−1). Silver [12] & Aster et al. [13] reported that mostly GT is detected incidentally and women have no symptoms. Sainio et al. [5] reported that cases of GT have no impact on either the mother or the fetus. GT was the most common cause in this study with a platelet count ranging from 65,000 to 135000 μl−1. 88.52% had platelet counts ≥100,000 μl−1. It followed a benign course without any adverse effect and need for intervention during pregnancy. Thus, prevalence and severity of GT in this study were comparable to the world literature.
ITP affects only 1–2 of every 10,000 pregnancies. Incidence of ITP was relatively higher in the present study. Kwon et al. [14] and Nadine Shehata et al. [1] concluded that detection of thrombocytopenia prior to 28 weeks of gestation is predictive of ITP but two cases of hypersplenism were diagnosed through early screening in this study. All 5 cases of ITP were being treated before pregnancy and so had mild thrombocytopenia. Platelets are transfused to ITP cases for bleeding complications or to raise platelet counts to 10,000 for delivery and 50,000 for LSCS [19, 20]. Suri et al. [21] studied 16 cases of ITP and found that none had fetomaternal morbidity. ACOG [17] recommended serial assessment of the maternal platelet count in every trimester for asymptomatic women in remission and more frequently for thrombocytopenic cases of ITP. Kathryn et al. [22] stated that therapy for pregnant mothers with ITP is similar to that for non-pregnant patients. Treatment is recommended when the platelet count is unacceptably low or when the patient has symptoms, such as petechiae or mucosal bleeding [23, 24]. All ITP cases were managed as per ACOG guidelines during antenatal period. None required immunoglobulin therapy or splenectomy. Significantly high morbidity and mortality in this group cannot be entirely attributed to thrombocytopenia alone due to the presence of associated medical/obstetric factors.
ACOG [17] recommended that the primary treatment of maternal thrombocytopenia in the setting of PIH with HELLP syndrome is delivery. Platelet transfusions are less effective in these women because of accelerated platelet destruction. Fonseca [18] found no significant improvement in clinical complication or recovery of laboratory parameters or overall length of hospital stay with steroids in HELLP syndrome. Steroids were not used to raise the platelet counts in any of the patients of this study. Among obstetric thrombocytopenia cases, one case of abruptio placentae with DIC had petechiae & purpura all over body and platelet count of 59,000 μl−1. She required 2 units of blood, 3 units of platelets and 2 units of FFP.
The preterm delivery rate (31.8%) among cases was higher than that (11.6%) in general population [15]. This was due to the associated obstetric and medical complications that indicate preterm delivery. The proportion of vaginal, instrumental and cesarean delivery among thrombocytopenia cases was similar to overall proportions of hospital statistics of 2007. Pre-delivery platelet transfusions were needed only for patients of medical thrombocytopenia. This is in line with recommendations from Richard Fischer [16] who found that bleeding associated with surgery is uncommon unless the platelet counts are lower than 50,000 μl−1. Incidence of PPH was significantly high (9.89%) among cases especially in medical thrombocytopenia (P = 0.008). This calls for preparation with all preventive and therapeutic measures for PPH.
In the postpartum period, thrombocytopenia persisted in 30% of medical thrombocytopenia and 5% of obstetric thrombocytopenia and none of GT cases (P < 0.001). Nadine Shehata [1] reported that in GT, platelet count typically returned to normal within 6 weeks of delivery, which also is the approximate length of time required for other haemostatic factors that are altered during a normal pregnancy to return to normal. However, in cases of hypertensive disorders, the return is much earlier except for occasional cases of persistent thrombocytopenia. No neonatal hemorrhagic complications were seen in newborns of thrombocytopenia cases suggesting an overall good neonatal outcome.
Conclusion
The baseline low platelet counts and declining trend with increasing gestational age predispose Indian women to increased risk of thrombocytopenia in pregnancy. Thus, platelet count estimation should be a routine at first antenatal visit for timely diagnosis and to achieve favorable fetomaternal outcome in all types of thrombocytopenia during pregnancy.
References
- 1.Shehata N, Burrows R, Kelton JG. Gestational thrombocytopenia. Clin Obst Gynaecol. 1999;42(2):327–334. doi: 10.1097/00003081-199906000-00017. [DOI] [PubMed] [Google Scholar]
- 2.Burrows RF, Kelton JG. Thrombocytopenia at delivery (a prospective survey of 6,715 deliveries) Am J Obstet Gynaecol. 1990;162:731–734. doi: 10.1016/0002-9378(90)90996-k. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan CA, Martin JN., Jr Management of the obstetric patients with thrombocytopenia. Clin Obstet Gynecol. 1995;38:521–534. doi: 10.1097/00003081-199509000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Magann EF, Martin JN. Twelve steps to optical management of HELLP syndrome. Mississippi & Tennessee classification systems for HELLP syndrome. Clin Obstet Gynecol. 1999;42(3):532–550. doi: 10.1097/00003081-199909000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Sainio S, Kekomaki R, Rikonen S, Teramo K. Maternal thrombocytopenia at term: a population-based study. Acta Obstetr Gynecol Scand. 2000;79(9):744–749. doi: 10.1034/j.1600-0412.2000.079009744.x. [DOI] [PubMed] [Google Scholar]
- 6.Mathews JH, Benjamin S, Gill DS, Smith NA. Pregnancy associated thrombocytopenia definition, incidence and natural history. Acta Hematol. 1990;84:24–29. doi: 10.1159/000205022. [DOI] [PubMed] [Google Scholar]
- 7.Boehlen F, Hohfeld P, et al. Platelet count at term pregnancy: a reappraisal of the threshold. Obstet Gynecol. 2000;95(1):29–33. doi: 10.1016/S0029-7844(99)00537-2. [DOI] [PubMed] [Google Scholar]
- 8.Karim R, Sacher RA. Thrombocytopenia in pregnancy. Curr Hematol Resp Mar. 2004;3(2):128–133. [PubMed] [Google Scholar]
- 9.Fay RA, Hughes AO, Farron NT. Platelets in pregnancy: hyper destruction in pregnancy. Obstet Gynecol. 1983;61:238–240. [PubMed] [Google Scholar]
- 10.Verdy E, Bessons V, Dreygus M, et al. Longitudinal analysis of platelet count and volume in normal pregnancy. Thromb Haemost. 1997;77:806–807. [PubMed] [Google Scholar]
- 11.Ajzenberg N, Dreyfus M, Kaplan C, Yvart J, Will B, Tichernia G. Pregnancy associated thrombocytopenia revisited: assessment and follow-up of 50 cases. Blood. 1998;92:4573–4580. [PubMed] [Google Scholar]
- 12.Silver RM, Branch DW, Scott JR. Maternal thrombocytopenia in pregnancy: time for a reassessment. Am J Obstet Gynecol. 1995;173:479–482. doi: 10.1016/0002-9378(95)90269-4. [DOI] [PubMed] [Google Scholar]
- 13.Aster RH. Gestational thrombocytopenia: a plea for conservative management. N Engl J Med. 1990;323:264. doi: 10.1056/NEJM199007263230409. [DOI] [PubMed] [Google Scholar]
- 14.Kwon JY, Shin JC, Lee JW, Lee JK, Kim SP, Rha JG. Predictors of ITP in pregnant women presenting with thrombocytopenia. Int J Gynecolo Obstet. 2007;96(2):85–88. doi: 10.1016/j.ijgo.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 15.F Gary Cunnigham, Kenneth J. Levenu, Steven L. Bloom, John C. Hawth, Larry C. Gilstrap III, Katharine D. Wenstrom. Hematological disorders: platelet disorders, 22nd edn. Williams Obstetrics, New York pp 1156–1158
- 16.Fischer R (2006) Thrombocytopenia in pregnancy. Emedicine article, www.emedicine.com/med/topic3480
- 17.American college of obstetricians and gynecologists (1999) ACOG practice bulletin. Thrombocytopenia in pregnancy. Number 6, September 1999. Clinical management guidelines for obstetrician-gynecologists. American college of obstetricians & gynecologists. Int J Gynaecol Obstet 67(2):117–28 [PubMed]
- 18.Fonseca JE, Mendez F, Catano C, Arias F. Dexamethasone treatment does not improve the outcome of women with HELLP syndrome: a double-blind, placebo-controlled, randomized clinical trial. Am J Obsetet Gynecol. 2005;193(5):1591–1598. doi: 10.1016/j.ajog.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 19.Laros RK Jr. (1994). Blood component therapy. ACOG Technical Bulletin, vol 199. The ACOG, Washinton DC, pp 1–5
- 20.McCrae KR, Samuals P, Schricber AD. Pregnancy associated thrombocytopenia: pathogenesis and management. Blood. 1992;80:2697–2714. [PubMed] [Google Scholar]
- 21.Suri V, Agrawal N, Saxena S, Malhotra P, Verma S. Maternal and perinatal outcome in ITP with pregnancy. Acta Obst Gynae Scand. 2006;85(12):1430–1435. doi: 10.1080/00016340600961116. [DOI] [PubMed] [Google Scholar]
- 22.Webert KE, Mittal R, Sigouin C, Heddle NM, Kelton JG. A retrospective 11 year analysis of obstetric patients with ITP. Blood. 2003;102(13):4306–4317. doi: 10.1182/blood-2002-10-3317. [DOI] [PubMed] [Google Scholar]
- 23.Kelton JG. Management of the pregnant patient with ITP. Ann Intern Med. 1983;99:796–800. doi: 10.7326/0003-4819-99-6-796. [DOI] [PubMed] [Google Scholar]
- 24.Letsky EA, Greaves M. Guidelines on the investigation and management of thrombocytopenia in pregnancy and neonatal alloimmune thrombocytopenia. Maternal and neonatal haemostasis working party of the haemostasis and thrombosis task force of the British Society for haematology. Br J Haematol. 1996;95:21–26. [PubMed] [Google Scholar]