Abstract
Treatment of relapsed or refractory multiple myeloma remains a challenge and novel treatment regimen are required. Here, a matched pair analysis was performed comparing TCID (thalidomide, cyclophosphamide, idarubicin, dexamethasone) treatment to the treatment of patients with VID (vincristine, idarubicin, dexamethasone) or with VRID (vinorelbine, idarubicin, dexamethasone) for relapsed or refractory multiple myeloma. In total, 197 patients were enrolled in multicenter trials. After matching for important prognostic variables 46 matched-pairs (total of 138 patients) could be analysed with regard to survival, toxicity and efficacy. Interestingly, a significant improvement of overall response rate (ORR) for TCID treatment compared to VID and VRID was found. In addition, TCID treatment also led to a significantly higher overall survival (OS) as well as progression-free survival (PFS) compared to VID and VRID. In conclusion, TCID treatment appears to be superior to VRID and VID treatment in patients with progressive or refractory myeloma.
Keywords: Myeloma, Thalidomide, Idarubicin, Vinorelbine, Dexamethasone
Keywords: Medicine & Public Health, Oncology, Human Genetics, Blood Transfusion Medicine, Hematology
Introduction
Treatment of relapsed or refractory multiple myeloma remains a challenge and novel treatment regimens are required.
For many years the combination of vincristine, doxorubicin (adriamycin) and dexamethasone (VAD) was the standard treatment in the therapy of multiple myeloma [1]. To avoid the complications of central venous line therapy a modification of this therapy by substituting doxorubicin by oral idarubicin was evaluated in various trials [2, 3]. Due to the fact that use of vincristine could not clearly show a significant efficacy in the treatment of multiple myeloma, vinorelbine, a new, semi-synthetic vinca-alkaloid, was evaluated in several trials [4].
The aim of our study was to evaluate the role of various combination partners of idarubicin and dexamethasone. Dexamethasone has been shown to be synergistic with chemotherapy and with thalidomide in myeloma patients [5]. Oral idarubicin has shown excellent effectiveness in preceding trials and can be applied easily [6]. Cyclophosphamide is highly effective in myeloma, even in refractory patients [7].
Thalidomide is highly effective, too, and has low hematotoxic side effects. Synergy has been shown with chemotherapy as well as with dexamethasone [5]. Recently, it has been reported that thalidomide has no negative effect on stem cell mobilization [8].
Here, the following combination partners of ID were compared: Vincristine (VID), vinorelbine (VRID) and thalidomide/cyclophosphamide (TCID).
Patients and Methods
Patients
One hundred and ninety seven patients with refractory, recurrent or de novo myeloma were gathered from three multicenter trials to compare the efficacy of different treatments in the therapy of multiple myeloma.
Treatment Regimens
VID
Data of 74 patients were collected from a prospective phase II trial, which revealed that VID was an effective and tolerable oral alternative to VAD treatment. A detailed analysis of this study was presented elsewhere [5].
Patients in the VID trial received the following treatment courses: 2 mg vincristine was given as an intravenous bolus injection on day 1. On days 1 and 4, 10 mg/m² idarubicin were given as a capsule p. o. Dose increases up to 13 mg/m2/day and also dose reductions to 8 mg/m2/day were possible. Furthermore, patients received dexamethasone at a dose of 40 mg p. o. on days 1–4, 9–12 and 17–20. On day 29 courses were repeated for a total of 6–8. The median number of courses was 4 in this trial.
VRID
In the VRID treatment arm 48 patients were included from 1998 to 2001 in a multicenter trial (manuscript submitted). In this study all patients were treated with a regimen consisting of VRID. 20 mg/m2/day vinorelbine was administered as an intravenous short infusion on days 1 and 10. Idarubicin in capsules at 10 mg/m2/day and 40 mg dexamethasone was given on days 1–4. In the first cycle patients received dexamethasone on days 8–11 and 15–18, too. Treatment cycles were repeated on day 22. In total, 6 cycles were applied. The median number of cycles patients obtained was 1.
TCID
Seventy five patients with refractory or recurrent myeloma were registered from August 2002 to July 2009, but due to exclusion of 8 patients, only 67 were fully included into this multicenter phase II trial. Reasons for exclusion were not refractory or not recurrent multiple myeloma (1 patient). Furthermore, 4 patients did not get therapy, 2 patients were not evaluable because lack of paraprotein value, 1 patient was included twice.
At last 65 patients have been documented carefully to evaluate the role of TCID as a standard therapy or thalidomide alone versus thalidomide in combination with oral idarubicin for salvage therapy.
Idarubicin was given in capsules at 8–10 mg/m2/day for days 1–4. 40 mg dexamethasone was given on days 1–4 and 15–18, cyclophosphamide at 200 mg/m2/day for days 1–4 and thalidomide 100 mg daily with scheduled increase to 400 mg. Treatment cycles were repeated every 28 days. 3–8 cycles were applied. Supportive therapy consisted of PEG-filgrastim, cotrimoxazol, dalteparin and ibandronate. In addition, patients were randomized between thalidomide and thalidomide/idarubicin maintenance treatment consisting of either thalidomide up to a dose of 400 mg/day p.o. according to compatibility and idarubicin 5 mg p.o. every second day. According to protocol patients should receive at least three cycles before HDT with autologous stem cell transplantation, or otherwise until stable remission, but eight cycles at most. The median of cycles was three.
Patient Characteristics of the Matched Pair Analysis
The inclusion criteria for all three trials were rather similar. The following parameters had to be fulfilled completely: evidence of multiple myeloma (International Myeloma Working Group) [5], stage II A/B or III A/B according to Durie and Salmon [9], symptomatic or progressive disease (progression of paraprotein/infiltration of bone marrow at least 10%).
The forth criteria described the type of multiple myeloma. Here only one of the following had to fit: primary or secondary refractory disease, failure of response after first line therapy (EBMT) [10], or subsequent relapse after high-dose chemotherapy (HDT) with autologous stem cell transplantation (ASCT). Additionally, control of therapy had to be guaranteed (evaluable results of paraprotein or infiltration of bone marrow) and informed consent had to be given.
Concerning inclusion criteria, in the TCID group 16 patients (24.6%) showed progressive disease after standard chemotherapy, 14 (21.5%) no change after standard chemotherapy, 9 (13.8%) recurrent disease after standard therapy and 26 (40%) recurrence after high dose chemotherapy followed by stem cell transplantation. In further analysis patients with PD or NC formed the refractory group (30 patients; 46.2%) and the relapsed part consisted of 35 patients (53.8%).
At start of the TCID treatment 17 patients (26.2%) were in stages II A, in stage II B were 2 patients (3.1%), in stage III A 44 patients (67.7%) and in stage III B 2 patients (3.1%). according to the staging system of Durie and Salmon.
Distribution of patients in the VRID treatment was as follows: 10 patients (20.8%) had primary refractory disease, 11 (22.9%) secondary refractory disease, 3 (6.3%) refractory disease after therapy with VAD and 1 patient had refractory myeloma after treatment with melphalan and prednisone in WMSG (Westdeutsche Myelomstudiengruppe) [11]. These groups formed the refractory disease group. Recurrence after high dose chemotherapy followed by stem cell transplantation was evaluated in 23 (47.9%).
At start of the VRID treatment 8 patients were in stage IIA (16.7%), in IIB 1 patient (2.1%), in IIIA 36 patients (75.0%) and in IIIB 3 patient (6.3%).
Evaluation in the VID trial showed that 12 patients (16.2%) were primary refractory, 20 patients (27.0%) secondary refractory, 10 patients (13.5%) refractory after therapy with VAD and 4 patients (5.4%) were refractory for ≥2 other standard chemotherapies. These patients were part of the refractory disease group. The second group consisted of 16 de novo patients with HDT (21.6%) and 12 de novo patients without other criteria (16.2%).
At the beginning of VID 16 patients (21.6%) had stage IIA, 1 patient (1.4%) had IIB, 45 (60.8%) were in stage IIIA and 12 patients (16.2%) in IIIB.
Statistical Analysis
The primary intention of this matched-pair analysis was to compare the overall and the progression-free survival of the patients treated with VRID versus patients in the TCID trial versus patients in the VID trial.
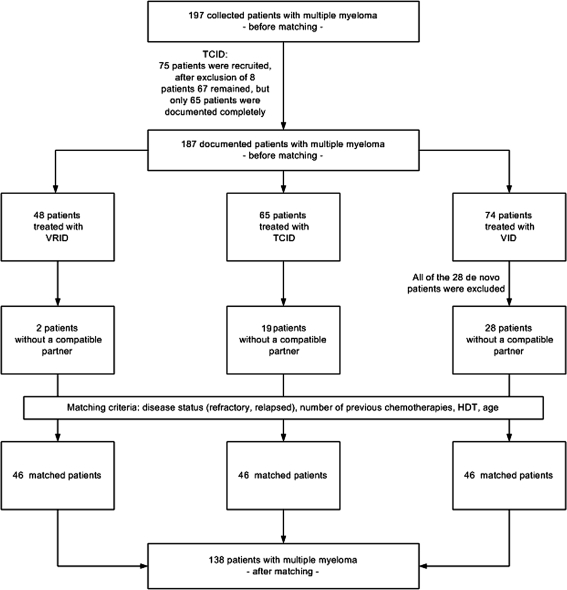
To enhance comparability of the analyzing population and thus the therapeutic value of this report, only patients with relapsed or refractory multiple myeloma were matched. Further information about the strategy of data collection and the matching process is given in Fig. 1.
Fig. 1.
Strategy of data collection and matching process
The criteria for the matching process were defined as follows: disease status (refractory, relapsed or de novo), number of previous chemotherapy cycles, HDT and the age of the patients.
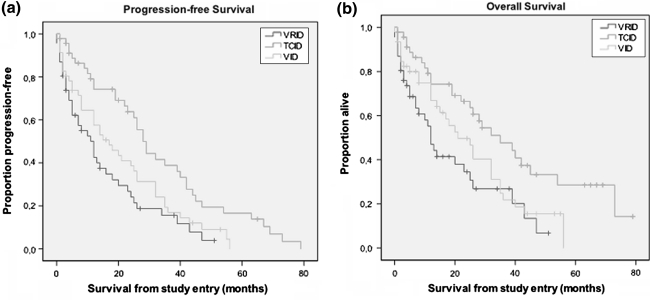
Overall survival (OS) was defined from beginning of the study until death. Progression-free survival was measured from beginning of trial to disease progression or death by any cause. Survival curves were estimated by the method of Kaplan–Meier (Fig. 2). To show significant differences between survivals of patients in the different trials the log rank test was used.
Fig. 2.
Kaplan–Meier estimate of a progression-free survival and b overall survival from the beginning of the study. Data are presented for TCID, VRID and the VID group separately. P values for progression-free survival were for TCID versus VRID P ≤ 0.001, for TCID versus VID P = 0.011 and for VID versus VRID P = 0.183, respectively. P values for overall survival were for TCID versus VRID P = 0.002, for TCID versus VID P = 0.03 and for VID versus VRID P = 0.191, respectively
Response criteria for the TCID group versus VID trial and versus VRID followed the recommendations of European Blood and Marrow Transplantation (EBMT)/IBMTR/ABMTR [12].
All data of the open multicenter trial were analysed in the University Hospital in Bonn using SPSS software (Version 14.0 und 17.0).
Univariate analyses were performed and the P values were calculated for all prognostic variables, once before the matching process (data not shown) and another time after the matching process (Table 1). To identify significant differences between the three treatment groups, the χ2-test and analysis of variances were used. All tests were two-sided and the level of significance was set to 0.05.
Table 1.
Distribution of patients’ characteristics among treatment groups after the matching process (n = 138)
| VRID n = 46 | TCID n = 46 | VID n = 46 | Significance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | P | ||||
| Age | ≤61 | 26 | 56.5 | ≤61 | 21 | 45.7 | ≤61 | 23 | 50.0 | 0.74 |
| 62+ | 20 | 43.5 | 62+ | 25 | 54.3 | 62+ | 23 | 50.0 | ||
| Min. = 36 Max. = 78 | Min. = 42 Max. = 78 | Min. = 37 Max. = 80 | ||||||||
| Median = 60 | Median = 62 | Median = 61 | ||||||||
| Sex | Male | 25 | 54.3 | Male | 29 | 63.0 | Male | 28 | 60.9 | 0.677 |
| Female | 21 | 45.7 | Female | 17 | 37.0 | Female | 18 | 39.1 | ||
| HDT | Yes | 25 | 54.3 | Yes | 17 | 37.0 | Yes | 9 | 19.6 | <0.05 |
| No | 21 | 45.7 | No | 29 | 63.0 | No | 37 | 80.4 | ||
| Number of previous chemotherapy cycles | 1–10 | 33 | 71.7 | 1–10 | 30 | 65.2 | 1–10 | 28 | 60.9 | 0.407 |
| 11–20 | 10 | 21.8 | 11–20 | 15 | 32.6 | 11–20 | 12 | 26.1 | ||
| ≥20 | 3 | 6.5 | ≥20 | 1 | 2.2 | ≥20 | 5 | 10.9 | ||
| Min. = 3 Max. = 31 | Min. = 3 Max. = 43 | Min = 1 Max. = 33 | ||||||||
| Median = 8 | Median = 8 | Median = 9 | ||||||||
| Disease status | Relapsed | 21 | 45.6 | Relapsed | 22 | 47.8 | Relapsed | 0 | 0 | <0.05 |
| Refractory | 25 | 54.3 | Refractory | 24 | 52,2 | Refractory | 46 | 100 | ||
VID: 1 patient without documentation of the number of previous chemotherapies (cycles)
Results
Here, 197 patients with refractory or recurrent myeloma were recruited in three open multicenter trials. Due to the reasons mentioned above, the whole group for analysis consisted of 187 patients. Univariate analyses were performed for this population before the matching process. For further analysis only the matched population (n = 138) was used.
Univariate Analysis of the Whole Patient Group
Before the matching process, the prognostic aspects of the patient population did not differ substantially as univariate analyses revealed no significant differences in age, gender and high-dose-chemotherapies between the trials, but there was an important distinction in patients treated with VID in comparison to the TCID and the VRID trial with regard to disease status and number of previous chemotherapies cycles as the VID trial included patients with de novo myeloma.
Details of patients’ characteristics and their distribution among the three treatment groups after the matching process are listed in Table 1.
Matched Pair Analysis
Seventy-four of the 197 patients were in the VID and 48 in the VRID trial. Seventy-five patients were recruited for the TCID trial, but the documentation of only 65 patients allowed further evaluation, 28 patients with de novo disease were excluded from participation in the VID trial.
Fully matched partners for the remaining 46 patients could be found with regard to the four matching parameters mentioned above. The detailed process of matching is shown in Fig. 1.
The patients’ characteristics of the three trials were widely balanced after matching, but significant differences still existed. The whole distribution of prognostic variables after this matching process is shown in Table 1.
Efficacy
Looking at the response rates of the three treatment regimens (Table 2) significant differences were detectable. Thirty patients (65.2%) in the TCID group achieved a partial remission (PR) compared to the VID group with PR in only 28.3% (13 patients) or the VRID trial with only 4 patients (8.7%) in PR.
Table 2.
Response rates to TCID versus VID versus VCID therapy
| Number of patients | ||||
|---|---|---|---|---|
| Refractory group | Relapsed group | Overall | Overall (%) | |
| A: TCID treatment | ||||
| Total | 24 | 22 | 46 | 100 |
| CR | 0 | 0 | 0 | 0.0 |
| PR | 15 | 15 | 30 | 65.2 |
| PR unconfirmed | 0 | 1 | 1 | 2.2 |
| MR | 2 | 3 | 5 | 10.9 |
| NC | 1 | 2 | 3 | 6.5 |
| PD | 2 | 0 | 2 | 4.4 |
| PD after PR | 0 | 1 | 1 | 2.2 |
| PD after MR | 0 | 0 | 0 | 0.0 |
| ED | 1 | 0 | 1 | 2.2 |
| n.e. | 3 | 0 | 3 | 6.5 |
| B: VID treatment | ||||
| Total | 46 | 0 | 46 | 100 |
| CR | 9 | 0 | 9 | 19.6 |
| PR | 13 | 0 | 13 | 28.3 |
| PR unconfirmed | 0 | 0 | 0 | 0.0 |
| MR | 11 | 0 | 11 | 23.9 |
| NC | 6 | 0 | 6 | 13.0 |
| PD | 4 | 0 | 4 | 8.7 |
| PD after PR | 0 | 0 | 0 | 0.0 |
| PD after MR | 0 | 0 | 0 | 0.0 |
| ED | 2 | 0 | 2 | 4.3 |
| n.e. | 1 | 0 | 1 | 2.2 |
| C: VRID treatment | ||||
| Total | 25 | 21 | 46 | 100 |
| CR | 0 | 0 | 0 | 0.0 |
| PR | 2 | 2 | 4 | 8.7 |
| PR unconfirmed | 0 | 0 | 0 | 0.0 |
| MR | 6 | 4 | 10 | 21.7 |
| NC | 4 | 2 | 6 | 13.0 |
| PD | 6 | 4 | 10 | 21.7 |
| PD after PR | 0 | 0 | 0 | 0.0 |
| PD after MR | 1 | 3 | 4 | 8.7 |
| ED | 3 | 3 | 6 | 13.0 |
| n.e. | 3 | 3 | 6 | 13.0 |
Response criteria according to EBMT definition. n.e. not evaluable due to discontinuance of treatment. Data are presented for the refractory group, the relapsed group and the overall group separately
In contrast, progressive disease (PD) was more often in the VRID trial (21.7%) and in the VID group (8.7%) as compared to TCID treatment (4.4%).
Comparing the ORR (overall remission rate) including CR, PR, PRu or MR with a second group of response rates including PD and PD after PR, a significant higher efficacy of TCID (TCID vs. VID, P = 0.026; TCID vs. VRID, P < 0.01) was detected.
The time period of remission in the TCID trial ranged from 3 to 71 months, the median duration of remission was 10 months. In the VRID group remission was 2–30 months with a median of 6 months. Patients treated with VID had a remission for 1–38 months with a median remission duration of 8 months (P = 0.390).
Median progression-free survival was 28.0 months (95% CI: 23.7–32.3) in the TCID versus 17 months (95% CI: 8.6–25.4) in the VID versus 12 months (95% CI: 7.2–16.8) in the VRID trial.
Median OS was 35 months (95% CI: 21.5–48.5) in the TCID versus 21 months (95% CI: 12.6–29.4) in the VID versus 12 months (95% CI: 9.2–14.8) in the VRID trial (Fig. 2).
Comparing the survivals of the three groups by using the log-rank test, significant differences between the OS of TCID and VRID (P = 0.002) and between TCID and VID (P = 0.03) could be detected. In addition, PFS of TCID versus VRID (P < 0.001) and TCID versus VID (P = 0.011) was significant. In contrast, VID versus VRID showed no significant differences for OS and PFS.
Toxicity
In all three trials toxicity was observed.
Hematological toxicity WHO grade III/IV, including leukopenia, anemia and thrombocytopenia, was discovered in 85% in VRID versus 59% in TCID versus 52% in VID. The p value demonstrated a significant difference (P = 0.002).
In the VID trial no further details of hematological toxicity were documented.
In the VRID trial leukopenia <1.0 G/l occurred in 17/47 (36%) patients in course 1 and declined to 0/14 patients in course 6. Infection was the most common non-hematologic toxicity and occurred in 20/48 (42%) patients. The dose of vinorelbine was reduced in 52/164 (32%) courses and that of idarubicin in 75/164 courses (46%). Further WHO III°/IV° toxicities were nausea and vomiting (2 patients) and alopecia (1 patient).
In the TCID population the following aspects were noticed: Leukopenia grade III (<2.0–1.0 × 109/l) or grade IV (<1.0 × 109/l) were discovered in 52.2% (24 patients), thrombocytopenia grade III (<50.0–25.0 × 109/l) or grade IV (<25.0 × 109/l) in 28.3% (13 patients) and least of all anemia grade III (Hb < 8.0–6.5 g/dl) or grade IV(Hb < 6.5 g/dl) in 19.6% (9 patients). 30.4% of patients treated with TCID required blood cell transfusions and 10.9% needed platelet transfusions during the study.
No information about non-hematological toxicity was given in VID, but in VRID and TCID. Non-hematological toxicity appeared less often than hematological toxicity during treatments. The most frequent non-hematological adverse events in the TCID trial were gastrointestinal. In the VRID treatment cardiac toxicity was very high (41%) compared to no documented case of cardiac toxicity in the TCID trial. Although differences between the non-hematological toxicities in the trials could be revealed, statistical significance was measured only in cardiac toxicity (P < 0.05). More details of treatment-related toxicity are shown in Table 3.
Table 3.
Highest WHO degree of treatment-related toxicity observed with TCID versus VID versus VCID therapy (percentage of patients)
| VRID (n = 46) | TCID (n = 46) | VID (n = 46) | P-value | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refractory (n = 25) | Relapsed (n = 21) | Overall (n = 46) | Refractory (n = 24) | Relapsed (n = 22) | Overall (n = 46) | Refractory (n = 46) | Relapsed (n = 0) | Overall (n = 46) | |||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | ||
| A | |||||||||||||||||||
| Hematological toxicity (WHO Grade III/IV) | 22 | 88 | 17 | 81 | 39 | 85 | 13 | 54 | 14 | 64 | 27 | 59 | 24 | 52 | – | – | 24 | 52 | 0.002 |
| B | |||||||||||||||||||
| Hematological toxicity (WHO Grade III/IV) | |||||||||||||||||||
| Anemia | 2 | 8.0 | 2 | 9.5 | 4 | 8.7 | 5 | 20.8 | 4 | 18.2 | 9 | 19.6 | – | – | – | – | – | – | 0.231 |
| Leukopenia | 1 | 4.0 | 0 | 0.0 | 1 | 2.2 | 11 | 45.8 | 13 | 59.1 | 24 | 52.2 | – | – | – | – | – | – | <0.05 |
| Thrombocytopenia | 2 | 8.0 | 4 | 19.0 | 6 | 13.0 | 5 | 20.8 | 8 | 36.4 | 13 | 28.3 | – | – | – | – | – | – | 0.121 |
| Red blood cell transfusion | 11 | 44.0 | 7 | 33.3 | 18 | 39.1 | 8 | 33.3 | 6 | 27.3 | 14 | 30.4 | – | – | – | – | – | – | 0.332 |
| Platelet transfusion | 1 | 4.0 | 3 | 14.3 | 4 | 8.7 | 3 | 12.5 | 2 | 9.1 | 5 | 10.9 | – | – | – | – | – | – | 1.000 |
| C | |||||||||||||||||||
| Non hematological toxicity (WHO Grade III/IV) | |||||||||||||||||||
| Cardiac toxicity | 12 | 48 | 7 | 33 | 19 | 41 | 0 | 0 | 0 | 0 | 0 | 0 | – | – | – | – | – | – | <0.05 |
| Nausea/emesis | 2 | 8 | 0 | 0 | 2 | 4 | 0 | 0 | 2 | 9 | 2 | 4 | – | – | – | – | – | – | 1.000 |
| Stomatitis | 4 | 16 | 0 | 0 | 4 | 9 | 1 | 4 | 0 | 0 | 1 | 2 | – | – | – | – | – | – | 0.361 |
| Diarrhoea | 4 | 16 | 0 | 0 | 4 | 9 | 1 | 4 | 1 | 5 | 2 | 4 | – | – | – | – | – | – | 0.677 |
| Obstipation | 1 | 4 | 0 | 0 | 1 | 2 | 1 | 4 | 1 | 5 | 2 | 4 | – | – | – | – | – | – | 1.000 |
Response criteria according to EBMT definition. n.e. not evaluable due to discontinuance of treatment. Data are presented for the refractory group, the relapsed group and the overall group separately. Refractory is here defined as PD or NC after standard treatment
* Data were measured before therapy with VRID, thus they do not demonstrate the hematological toxicity of VRID therapy
Reasons for Discontinuance of Treatment
The single most frequent reason for discontinuation of treatment with TCID was HDT in 19 patients (41.3%). Furthermore, TCID treatment had to be stopped due to progressive disease in 3 patients (6.5%) and because of serious adverse events in 6 patients (13.0%). Other reasons were leukopenia in 1 patient (2.2%) and death in 1 patient (2.2%). Four patients (8.7%) requested to stop treatment with TCID and 12 patients (26.1%) received treatment regularly according to protocol.
In the VID trial no reasons for discontinuance of therapy were documented. In the VRID trial eight patients stopped treatment due to PD (17.4%), had serious adverse events (6 patients, 13.0%), or had progression after initial response to treatment (2 patients, 4.3%).
Discussion
Patients with recurrent or refractory myeloma have a poor prognosis. Consequently, more effective treatments should be established.
This matched-pair analysis intended to reveal differences in efficacy and tolerance of three various combinations of chemotherapy in the therapy of relapsed or refractory multiple myeloma and to evaluate the role of various combination partners of idarubicin and dexamethasone. Dexamethasone has been shown to be synergistic with chemotherapy and with thalidomide in myeloma patients [5]. Oral idarubicin has shown excellent effectiveness in preceding trials and can be applied easily [6]. Cyclophosphamide is highly effective in myeloma, even in refractory patients [7].
Here, the following combination partners of ID were compared: VID, VRID and TCID.
Vincristine has been the standard treatment combined with adriamycin and dexamethasone in the therapy of multiple myeloma for many years (VAD) [1]. While VAD was widely accepted as an effective treatment regimen for patients with multiple myeloma, several aspects of this regimen have caused problems: Its administration via a central venous line which led to the replacement of adriamycin by oral idarubicin in the similar effective VID regimen. And further, the use of vincristine despite the lack of any evidence of activity in myeloma and its high rate of peripheral neuropathy. However, in vitro and clinical reports have demonstrated activity of vinorelbine, a semi-synthetic vinca alkaloid, in advanced multiple myeloma.
Thalidomide has significant activity both as single agent and in combination with other drugs in patients with de novo and advanced myeloma [13]. In view of recent encouraging data with thalidomide based regimens, demonstrating response rates of 32–37% in patients with refractory multiple myeloma [14, 15], we evaluated a completely oral treatment regimen with TCID in a similar patient population. The overall remission rate (CR, PR, PRu or MR) of 78.3% in the TCID population was encouraging, compared to remission in VID in 71.7% and 30.4% in VRID. Moreover, PD, PD after PR or PD after MR was seen in TCID only in 6.5% versus 8.7% in VID and 30.4% in VRID. The results are also comparable to other protocols such as CTD [16], oral TCD [17], TCPred [18] or TCED [19]. In the VRID treatment group the median of cycles patients obtained was one which may be one reason for the treatment being less effective.
Keeping in mind that improvement of response is an important prognostic criterion for survival after HDT in patients with myeloma as Attal et al. demonstrated [20], the results of response in the TCID trial confirm the efficacy of thalidomide.
Even concerning OS and progression-free survival (PFS) the TCID population showed a significant improvement compared to VRID treatment. The TCID group had maintenance treatment and SCT. This is likely to improve the results and PFS. The patients’ characteristics of the three trials were widely balanced after matching, but significant differences still existed. So the better PFS could be due to the treatment difference.
Another study published recently, using thalidomide as monotherapy [21], noted an OS of 22 months and a progression-free survival of 15.7 months. This underlines the advantage of thalidomide in combination with other agents. In conclusion, TCID was feasible and seemed to be more effective in relapsed or refractory myeloma patients compared to VID and VRID.
Side effects of the three different trials were similar to literature reported previously, e.g., in Alexanian et al. [22]. The main toxicity occurring in the TCID regime was the hematological toxicity, most likely due to cyclophosphamide. Even non-hematological toxicity seemed to be more acceptable in TCID than in VRID, as no relevant cardiac toxicity was frequently observed.
In a recently published report, Corso et al. [23] demonstrated that the most frequent serious adverse events were neuropathy in 40% and constipation in 26%. Hematological toxicity, as anemia, thrombocytopenia and leukopenia appeared only in 9.5% and 4%. Besides, the same group observed a better efficacy of thalidomide, when combined with dexamethasone. Likewise, Palumbo et al. [24] came to the same conclusion.
Looking at the results, one has to be aware that results of a matched-pair analysis are not comparable to those of a randomized trial, even if a large number of patients is included and patients’ characteristics are well balanced. Consequently, results of a matched-pair analysis should be considered with care.
One of the limitations of this report are the remaining significant differences between the three trials after the matching process that are mainly due to the better disease status and thus the less frequent HDT in the VID population. Even so, matching facilitates comparability between the trials.
An additional aspect, which has to be kept in mind, is the small number of the evaluated patient population. Consequently, only huge differences between the three trials could be revealed. Still, with regards to the low prevalence of patients with multiple myeloma at all, this report is nevertheless able to show that a combination of thalidomide, cyclophosphamide, idarubicin and dexamethasone is highly effective in treatment of patients with refractory or recurrent multiple myeloma. Despite of all the limitations mentioned above, the encouraging results of this analysis are to be taken into account as they support the statement that TCID is superior to VID and VRID.
Acknowledgments
We kindly acknowledge the support of all participating centers of our myeloma group. In particular, we thank the patients and their families for their participation. In addition, the excellent work of Dr. C. Hahn-Ast, Bonn, Germany is kindly acknowledged.
Conflict of interest
A.G. is affiliated to Celgene. All other authors state that they have no conflicts of interest.
References
- 1.Barlogie B, Smith L. Effective treatment of advanced multiple myeloma refractory to alkylating agents. N Engl J Med. 1984;310:1353–1356. doi: 10.1056/NEJM198405243102104. [DOI] [PubMed] [Google Scholar]
- 2.Glasmacher A, Goldschmidt H, Mezger J, Haferlach T, Schmidt-Wolf IGH, Gieseler F. Oral idarubicin, dexamethason and vincristine (VID) in the treatment of multiple myeloma. Leukemia. 1997;11:22–26. doi: 10.1038/sj.leu.2400517. [DOI] [PubMed] [Google Scholar]
- 3.Cook G, Sharp RA, Tansey P, Franklin IM. A phase I/II trial of Z-Dex (oral idarubicin and dexamethasone), an oral equivalent of VAD, as initial therapy at diagnosis or progression in multiple myeloma. Br J Haematol. 1996;93:931–934. doi: 10.1046/j.1365-2141.1996.d01-1715.x. [DOI] [PubMed] [Google Scholar]
- 4.Harousseau Jl, Dammacco F, San-Miguel J et al (1999) An alternative treatment for relapsed/resistant multiple myeloma (MM): vinorelbin (VRL) plus high-dose dexamethasone (DEX): two phase II studies. Proc Annu Meet Am Soc Clin Oncol 18 (abstract)
- 5.Glasmacher A, Goldschmidt H, Mezger J, Haferlach T, Schmidt-Wolf IG, Gieseler F. Oral idarubicin, dexamethasone and vincristine in the treatment of multiple myeloma: final analysis of a phase II trial. Haematologica. 2004;89(3):371–373. [PubMed] [Google Scholar]
- 6.Glasmacher A, Hahn C, Hoffmann F, Naumann R, Goldschmidt H, Lilienfeld-Toal M, Orlopp K, Schmidt-Wolf I, Gorschlüter M. A systematic review of phase-II trials of thalidomide monotherapy in patients with relapsed or refractory multiple myeloma. Br J Haematol. 2006;132:584–593. doi: 10.1111/j.1365-2141.2005.05914.x. [DOI] [PubMed] [Google Scholar]
- 7.Davies FE, Wu P, Jenner M, Srikanth M, Saso R, Morgan GJ. The combination of cyclophosphamide, velcade and dexamethasone induces high response rates with comparable toxicity to velcade alone and velcade plus dexamethasone. Haematologica. 2007;92:1149–1150. doi: 10.3324/haematol.11228. [DOI] [PubMed] [Google Scholar]
- 8.Breitkreutz I, Lokhorst HM, Raab MS, Holt B, Cremer FW, Herrmann D, Glasmacher A, Schmidt-Wolf IG, Blau IW, Martin H, Salwender H, Haenel A, Sonneveld P, Goldschmidt H. Thalidomide in newly diagnosed multiple myeloma: influence of thalidomide treatment on peripheral blood stem cell collection yield. Leukemia. 2007;21:1294–1299. doi: 10.1038/sj.leu.2404661. [DOI] [PubMed] [Google Scholar]
- 9.Ong F, Hermans J, Noordijk EM, Kliun-Nelemans JC. Is the Durie and Salmon diagnostic classification system for plasma cell dyscrasias still the best choice? Application of three classification systems to a large population-based registry of paraproteinemia and multiple myeloma. Ann Hematol. 1995;70:19–24. doi: 10.1007/BF01715377. [DOI] [PubMed] [Google Scholar]
- 10.Durie BGM, Salmon SE. A clinical staging system for multiple myeloma: correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975;36:842–884. doi: 10.1002/1097-0142(197509)36:3<842::AID-CNCR2820360303>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 11.Bladé J, Samson D, Reece D, Apperley J, Björkstrand B, Gahrton G, Gertz M, Giralt S, Jagannath S, Vesole D. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoetic stem cell transplantation. Br J Haematol. 1998;102:1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 12.Glasmacher A, Hahn C, Fiegl M, et al. VRID as induction therapy in patients with refractory multiple myeloma. Blood. 2000;100:385b. [Google Scholar]
- 13.Cavenagh JD, Oakervee H, UK Myeloma Forum and the BCSH Haematology/Oncology Task Forces (2003) Thalidomide in multiple myeloma: current status and future prospects. Br J Haematol 120:18–26 [DOI] [PubMed]
- 14.Singhal S, Mehta J, Desikan R, et al. Antitumor activity of thalidomide alone or with dexamethasone for multiple myeloma. N Engl J Med. 2003;341:1565–1571. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- 15.Barlogie B, Desikan R, Eddlemon P, Spencer T, Zeldis J, Munshi N, Badros A, Zangari M, Anaissie E, Epstein J, Shaughnessy J, Ayers D, Spoon D, Tricot G. Extended survival in advanced and refractory multiple myeloma after single-agent thalidomide: identification of prognostic factors in a phase 2 a study of 169 patients. Blood. 2001;98:492–494. doi: 10.1182/blood.V98.2.492. [DOI] [PubMed] [Google Scholar]
- 16.Sidra G, Williams CD, Russell NH, Zaman S, Myers B, Byrne JL. Combination chemotherapy with cyclophosphamide, thalidomide and dexamethasone for patients with refractory, newly diagnosed or relapsed myeloma. Haematologica. 2006;91:862–863. [PubMed] [Google Scholar]
- 17.Kyriakou C, Thomson K, D’Sa S, Flory A, Hanslip J, Goldstone AH, Yong KL. Low-dose thalidomide in combination with oral weekly cyclophosphamide and pulsed dexamethasone is a well tolerated and effective regimen in patients with relapsed and refractory multiple myeloma. Br J Haematol. 2005;129:763–770. doi: 10.1111/j.1365-2141.2005.05521.x. [DOI] [PubMed] [Google Scholar]
- 18.Suvannasankha A, Fausel C, Juliar BE, Yiannoutsos CT, Fisher WB, Ansari RH, Wood LL, Smith GG, Cripe LD, Abonour R. Final report of toxicity and efficacy of a phase II study of oral cyclophosphamide, thalidomide, and prednisone for patients with relapsed or refractory multiple myeloma: a hoosier oncology group trial, HEM01–21. Oncologist. 2007;12:99–106. doi: 10.1634/theoncologist.12-1-99. [DOI] [PubMed] [Google Scholar]
- 19.Moehler TM, Neben K, Benner A, Egerer G, Krasniqi F, Ho AD, Goldschmidt H. Salvage therapy for multiple myeloma with thalidomide and CED chemotherapy. Blood. 2001;98:3846–3848. doi: 10.1182/blood.V98.13.3846. [DOI] [PubMed] [Google Scholar]
- 20.Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, Casassus P, Maisonneuve H, Facon T, Ifrah N, Payen C, Bataille R. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 2001;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 21.Kumar S, Gertz MA, Dispenzieri A, Lacy MQ, Geyer SM, Iturria NL, Fonseca R, Hayman SR, Lust JA, Kyle RA, Greipp PR, Witzig TE, Rajkumar SV. Response rate, durability of response and survival after thalidomide therapy for relapsed multiple myeloma. Mayo Clin Proc. 2003;78:34–39. doi: 10.4065/78.1.34. [DOI] [PubMed] [Google Scholar]
- 22.Alexanian R, Weber D, Giralt S, Delasalle K. Consolidation therapy of multiple myeloma with thalidomide–dexamethasone after intensive chemotherapy. Ann Oncol. 2002;13:1116–1119. doi: 10.1093/annonc/mdf188. [DOI] [PubMed] [Google Scholar]
- 23.Corso A, Zappasodi P, Barbarano L, Petrucci MT, Palumbo A, Caravita T, Mangiacavalli S, Cafro AM, Varettoni M, Gay F, Morra E, Lazzarino M. Long-term outcome in relapsed and refractory multiple myeloma treated with thalidomide. Balancing efficacy and side-effects. Leuk Res. 2009;33:145–149. doi: 10.1016/j.leukres.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 24.Palumbo A, Giaccone L, Bertola A, Pregno P, Bringhen S, Rus C, Triolo S, Gallo E, Pileri A, Boccadoro M. Low-dose thalidomide plus dexamethasone is an effective salvage therapy for advanced myeloma. Haematologica. 2001;86:399–403. [PubMed] [Google Scholar]