Abstract
AIM: To evaluate the effects of diazoxide on ischemia/reperfusion (I/R)-injured hepatocytes and further elucidate its underlying mechanisms.
METHODS: Male Sprague-Dawley rats were randomized (8 for donor and recipient per group) into five groups: I/R group (4 h of liver cold ischemia followed by 6 h of reperfusion); heme oxygenase-1 (HO-1) small interfering RNA (siRNA) group (injection of siRNA via donor portal vein 48 h prior to harvest); diazoxide (DZ) group (injection of DZ via donor portal vein 10 min prior to harvest); HO-1 siRNA + DZ group; and siRNA control group. Blood and liver samples were collected at 6 h after reperfusion. The mRNA expressions and protein levels of HO-1 were determined by reverse transcription polymerase chain reaction and Western blotting, and tissue morphology was examined by light and transmission electron microscopy. Serum transaminases level and cytokines concentration were also measured.
RESULTS: We observed that a significant reduction of HO-1 mRNA and protein levels in HO-1 siRNA and HO-1 siRNA + DZ group when compared with I/R group, while the increases were prominent in the DZ group. Light and transmission electron microscopy indicated severe disruption of tissue with lobular distortion and mitochondrial cristae damage in the HO-1 siRNA and HO-1 siRNA + DZ groups compared with DZ group. Serum alanine aminotransferase, aspartate transaminase, tumor necrosis factor-α and interleukin-6 levels increased in the HO-1 siRNA and HO-1 siRNA + DZ groups, and decreased in the DZ group.
CONCLUSION: The protective effect of DZ may be induced by upregulation of HO-1. By inhibiting expression of HO-1, this protection pretreated with DZ was abolished.
Keywords: Ischemia/reperfusion injury, Diazoxide, Heme oxygenase-1, Liver transplantation, Rat
INTRODUCTION
The ATP-sensitive potassium (KATP) channel was identified in cardiac muscle[1]. Later, a similar channel was described in liver[2], brain[3,4] and skeletal muscle mitochondria[5]. The primary function of this channel is to allow K+ transport into the mitochondrial matrix and this phenomenon could be involved in maintaining mitochondrial volume homeostasis[6]. KATP channels that exist in the inner membrane of mitochondria[2] have been implicated to mediate the protective effects of ischemic preconditioning (IPC) in ischemic heart[7,8]. Diazoxide (DZ) is a selective mitochondria ATP-sensitive potassium (mitoKATP) channel opener, which has been reported to have a protective effect on the heart[9,10], brain[11,12] and spinal cord[13] following ischemia/reperfusion (I/R) injury. Several recent studies have suggested that mitoKATP channels may be only a trigger, but not a mediator of protection[14]. The exact mechanisms have not been fully clarified.
IPC is a well-known phenomenon in which brief episodes of ischemia and reperfusion confer a state of protection against subsequent sustained long-term I/R injury[15,16]. Although the exact underlying mechanisms of IPC are unknown, activation of mitoKATP channels has been proposed to play a pivotal role in preconditioning[17]. More studies have indicated that pretreatment with mitoKATP channel openers induces IPC-like protective effects[18], and that IPC-induced protection is antagonized by mitoKATP channel inhibitors[19,20]. These findings support the hypothesis that IPC is mediated through the opening of mitoKATP channels.
Heme oxygenase-1 (HO-1) is the rate-limiting step in the oxidative degradation of heme. Overexpression of HO-1 exerts a cytoprotective function in a number of I/R injury and liver transplant models[21-23]. Recent studies have suggested that IPC may protect against systemic inflammatory response via enhanced HO-1 overexpression[24-27]. The purpose of our study was to investigate the protective action of DZ on I/R injury and expression of HO-1 after liver transplantation in rats. We explored the potential molecular mechanisms for DZ to reduce I/R injury. Using HO-1 small interfering RNA (siRNA), we further observed the roles of HO-1 in DZ-induced action.
MATERIALS AND METHODS
Animals
Adult male Sprague-Dawley (S-D) rats (200-250 g) (Kunming Medical College Laboratory Animal Center, China) were used. Rats were maintained on standard rodent chow and water ad libitum in a room according to the local animal welfare guidelines. All experiments were approved by the ethics committee for the use of experimental animals at Kunming Medical College.
HO-1 siRNA design
The design of HO-1 siRNA was based on the characterization by Zhang et al[28]. Additionally, a scrambled sequence was designed as a negative control siRNA. The targeted sequence of rat HO-1 siRNA was 5’-AAGCCACACAGCACUAUGUdTdT-3’ (sense) and 5’-ACAUAGUGCUGUGUGGCUUdTdT-3’ (antisense). siRNA duplexes were chemically synthesized by Guangzhou Rui Bo Biological Technology Co (Guangzhou, China).
Experimental protocol
S-D rats underwent ether anesthesia. The basic techniques of liver harvesting and orthotopic transplantation without hepatic arterial reconstruction were according to the method described previously by Kamada et al[29]. After organ harvest, liver grafts were stored for 4 h in cold University of Wisconsin solution at 4 °C. Subsequently, syngeneic orthotopic liver transplantation (OLT) was performed and the anhepatic phase was estimated to be 11-13 min for all recipients. All transplant experiments in this study were performed by a single person. Separate groups of rats were killed at 6 h after their vessels were unclamped, then samples of blood and liver tissue were taken for further analysis.
Eighty rats (16 for every group) were divided randomly into five groups (Figure 1): (1) I/R group: OLT was performed according to method described previously; (2) siRNA group: same treatment as I/R group, but donors were injected with HO-1 siRNA (200 nmol/kg body weight) via the portal vein 48 h before liver harvest; (3) DZ group: same as I/R group, but donors were injected with DZ (5 mg/kg body weight), a mitoKATP channel opener, via the portal vein 10 min before liver harvest; (4) siRNA + DZ group: same treatment as I/R group, donors were treated with HO-1siRNA and DZ; and (5) siRNA control group: same as I/R group, donors were injected with negative control siRNA (200 nmol/kg body weight). DZ was purchased from Sigma Chemical Co. (St Louis, MO, United States) and dissolved in dimethyl sulfoxide (DMSO) (Sigma, United States) before addition to experimental solutions. The final concentration of DMSO in the solution was less than 0.05%.
Figure 1.
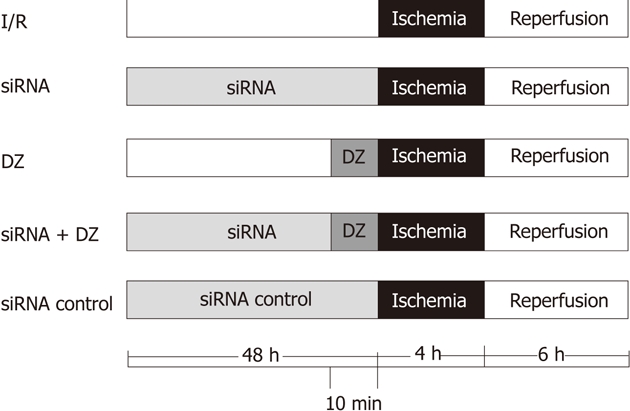
Experimental protocol. DZ: Diazoxide; siRNA: Small interfering RNA.
Serum enzymes
At 6 h following liver reperfusion, blood was collected via the abdominal aorta. After centrifugation of whole blood (3000 rpm, 15 min), serum was extracted and stored at -70 °C until analysis. Alanine aminotransferase (ALT) and aspartate transaminase (AST) levels were measured using a clinical chemistry system (7060 automatic analyzer, Hitachi, Japan).
Reverse-transcriptase polymerase chain reaction
Animals were sacrificed 6 h after transplantation. Liver samples were obtained, shock-frozen in liquid nitrogen and stored at -80 °C for further RNA isolation. Total RNA was extracted from 100 mg liver tissues with Trizol reagents (Invitrogen, United States) according to the manufacturer's guidelines and quantified by UV absorption. Following DNase I (Invitrogen, United States) digestion, 1 μg total RNA was subjected to reverse transcription polymerase chain reaction (RT-PCR) with M-MLV reverse transcriptase (Promega, United States), using oligo-dT as primers. The mixture was inactivated at 42 °C for 60 min, and then the reverse transcriptase was inactivated by heating at 70 °C for 10 min. Specific primers for rat HO-1 and β-actin (an internal standard) were designed by Primer Premier 5.0. For HO-1, the primers were 5’-TGGAAGAGGAGATAGAGCGA-3’ (sense) and 5’-TGTTGAGCAGGAAGGCGGTC-3’ (antisense), generating a 451-bp fragment. For β-actin, the primers used were 5’-CACGATGGAGGGGCCGGACTCATC3’ (sense) and 5’-TAAAGACCTCTATGCCAACACAGT-3’ (antisense), generating a 240-bp fragment. PCR constituents were as follows: 2 μL cDNA mixture was subjected to amplification in 20 μL final volume, 94 °C, 2 min; then 94 °C, 1 min; 60 °C, 1 min; 72 °C, 2 min, for 40 cycles; and 72 °C, 5 min to end the reaction. The PCR products were subjected to electrophoresis on 2% agarose gel containing ethidium bromide and visualized by UV illumination. This semi- quantitative measure for HO-1 was expressed as ratios to β-actin.
Western blotting analysis
Protein extracts were isolated from liver tissue with radioimmunoprecipitation containing phenylmethyl sulfonylfluoride. Protein concentration was determined by a bicinchoninic acid protein assay reagent (Pierce, Rockford, IL, United States). Proteins (20 μg/sample) in SDS-loading buffer were heated to 100 °C for 5 min, and separated by 12% SDS-PAGE and transferred to nitrocellulose membranes using an electroblotting apparatus. The membrane was blocked overnight at 4 °C in 5% nonfat dry milk and TBST buffer to block nonspecific binding sites. Western blots were probed with primary antibodies (anti-HO-1, 1:200 dilution; Millipore, United States) at room temperature (RT) for 2 h. After being washed, blots were incubated with horseradish-peroxidase-labeled goat anti-rabbit IgG antibodies (dilution, 1:50 000; Pierce) for 2 h at RT. Thereafter, the proteins were visualized by ECL detection system (Amersham Pharmacia Biotech, Piscataway, NJ, United States) and analyzed by the Quantity One Analysis Software (Bio-Rad, United States). β-actin was used as protein loading control.
Histology and electron microscopic examination
Livers were excised rapidly and fixed in conventional fixing solutions (10% neutral-buffered formalin) after 6 h reperfusion. Livers were paraffin embedded and sectioned into 3-μm-thick slices with a tissue chopper. The sections were examined by HE staining. The histological severity of I/R injury was graded according to Suzuki’s classification[30]. All slides were judged by the same investigator who had been blinded to the corresponding study group. The excised liver samples were cut into small pieces, and immersed in excessive volumes of 2.5% glutaraldehyde with 0.1 mol phosphate buffer (pH 7.2). Following fixation for 24 h, samples were further immersed in 2% osmium tetroxide (OsO4) in 0.1 mol cacodylate buffer (pH 7.2) for 2 h at 4 °C, dehydrated, and embedded in epoxide resin (EPON 812). Ultrathin sections were stained with lead citrate and uranyl acetate, and examined using a JEM-1200EX transmission electron microscope.
Enzyme-linked immunosorbent assay
Serum interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) levels of different groups were measured using commercially available enzyme-linked immunosorbent assay (ELISA) kits (R and D System, United States) according to the test protocols. All samples, including standard and control solution, were assayed in duplicate. Values were expressed as pg/mL.
Statistical analysis
Statistical analysis was performed using SPSS version 13.0 for Windows (SPSS, Inc, Chicago, IL, United States). Values are expressed as means ± SD. Difference between experimental groups were analyzed by one-way analysis of variance. P < 0.05 was considered statistically significant.
RESULTS
Hepatic transaminases
The hepatocellular damage was measured with the determination of ALT and AST levels. Rats treated with siRNA showed a significant increase in ALT and AST compared with I/R rats. Liver function measurement in the DZ group was significantly lower than that in the I/R group. There was no significant difference between the siRNA and siRNA + DZ groups (P > 0.05) (Figure 2).
Figure 2.
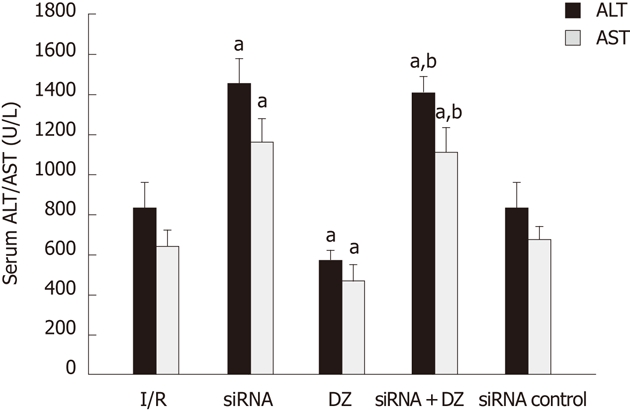
Serum alanine aminotransferase and aspartate transaminase levels after reperfusion. aP < 0.05 vs ischemia/reperfusion (I/R) group, bP < 0.01 vs diazoxide (DZ) group. ALT: Alanine aminotransferase; AST: Aspartate transaminase.
HO-1 mRNA and protein expression levels
The PCR products were separated on agarose gel and the relative expression of HO-1 were shown. As shown in Figure 3A, HO-1 mRNA expression was decreased in both siRNA and siRNA + DZ groups compared with the I/R group at 6 h after I/R injury. In contrast, DZ treatment significantly enhanced the hepatic HO-1 mRNA level. No significant difference was observed between the siRNA and siRNA + DZ groups. HO-1 protein level was determined by western blotting. The results were almost the same as those obtained in the RT-PCR analyses. Compared with the I/R group, HO-1 protein expression was markedly downregulated in both siRNA and siRNA + DZ groups. The level of HO-1 was significantly higher in the DZ group than all other groups. Moreover, the difference between siRNA and siRNA + DZ groups did not attain statistical significance (Figure 3B).
Figure 3.
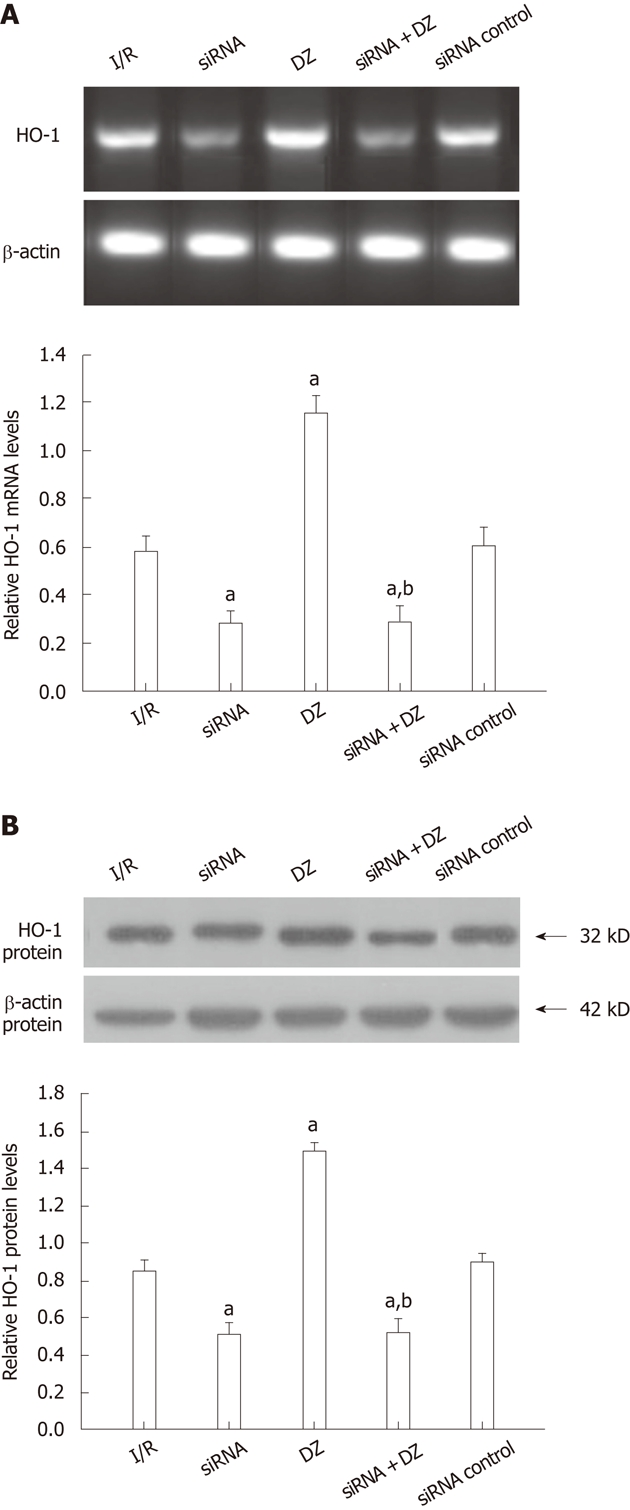
Liver heme oxygenase-1 mRNA and protein levels at 6 h after transplantation. aP < 0.05 vs ischemia/reperfusion (I/R) group, bP < 0.01 vs diazoxide (DZ) group. A: Liver heme oxygenase-1 (HO-1) mRNA expression; B: Liver HO-1 protein levels.
Histology
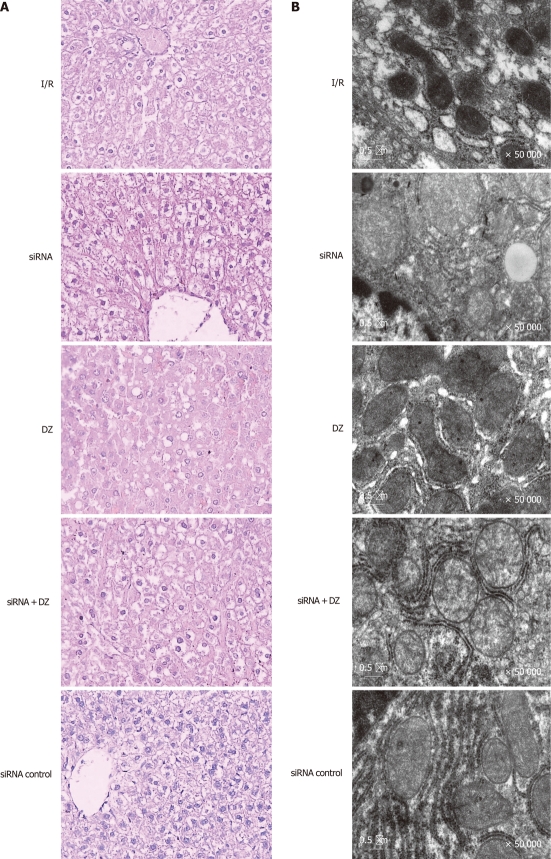
Necrosis and liver damage were assessed with HE-stained liver tissue 6 h after reperfusion. Liver specimens from rats subjected to I/R showed macrovesicular fatty changes, nuclear fragmentation, and sinusoidal congestion and congested with many red blood cells. In siRNA and siRNA + DZ groups, histological tissue damage was significantly aggravated. Moreover, multiple and extensive ballooning/hepatocellular necrosis and massive infiltration of neutrophils were observed. However, these pathological changes were significantly decreased in the DZ group (data not shown). There was no significant difference between the I/R and siRNA control groups (Figure 4A).
Figure 4.
Liver histology and ultrastructural changes of hepatocytes. A: Ischemia/reperfusion (I/R) treated livers showed vacuolization, nuclear fragmentation, sinusoidal congestion, and hepatocyte necrosis. However, histological tissue damage was aggravated in the small interfering RNA (siRNA) and siRNA + diazoxide (DZ) groups. In contrast, DZ treatment relieved liver damage (HE, × 400); B: The mitochondria and the smooth endoplasmic reticulum were dilated and even destroyed, and the mitochondrial cristae were disorganized in I/R treated hepatocytes.
Transmission electron microscopy
To confirm hepatocyte ultrastructural alterations, we examined the liver grafts during cold ischemia and reperfusion, by electron microscopy. In the siRNA group, the nuclei of the hepatocytes were irregular in shape and contained damaged nuclear chromatin in the nucleoplasm. The hepatocytes appeared largely intact vacuolar structures with lipid droplets present in the cytoplasm. The mitochondria and the smooth endoplasmic reticulum were dilated and even destroyed, and the mitochondrial cristae were disorganized. By contrast, mitochondria from rats treated with DZ appeared markedly better with less mitochondrial membrane damage and swelling in the rat hepatocytes after I/R. However, siRNA + DZ group treatment had markedly aggravated these damages as compared with DZ group. The I/R and siRNA control groups revealed less hepatocyte damage than the siRNA group (Figure 4B).
Cytokines assay
Serum cytokine levels (IL-6/TNF-α) were determined by ELISA. The IL-6/TNF-α levels revealed a substantial increase in the siRNA group as compared with the I/R group. Groups pretreated with DZ had a marked decrease in these products. Moreover, the cytokine levels (IL-6/TNF-α) in the siRNA + DZ group were significantly higher, when compared to the DZ group. There was no statistical difference between the I/R and siRNA control groups (P > 0.05) (Figure 5).
Figure 5.
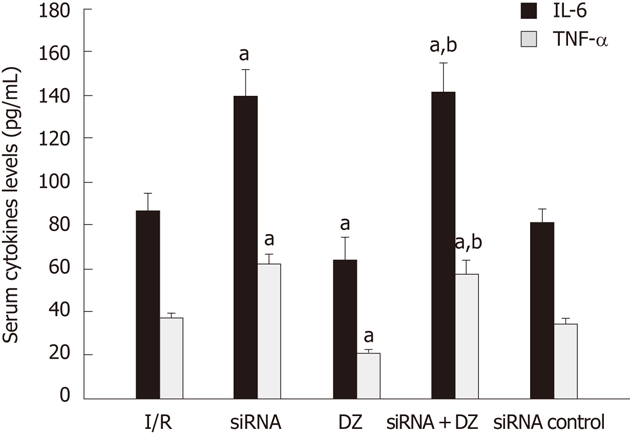
Serum cytokine levels (interleuki-6/ tumor necrosis factor-α) were measured. aP < 0.05 vs ischemia/reperfusion (I/R) group, bP < 0.01 vs diazoxide (DZ) group. IL-6: Interleukin-6; TNF-α: Tumor necrosis factor-α.
DISCUSSION
HO-1 is up-regulated in response to oxidative stress and catalyzes the degradation of pro-oxidant heme to carbon monoxide (CO), iron and bilirubin[31]. Three HO isoforms have been identified to date: HO-1, HO-2 and HO-3. HO-1 is inducible and distributed ubiquitously in mammalian tissue[32], while HO-2 is expressed constitutively and found mainly in the central nervous system[33]. HO-3 is only described in the rat brain and has no activity. HO-1 expression is an adaptive and protective response to oxidative stress in a wide variety of cells[34,35]. HO-1 is induced by a variety of stimuli, including nonheme inducers and various agents that cause oxidative stress, and prevents the oxidative DNA damage caused by heat shock and reactive oxygen species (ROS).
MitoKATP channel opener is responsible for more effective oxidative phosphorylation[36] and regulation of ROS generation[37,38] in IPC. Prevention of mitochondrial failure improves cellular survival after an ischemic event. Pharmacological agents that maintain mitoKATP channels can prevent mitochondrial failure in a manner similar to that induced by IPC[13,39]. MitoKATP is shown to have protective effect on the heart, kidney and brain following I/R injury[40,41]. However, the detailed effect of mitoKATP in liver I/R injury has not been fully studied. DZ is mainly used as a vasodilator in the treatment of clinical hypertension. However, in our study, we used DZ, the putative mitoKATP channel opener to evaluate the effects of mitoKATP channels in liver I/R injury in OLT.
In the present investigation, rats pretreated with DZ results in enhanced resistance to liver cold I/R injury and increased HO-1 expression levels. To examine whether HO-1 was responsible for the protective effect of DZ in liver I/R injury, we used HO-1 siRNA, which interferes with the enzymatic expression of HO-1 to examine its possible effect. Inhibition of the expression of HO-1 almost completely blocked the protection afforded by DZ. These results strongly indicated that the protection of DZ was mediated through upregulation of HO-1. We speculate that DZ induces an increase in HO-1 levels that protect liver I/R injury. The K+ channel-independent effects of DZ to inhibit succinate oxidation[42] and succinate dehydrogenase[43] or inhibit opening of the mitochondrial membrane pore[44] may be responsible for its protective effects. When we inhibited the expression of HO-1 and the above effects of DZ remained, the protection of DZ against hepatic I/R injury in rats almost disappeared. It is possible that DZ, through opening KATP channels, activated an unknown pathway, which directly or indirectly upregulated the expression of HO-1. HO-1 plays a critical role in protection against liver I/R injury in rat OLT, thus, inhibition of HO-1 activity may exaggerate I/R injury.
We measured serum cytokine levels (IL-6/TNF-α) by ELISA to determine the effect on anti-inflammatory activity. Rats pretreated with DZ significantly decreased serum IL-6/TNF-α levels. However, they were markedly elevated in the siRNA + DZ group than those in the DZ group. Xu et al[45] showed that DZ inhibits the production of the proinflammatory cytokines TNF-α and IL-6 by peripheral blood mononuclear cells in normal pregnancy. However, our study showed that DZ was able to reduce the release of cytokines. However, when the expression of HO-1 was inhibited, the release of cytokines was not reduced. HO-1 has been shown to be expressed principally in Kupffer cells[21,46,47]. Our study suggests that the potential utility of HO-1 overexpression in preventing I/R injury results from inhibition of Kupffer cells activation[48]. Activated Kupffer cells release a large amount of proinflammatory cytokines[49-51], which lead to aggravation of I/R injury. Devey et al[52,53] reported that depletion of Kupffer cells using liposomal clodronate led to loss of hepatic HO-1 expression and much more severe injury. It has been demonstrated that overexpression of HO-1 plays an important role in a number of I/R injury and liver transplant models. Therefore, we could think that the protective effect of DZ was by increasing HO-1 expression in our study.
In summary, DZ can attenuate hepatic I/R injury in rat liver transplantation and induce hepatic HO-1 expression. In addition, by inhibiting HO-1 expression with a specific siRNA, this protection pretreated with DZ was abolished. Thus, the mechanism of the protective effects of DZ is dependent on HO-1. The upregulation of HO-1 results in alleviation of hepatic I/R injury, as well as a decrease of the production of inflammatory cytokine such as IL-6 and TNF-α. Our results provide an insight into induction of HO-1, and suggest that DZ may have the potential to be developed as a hepatic HO-1 inducer for therapeutic purposes. Our work also raises the possibility that HO-1 can be induced by DZ to stimulate protective process that attenuates I/R injury.
COMMENTS
Background
Diazoxide (DZ) is a selective mitochondria ATP-sensitive potassium channel opener, which has been reported to have a protective effect on the heart, brain and spinal cord following ischemia/reperfusion (I/R) injury. However, the protective action of DZ on liver I/R injury is unknown and the exact underlying mechanisms are poorly understood.
Research frontiers
Heme oxygenase-1 (HO-1) is the rate-limiting step in the oxidative degradation of heme. Overexpression of HO-1 exerts a cytoprotective function in a number of I/R injury and liver transplant model. HO-1 expression is an adaptive and protective response to oxidative stress in a wide variety of cells.
Innovations and breakthroughs
The data represent a major advance in our understanding of ATP-sensitive potassium channel. DZ, can attenuate hepatic I/R injury in rat liver transplantation and induce hepatic HO-1 overexpression. In addition, the authors clearly demonstrated a direct correlation between expression of HO-1 and DZ. Thus, DZ may have the potential to be developed as a hepatic HO-1 inducer for therapeutic purposes.
Applications
The study results suggest that the mechanism of the protective effects of DZ is dependent on HO-1. Further studies are needed to evaluate the safety and efficiency of DZ used in clinical.
Peer review
This was a well designed study with excellent results. It was well written. It seemed almost too perfect. However, as long as they have a history of academic integrity.
Footnotes
Supported by Social Development Projects of Yunnan Province, No. 2008CA026
Peer reviewer: Yasuhiko Sugawara, University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113, Japan
S- Editor Gou SX L- Editor Kerr C E- Editor Xiong L
References
- 1.Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- 2.Inoue I, Nagase H, Kishi K, Higuti T. ATP-sensitive K+ channel in the mitochondrial inner membrane. Nature. 1991;352:244–247. doi: 10.1038/352244a0. [DOI] [PubMed] [Google Scholar]
- 3.Bajgar R, Seetharaman S, Kowaltowski AJ, Garlid KD, Paucek P. Identification and properties of a novel intracellular (mitochondrial) ATP-sensitive potassium channel in brain. J Biol Chem. 2001;276:33369–33374. doi: 10.1074/jbc.M103320200. [DOI] [PubMed] [Google Scholar]
- 4.Debska G, May R, Kicińska A, Szewczyk A, Elger CE, Kunz WS. Potassium channel openers depolarize hippocampal mitochondria. Brain Res. 2001;892:42–50. doi: 10.1016/s0006-8993(00)03187-5. [DOI] [PubMed] [Google Scholar]
- 5.Debska G, Kicinska A, Skalska J, Szewczyk A, May R, Elger CE, Kunz WS. Opening of potassium channels modulates mitochondrial function in rat skeletal muscle. Biochim Biophys Acta. 2002;1556:97–105. doi: 10.1016/s0005-2728(02)00340-7. [DOI] [PubMed] [Google Scholar]
- 6.Kicińska A, Szewczyk A. Protective effects of the potassium channel opener-diazoxide against injury in neonatal rat ventricular myocytes. Gen Physiol Biophys. 2003;22:383–395. [PubMed] [Google Scholar]
- 7.Gross GJ, Fryer RM. Sarcolemmal versus mitochondrial ATP-sensitive K+ channels and myocardial preconditioning. Circ Res. 1999;84:973–979. doi: 10.1161/01.res.84.9.973. [DOI] [PubMed] [Google Scholar]
- 8.Rodrigo GC, Davies NW, Standen NB. Diazoxide causes early activation of cardiac sarcolemmal KATP channels during metabolic inhibition by an indirect mechanism. Cardiovasc Res. 2004;61:570–579. doi: 10.1016/j.cardiores.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Wakahara N, Katoh H, Yaguchi Y, Uehara A, Satoh H, Terada H, Fujise Y, Hayashi H. Difference in the cardioprotective mechanisms between ischemic preconditioning and pharmacological preconditioning by diazoxide in rat hearts. Circ J. 2004;68:156–162. doi: 10.1253/circj.68.156. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki M, Saito T, Sato T, Tamagawa M, Miki T, Seino S, Nakaya H. Cardioprotective effect of diazoxide is mediated by activation of sarcolemmal but not mitochondrial ATP-sensitive potassium channels in mice. Circulation. 2003;107:682–685. doi: 10.1161/01.cir.0000055187.67365.81. [DOI] [PubMed] [Google Scholar]
- 11.He X, Mo X, Gu H, Chen F, Gu Q, Peng W, Qi J, Shen L, Sun J, Zhang R, et al. Neuroprotective effect of diazoxide on brain injury induced by cerebral ischemia/reperfusion during deep hypothermia. J Neurol Sci. 2008;268:18–27. doi: 10.1016/j.jns.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 12.Lenzsér G, Kis B, Bari F, Busija DW. Diazoxide preconditioning attenuates global cerebral ischemia-induced blood-brain barrier permeability. Brain Res. 2005;1051:72–80. doi: 10.1016/j.brainres.2005.05.064. [DOI] [PubMed] [Google Scholar]
- 13.Roseborough G, Gao D, Chen L, Trush MA, Zhou S, Williams GM, Wei C. The mitochondrial K-ATP channel opener, diazoxide, prevents ischemia-reperfusion injury in the rabbit spinal cord. Am J Pathol. 2006;168:1443–1451. doi: 10.2353/ajpath.2006.050569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang S, Cone J, Liu Y. Dual roles of mitochondrial K(ATP) channels in diazoxide-mediated protection in isolated rabbit hearts. Am J Physiol Heart Circ Physiol. 2001;280:H246–H255. doi: 10.1152/ajpheart.2001.280.1.H246. [DOI] [PubMed] [Google Scholar]
- 15.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 16.Yin DP, Sankary HN, Chong AS, Ma LL, Shen J, Foster P, Williams JW. Protective effect of ischemic preconditioning on liver preservation-reperfusion injury in rats. Transplantation. 1998;66:152–157. doi: 10.1097/00007890-199807270-00002. [DOI] [PubMed] [Google Scholar]
- 17.Oldenburg O, Cohen MV, Yellon DM, Downey JM. Mitochondrial K(ATP) channels: role in cardioprotection. Cardiovasc Res. 2002;55:429–437. doi: 10.1016/s0008-6363(02)00439-x. [DOI] [PubMed] [Google Scholar]
- 18.Garlid KD, Paucek P, Yarov-Yarovoy V, Murray HN, Darbenzio RB, D’Alonzo AJ, Lodge NJ, Smith MA, Grover GJ. Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K+ channels. Possible mechanism of cardioprotection. Circ Res. 1997;81:1072–1082. doi: 10.1161/01.res.81.6.1072. [DOI] [PubMed] [Google Scholar]
- 19.Toombs CF, Moore TL, Shebuski RJ. Limitation of infarct size in the rabbit by ischaemic preconditioning is reversible with glibenclamide. Cardiovasc Res. 1993;27:617–622. doi: 10.1093/cvr/27.4.617. [DOI] [PubMed] [Google Scholar]
- 20.Fryer RM, Eells JT, Hsu AK, Henry MM, Gross GJ. Ischemic preconditioning in rats: role of mitochondrial K(ATP) channel in preservation of mitochondrial function. Am J Physiol Heart Circ Physiol. 2000;278:H305–H312. doi: 10.1152/ajpheart.2000.278.1.H305. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi T, Hirano K, Yamamoto T, Hasegawa G, Hatakeyama K, Suematsu M, Naito M. The protective role of Kupffer cells in the ischemia-reperfused rat liver. Arch Histol Cytol. 2002;65:251–261. doi: 10.1679/aohc.65.251. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi T, Sato Y, Yamamoto S, Takeishi T, Hirano K, Watanabe T, Takano K, Naito M, Hatakeyama K. Augmentation of heme oxygenase-1 expression in the graft immediately after implantation in adult living-donor liver transplantation. Transplantation. 2005;79:977–980. doi: 10.1097/01.tp.0000155245.85967.ad. [DOI] [PubMed] [Google Scholar]
- 23.Kato H, Amersi F, Buelow R, Melinek J, Coito AJ, Ke B, Busuttil RW, Kupiec-Weglinski JW. Heme oxygenase-1 overexpression protects rat livers from ischemia/reperfusion injury with extended cold preservation. Am J Transplant. 2001;1:121–128. [PubMed] [Google Scholar]
- 24.Tamion F, Richard V, Lacoume Y, Thuillez C. Intestinal preconditioning prevents systemic inflammatory response in hemorrhagic shock. Role of HO-1. Am J Physiol Gastrointest Liver Physiol. 2002;283:G408–G414. doi: 10.1152/ajpgi.00348.2001. [DOI] [PubMed] [Google Scholar]
- 25.Tamion F, Richard V, Renet S, Thuillez C. Intestinal preconditioning prevents inflammatory response by modulating heme oxygenase-1 expression in endotoxic shock model. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1308–G1314. doi: 10.1152/ajpgi.00154.2007. [DOI] [PubMed] [Google Scholar]
- 26.Jancsó G, Cserepes B, Gasz B, Benkó L, Borsiczky B, Ferenc A, Kürthy M, Rácz B, Lantos J, Gál J, et al. Expression and protective role of heme oxygenase-1 in delayed myocardial preconditioning. Ann N Y Acad Sci. 2007;1095:251–261. doi: 10.1196/annals.1397.029. [DOI] [PubMed] [Google Scholar]
- 27.Lai IR, Chang KJ, Chen CF, Tsai HW. Transient limb ischemia induces remote preconditioning in liver among rats: the protective role of heme oxygenase-1. Transplantation. 2006;81:1311–1317. doi: 10.1097/01.tp.0000203555.14546.63. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Shan P, Jiang D, Noble PW, Abraham NG, Kappas A, Lee PJ. Small interfering RNA targeting heme oxygenase-1 enhances ischemia-reperfusion-induced lung apoptosis. J Biol Chem. 2004;279:10677–10684. doi: 10.1074/jbc.M312941200. [DOI] [PubMed] [Google Scholar]
- 29.Kamada N, Calne RY. A surgical experience with five hundred thirty liver transplants in the rat. Surgery. 1983;93:64–69. [PubMed] [Google Scholar]
- 30.Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, Cejalvo D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation. 1993;55:1265–1272. doi: 10.1097/00007890-199306000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Maines MD. The heme oxygenase system: past, present, and future. Antioxid Redox Signal. 2004;6:797–801. doi: 10.1089/ars.2004.6.797. [DOI] [PubMed] [Google Scholar]
- 32.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 33.Bae JW, Kim MJ, Jang CG, Lee SY. Protective effects of heme oxygenase-1 against MPP(+)-induced cytotoxicity in PC-12 cells. Neurol Sci. 2010;31:307–313. doi: 10.1007/s10072-010-0216-6. [DOI] [PubMed] [Google Scholar]
- 34.Kapitulnik J. Bilirubin: an endogenous product of heme degradation with both cytotoxic and cytoprotective properties. Mol Pharmacol. 2004;66:773–779. doi: 10.1124/mol.104.002832. [DOI] [PubMed] [Google Scholar]
- 35.Kirkby KA, Adin CA. Products of heme oxygenase and their potential therapeutic applications. Am J Physiol Renal Physiol. 2006;290:F563–F571. doi: 10.1152/ajprenal.00220.2005. [DOI] [PubMed] [Google Scholar]
- 36.Dos Santos P, Kowaltowski AJ, Laclau MN, Seetharaman S, Paucek P, Boudina S, Thambo JB, Tariosse L, Garlid KD. Mechanisms by which opening the mitochondrial ATP- sensitive K(+) channel protects the ischemic heart. Am J Physiol Heart Circ Physiol. 2002;283:H284–H295. doi: 10.1152/ajpheart.00034.2002. [DOI] [PubMed] [Google Scholar]
- 37.Krenz M, Oldenburg O, Wimpee H, Cohen MV, Garlid KD, Critz SD, Downey JM, Benoit JN. Opening of ATP-sensitive potassium channels causes generation of free radicals in vascular smooth muscle cells. Basic Res Cardiol. 2002;97:365–373. doi: 10.1007/s003950200045. [DOI] [PubMed] [Google Scholar]
- 38.Ferranti R, da Silva MM, Kowaltowski AJ. Mitochondrial ATP-sensitive K+ channel opening decreases reactive oxygen species generation. FEBS Lett. 2003;536:51–55. doi: 10.1016/s0014-5793(03)00007-3. [DOI] [PubMed] [Google Scholar]
- 39.Sato T, Marbán E. The role of mitochondrial K(ATP) channels in cardioprotection. Basic Res Cardiol. 2000;95:285–289. doi: 10.1007/s003950070047. [DOI] [PubMed] [Google Scholar]
- 40.Zarch AV, Toroudi HP, Soleimani M, Bakhtiarian A, Katebi M, Djahanguiri B. Neuroprotective effects of diazoxide and its antagonism by glibenclamide in pyramidal neurons of rat hippocampus subjected to ischemia-reperfusion-induced injury. Int J Neurosci. 2009;119:1346–1361. doi: 10.1080/00207450802338721. [DOI] [PubMed] [Google Scholar]
- 41.Cancherini DV, Trabuco LG, Rebouças NA, Kowaltowski AJ. ATP-sensitive K+ channels in renal mitochondria. Am J Physiol Renal Physiol. 2003;285:F1291–F1296. doi: 10.1152/ajprenal.00103.2003. [DOI] [PubMed] [Google Scholar]
- 42.Dröse S, Brandt U, Hanley PJ. K+-independent actions of diazoxide question the role of inner membrane KATP channels in mitochondrial cytoprotective signaling. J Biol Chem. 2006;281:23733–23739. doi: 10.1074/jbc.M602570200. [DOI] [PubMed] [Google Scholar]
- 43.Dzeja PP, Bast P, Ozcan C, Valverde A, Holmuhamedov EL, Van Wylen DG, Terzic A. Targeting nucleotide-requiring enzymes: implications for diazoxide-induced cardioprotection. Am J Physiol Heart Circ Physiol. 2003;284:H1048–H1056. doi: 10.1152/ajpheart.00847.2002. [DOI] [PubMed] [Google Scholar]
- 44.Hausenloy D, Wynne A, Duchen M, Yellon D. Transient mitochondrial permeability transition pore opening mediates preconditioning-induced protection. Circulation. 2004;109:1714–1717. doi: 10.1161/01.CIR.0000126294.81407.7D. [DOI] [PubMed] [Google Scholar]
- 45.Xu B, Makris A, Thornton C, Ogle R, Horvath JS, Hennessy A. Antihypertensive drugs clonidine, diazoxide, hydralazine and furosemide regulate the production of cytokines by placentas and peripheral blood mononuclear cells in normal pregnancy. J Hypertens. 2006;24:915–922. doi: 10.1097/01.hjh.0000222762.84605.03. [DOI] [PubMed] [Google Scholar]
- 46.Geuken E, Buis CI, Visser DS, Blokzijl H, Moshage H, Nemes B, Leuvenink HG, de Jong KP, Peeters PM, Slooff MJ, et al. Expression of heme oxygenase-1 in human livers before transplantation correlates with graft injury and function after transplantation. Am J Transplant. 2005;5:1875–1885. doi: 10.1111/j.1600-6143.2005.00960.x. [DOI] [PubMed] [Google Scholar]
- 47.Kiemer AK, Gerwig T, Gerbes AL, Meissner H, Bilzer M, Vollmar AM. Kupffer-cell specific induction of heme oxygenase 1 (hsp32) by the atrial natriuretic peptide--role of cGMP. J Hepatol. 2003;38:490–498. doi: 10.1016/s0168-8278(03)00056-4. [DOI] [PubMed] [Google Scholar]
- 48.Zeng Z, Huang HF, Chen MQ, Song F, Zhang YJ. Heme oxygenase-1 protects donor livers from ischemia/reperfusion injury: the role of Kupffer cells. World J Gastroenterol. 2010;16:1285–1292. doi: 10.3748/wjg.v16.i10.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cutrn JC, Perrelli MG, Cavalieri B, Peralta C, Rosell Catafau J, Poli G. Microvascular dysfunction induced by reperfusion injury and protective effect of ischemic preconditioning. Free Radic Biol Med. 2002;33:1200–1208. doi: 10.1016/s0891-5849(02)01017-1. [DOI] [PubMed] [Google Scholar]
- 50.Thomas P, Hayashi H, Lazure D, Burke PA, Bajenova O, Ganguly A, Forse RA. Inhibition of lipopolysaccharide activation of Kupffer cells by transition metals. J Surg Res. 2008;148:116–120. doi: 10.1016/j.jss.2007.11.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hanschen M, Zahler S, Krombach F, Khandoga A. Reciprocal activation between CD4+ T cells and Kupffer cells during hepatic ischemia-reperfusion. Transplantation. 2008;86:710–718. doi: 10.1097/TP.0b013e3181821aa7. [DOI] [PubMed] [Google Scholar]
- 52.Devey L, Mohr E, Bellamy C, Simpson K, Henderson N, Harrison EM, Ross JA, Wigmore SJ. c-Jun terminal kinase-2 gene deleted mice overexpress hemeoxygenase-1 and are protected from hepatic ischemia reperfusion injury. Transplantation. 2009;88:308–316. doi: 10.1097/TP.0b013e3181ae3067. [DOI] [PubMed] [Google Scholar]
- 53.Devey L, Ferenbach D, Mohr E, Sangster K, Bellamy CO, Hughes J, Wigmore SJ. Tissue-resident macrophages protect the liver from ischemia reperfusion injury via a heme oxygenase-1-dependent mechanism. Mol Ther. 2009;17:65–72. doi: 10.1038/mt.2008.237. [DOI] [PMC free article] [PubMed] [Google Scholar]