Abstract
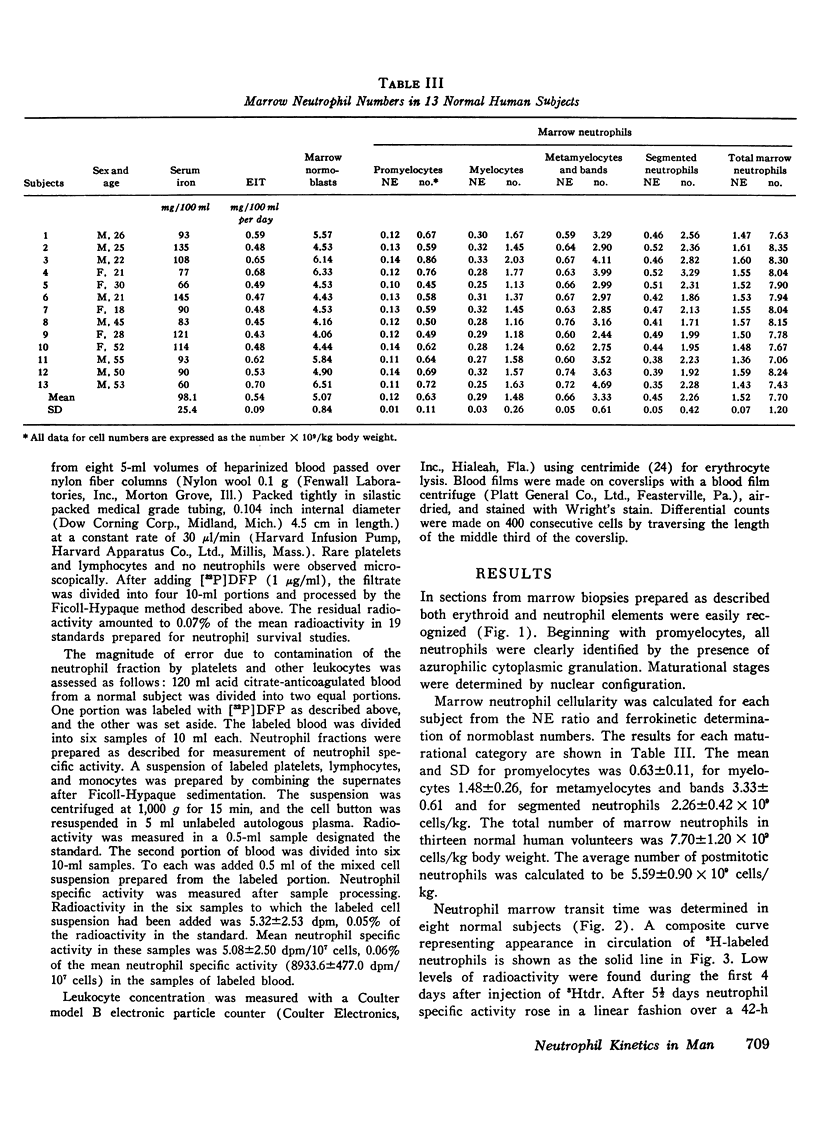
A method has been developed for measuring neutrophil cellularity in normal human bone marrow, in which the neutrophil-erythroid ratio was determined from marrow sections and marrow normoblasts were estimated by the erythron iron turnover. Neutrophil maturational categories, defined by morphologic criteria, were supported by autoradiographs of marrow flashed-labeled with 3H-thymidine. Correction for multiple counting error was empirically derived by counting serial sections through cells of each maturational category. The normal neutrophil-erythroid ratio in 13 normal human subjects was 1.5 +/- 0.07. The mean number of normoblasts in the same subjects was estimated to be 5.07 +/- 0.84 X 10(9) cells/kg. Total marrow neutrophils (X 10(9) cells/kg) were 7.70 +/- 1.20, the postmitotic pool (metamyelocytes, bands, and segmented forms) was 5.59 +/- 0.90 and the mitotic pool (promyelocytes + myelocytes) was 2.11 +/- 0.36. Marrow neutrophil ("total") production has been determined from the number of neutrophils comprising the postmitotic marrow pool divided by their transit time Transit time was derived from the appearance in circulating neutrophils of injected 3H-thymidine. The postmitotic pool comprised 5.59 +/- 0.90 X 10(9) neutrophils/kg, and the transit time was 6.60 +/- 0.03 days. From these data marrow neutrophil production was calculated to be 0.85 X 10(9) cells/kg per day. Effective production, measured as the turnover of circulating neutrophils labeled with 3H-thymidine, was 0.87 +/- 0.13 X 10(9) cells/kg per day. This value correlated well with the calculation of marrow neutrophil production. A larger turnover of 1.62 +/- 0.46 X 10(9) cells/kg per day was obtained when diisopropylfluorophosphate-32P was used to label circulating neutrophils. Studies using isologous cells doubly labeled with 3H-thymidine and diisopropylfluorophosphate-32P demonstrated a lower recovery and shorter t1/2 of the 32P label.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bithell T. C., Athens J. W., Cartwright G. E., Wintrobe M. M. Radioactive diisopropyl fluorophosphate as a platelet label: an evaluation of in vitro and in vivo technics. Blood. 1967 Mar;29(3):354–372. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- CARTWRIGHT G. E., ATHENS J. W., WINTROBE M. M. THE KINETICS OF GRANULOPOIESIS IN NORMAL MAN. Blood. 1964 Dec;24:780–803. [PubMed] [Google Scholar]
- CHAPLIN H., Jr, MOLLISON P. L., VETTER H. The body/venous hematocrit ratio: its constancy over a wide hematocrit range. J Clin Invest. 1953 Dec;32(12):1309–1316. doi: 10.1172/JCI102859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COPELAND B. E. Standard deviation; a practical means for the measurement and control of the precision of clinical laboratory determinations. Am J Clin Pathol. 1957 May;27(5):551–558. doi: 10.1093/ajcp/27.5.551. [DOI] [PubMed] [Google Scholar]
- Cook J. D., Marsaglia G., Eschbach J. W., Funk D. D., Finch C. A. Ferrokinetics: a biologic model for plasma iron exchange in man. J Clin Invest. 1970 Feb;49(2):197–205. doi: 10.1172/JCI106228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONOHUE D. M., GABRIO B. W., FINCH C. A. Quantitative measurement of hematopoietic cells of the marrow. J Clin Invest. 1958 Nov;37(11):1564–1570. doi: 10.1172/JCI103749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONOHUE D. M., REIFF R. H., HANSON M. L., BETSON Y., FINCH C. A. Quantitative measurement of the erythrocytic and granulocytic cells of the marrow and blood. J Clin Invest. 1958 Nov;37(11):1571–1576. doi: 10.1172/JCI103750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deubelbeiss K. A., Dancey J. T., Harker L. A., Finch C. A. Neutrophil kinetics in the dog. J Clin Invest. 1975 Apr;55(4):833–839. doi: 10.1172/JCI107994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deubeleiss K. A., Dancey J. T., Harker L. A., Cheney B., Finch C. A. Marrow erythroid and neutrophil cellularity in the dog. J Clin Invest. 1975 Apr;55(4):825–832. doi: 10.1172/JCI107993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresch C., Faille A., Bauchet J., Najean Y. Granulopöse: comparaison de diffèrentes mèthodes d'ètude de la durèe de maturation et des rèserves mèdullaires. Nouv Rev Fr Hematol. 1973 Jan-Feb;13(1):5–22. [PubMed] [Google Scholar]
- ELLIS L. D., JENSEN W. N., WESTERMAN M. P. NEEDLE BIOPSY OF BONE AND MARROW; AN EXPERIENCE WITH 1,445 BIOPSIES. Arch Intern Med. 1964 Aug;114:213–221. doi: 10.1001/archinte.1964.03860080063005. [DOI] [PubMed] [Google Scholar]
- Finch C. A., Deubelbeiss K., Cook J. D., Eschbach J. W., Harker L. A., Funk D. D., Marsaglia G., Hillman R. S., Slichter S., Adamson J. W. Ferrokinetics in man. Medicine (Baltimore) 1970 Jan;49(1):17–53. doi: 10.1097/00005792-197001000-00002. [DOI] [PubMed] [Google Scholar]
- GALBRAITH P. R., VALBERG L. S., BROWN M. PATTERNS OF GRANULOCYTE KINETICS IN HEALTH, INFECTION AND IN CARCINOMA. Blood. 1965 May;25:683–692. [PubMed] [Google Scholar]
- GIBLETT E. R., COLEMAN D. H., PIRZIOBIROLI G., DONOHUE D. M., MOTULSKY A. G., FINCH C. A. Erythrokinetics: quantitative measurements of red cell production and destruction in normal subjects and patients with anemia. Blood. 1956 Apr;11(4):291–309. [PubMed] [Google Scholar]
- HARRISON W. J. The total cellularity of the bone marrow in man. J Clin Pathol. 1962 May;15:254–259. doi: 10.1136/jcp.15.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HJORT P. F., PAPUTCHIS H., CHENEY B. Labeling of red blood cells with radioactive diisopropylfluorophosphate (DFP32): evidence for an initial release of label. J Lab Clin Med. 1960 Mar;55:416–424. [PubMed] [Google Scholar]
- Hansen N. E. The relationship between the turnover rate of neutrophilic granulocytes and plasma lysozyme levels. Br J Haematol. 1973 Dec;25(6):771–782. doi: 10.1111/j.1365-2141.1973.tb01789.x. [DOI] [PubMed] [Google Scholar]
- Harker L. A., Finch C. A. Thrombokinetics in man. J Clin Invest. 1969 Jun;48(6):963–974. doi: 10.1172/JCI106077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker L. A. Magakaryocyte quantitation. J Clin Invest. 1968 Mar;47(3):452–457. doi: 10.1172/JCI105741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman R. S. Characteristics of marrow production and reticulocyte maturation in normal man in response to anemia. J Clin Invest. 1969 Mar;48(3):443–453. doi: 10.1172/JCI106001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamshidi K., Swaim W. R. Bone marrow biopsy with unaltered architecture: a new biopsy device. J Lab Clin Med. 1971 Feb;77(2):335–342. [PubMed] [Google Scholar]
- LONDON I. M., WEST R., SHEMIN D., RITTENBERG D. On the origin of bile pigment in normal man. J Biol Chem. 1950 May;184(1):351–358. [PubMed] [Google Scholar]
- Labardini J., Papayannopoulou T., Cook J. D., Adamson J. W., Woodson R. D., Eschbach J. W., Hillman R. S., Finch C. A. Marrow radioiron kinetics. Haematologia (Budap) 1973;7(3):301–312. [PubMed] [Google Scholar]
- Lichtman M. A., Weed R. I. Alteration of the cell periphery during granulocyte maturation: relationship to cell function. Blood. 1972 Mar;39(3):301–316. [PubMed] [Google Scholar]
- Mauer A. M., Athens J. W., Ashenbrucker H., Cartwright G. E., Wintrobe M. M. LEUKOKINETIC STUDIES. II. A METHOD FOR LABELING GRANULOCYTES IN VITRO WITH RADIOACTIVE DIISOPROPYLFLUOROPHOSPHATE (DFP). J Clin Invest. 1960 Sep;39(9):1481–1486. doi: 10.1172/JCI104167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSGOOD E. E. Number and distribution of human hemic cells. Blood. 1954 Dec;9(12):1141–1154. [PubMed] [Google Scholar]
- PATT H. M. A consideration of myeloid-erythroid balance in man. Blood. 1957 Sep;12(9):777–787. [PubMed] [Google Scholar]
- Perry S., Moxley J. H., 3rd, Weiss G. H., Zelen M. Studies of leukocyte kinetics by liquid scintillation counting in normal individuals and in patients with chronic myelocytic leukemia. J Clin Invest. 1966 Sep;45(9):1388–1399. doi: 10.1172/JCI105447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REEVE E. B., VEALL N. A simplified method for the determination of circulating red-cell volume with radioactive phosphorus. J Physiol. 1949 Mar 1;108(1):12–23. doi: 10.1113/jphysiol.1949.sp004307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REIFF R. H., NUTTER J. Y., DONOHUE D. M., FINCH C. A. The relative number of marrow reticulocytes. Am J Clin Pathol. 1958 Sep;30(3):199–203. doi: 10.1093/ajcp/30.3.199. [DOI] [PubMed] [Google Scholar]
- RUTENBURG M., ROSALES C. L., BENNETT J. M. AN IMPROVED HISTOCHEMICAL METHOD FOR THE DEMONSTRATION OF LEUKOCYTE ALKALINE PHOSPHATASE ACTIVITY: CLINICAL APPLICATION. J Lab Clin Med. 1965 Apr;65:698–705. [PubMed] [Google Scholar]
- Rothstein G., Bishop C. R., Athens J. W., Ashenbrucker H. E. A method for leukokinetic study in the nonsteady state. Blood. 1971 Sep;38(3):302–311. [PubMed] [Google Scholar]
- WADSWORTH G. R. The blood volume of normal women. Blood. 1954 Dec;9(12):1205–1207. [PubMed] [Google Scholar]
- YOFFEY J. M. Bone marrow. Br Med J. 1954 Jul 24;2(4881):193–197. doi: 10.1136/bmj.2.4881.193. [DOI] [PMC free article] [PubMed] [Google Scholar]




