The two largest pools of carbon on Earth, carbonates and organic matter (kerogens, preserved primarily in shales), are both contained in the lithosphere (1). Both pools contain signatures of the two stable carbon isotopes, 13C and 12C; however, the processes responsible for the resulting isotopic fractionation differ. In the precipitation of calcite (the primary preserved form of carbonate in marine sediments), isotopic fractionation is dictated by thermodynamics; however, the isotopic discrimination is relatively small (2, 3). Hence, to first approximation, the 13C/12C composition of carbonates reflects the availability and isotopic signature of the source carbon (sea water). In contrast, the precipitation of organic carbon is dictated by kinetics; that is, when inorganic carbon is not limiting, the fixation of inorganic carbon by the enzyme ribulose 1,5 bis-phosphate carboxylase/oxygenase (RubisCO) leads to a ≈25–30‰ discrimination against the heavier isotope in the source carbon (4). As CO2 becomes limiting, the fractionation decreases. As all photosynthetic organisms contain RubisCO, and photosynthesis is by far the major route of entry of inorganic carbon into the organic realm, photosynthetic organisms potentially influence both the total pool of inorganic carbon and its isotopic distribution at any instance in time in Earth's history. The isotopic difference, ɛtoc, between the inorganic and organic carbon pools is, in principle, a semiquantitative proxy of the total inorganic carbon in the ocean–atmosphere system. In this issue of PNAS, Rothman (5) demonstrates that, as marine animal and terrestrial plant diversity increased in the latter part of the Phanaerozoic, ɛtoc values decreased, and the changes are significantly correlated. Using a model of marine phytoplankton isotope fractionation (6), Rothman suggests the relationship between ɛtoc and the diversity of both marine animals and terrestrial plants in the fossil record is causal and inverted the model (using the diversity index) to reconstruct Earth's atmospheric CO2 through the Phanaerozoic. Whether the model assumptions are accurate is anybody's guess, but the apparent correlation among these three proxies deserves inspection.
Photosynthesis is by far the major route of entry of inorganic carbon into the organic realm.
Geochemical reconstructions of Earth's CO2 on multimillion-year time scales call on vulcanism and metamorphism to supply the gas and on weathering reactions driven primarily by tectonic uplift to remove it (7, 8). Over geological time, it is generally believed that CO2 in the atmosphere–ocean system has largely decreased as weathering has increased. Weathering is accelerated by terrestrial plants (9), both physically by root extension (and hence fracture of rocks) and biochemically through local acidification in root metabolism and in the associated rhizospheric degradation of terrestrial organic matter. In this process, not only are silicates exposed to weathering reactions, but mineral phosphates are extracted and mobilized from the lithosphere, where a fraction eventually fluxes to the oceans. The enhanced phosphate flux, coupled with biological nitrogen fixation, potentially increases primary productivity and subsequent export and sequestration of organic carbon in marine sediments, thereby (on time scales of millions of years) further removing CO2 from the atmosphere–ocean pool (10). Indeed, the reconstruction of ɛtoc suggests that over the past ≈100 million years (Ma) of Earth's history, the isotopic fractionation of carbon has decreased by ≈6–7‰ and has been rapidly accelerating through the Cenozoic (the last 65 Ma).
That the overall depletion in atmospheric CO2 affected the diversity of land plants is not too surprising. Ecologically, diversity is sustained by a tension between competition for a limiting resource and the selection pressures such a limitation provides on genotypic variation. For example, terrestrial plant diversity reaches a maximum in tropical rain forests, where sufficient water is available to satisfy most flora, yet soil nutrient supplies are depleted. If more water is added to the system, such that bog or swamp conditions prevail, diversity decreases; similarly, if water is removed from the system (e.g., by deforestation, leading to loss of local rain), diversity decreases. This “unimodal” model of diversity and resource availability should not be confused with the relationship between diversity and productivity in terrestrial ecosystems (11, 12). As terrestrial plants assimilate CO2 through their stomates by diffusion into the leaf tissue, where it is assimilated by RubisCO, when CO2 concentrations decrease below ≈190 ppm, little net carbon is fixed. (This is approximately the ecological compensation point for C3 plants.) The decline in CO2 over the past 65 Ma was such that ≈15 Ma ago, another strategy evolved whereby CO2 (as bicarbonate anion) was fixed at lower concentrations and then “pumped” into the Calvin cycle. This so-called C4 pathway became a prevalent form of photosynthetic carbon assimilation in tropical and subtropical grasses in the late Miocene. In other words, the general trend, shown in Rothman's figure 2 (5), “makes sense” in that the Cenozoic CO2 record suggested by Rothman's model is consistent with the reconstruction from ɛtoc (13) and paleosol carbonates (14), as may be expected from the high degree of correlation between the biodiversity and ɛtoc curves. However, the relationship appears to weaken in the earlier part of the Phanaerozoic, especially in the Paleozoic, and the CO2 reconstructions by Berner et al. (15) and Rothman (5) may not be a complete picture.
The fossil record clearly indicates that vascular lignin-forming terrestrial plants increased in spatial range and depth of rooting during the early Devonian, leading to enhanced burial of organic matter (9) and the formation of the first identifiable forest soils (16). The accumulation of soils would have resulted in decrease in CO2 through the reaction:
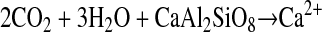 |
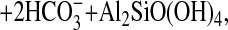 |
where the moblization of HCO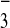 in the
aqueous phase delivers the inorganic carbon to the oceans to be
precipitated as Mg and Ca carbonates (dolomites and limestones).
Assuming that the rate of CO2 resupplied to the
atmosphere/ocean system by vulcanism and metamorphism did not exactly
match the drawdown caused by weathering, and that the enhanced burial
of organic carbon the overall pool of CO2 in the
two mobile phases (air and water) must have declined. The drawdown of
CO2 during this period is thought to have led to
the Permo-Carboniferous glaciation, the most extensive and
longest-lived glaciation of the Phanaerozoic. The drawdown in
CO2 during this period is further supported by
the δ13C record of carbonates and sulfur isotopes, as
well as stomatal density analyses of fossil leaves. Although these
proxies are by no means bulletproof, they provide self-consistent
metrics of the relative CO2 levels in the
ocean/atmosphere system during in the Paleozoic. However, taken at
face value, the ɛtoc data run paradoxically against this
hypothesis. The maximum isotopic fractionation of carbon in organic
matter, evident from the ɛtoc record, could be
interpreted as suggesting high atmospheric CO2
levels. This simple inference is at odds with the data and the
diversity of terrestrial plants and marine animals in the fossil record
(15). The reconstruction provided by Rothman does not resolve this
apparent paradox (5).
in the
aqueous phase delivers the inorganic carbon to the oceans to be
precipitated as Mg and Ca carbonates (dolomites and limestones).
Assuming that the rate of CO2 resupplied to the
atmosphere/ocean system by vulcanism and metamorphism did not exactly
match the drawdown caused by weathering, and that the enhanced burial
of organic carbon the overall pool of CO2 in the
two mobile phases (air and water) must have declined. The drawdown of
CO2 during this period is thought to have led to
the Permo-Carboniferous glaciation, the most extensive and
longest-lived glaciation of the Phanaerozoic. The drawdown in
CO2 during this period is further supported by
the δ13C record of carbonates and sulfur isotopes, as
well as stomatal density analyses of fossil leaves. Although these
proxies are by no means bulletproof, they provide self-consistent
metrics of the relative CO2 levels in the
ocean/atmosphere system during in the Paleozoic. However, taken at
face value, the ɛtoc data run paradoxically against this
hypothesis. The maximum isotopic fractionation of carbon in organic
matter, evident from the ɛtoc record, could be
interpreted as suggesting high atmospheric CO2
levels. This simple inference is at odds with the data and the
diversity of terrestrial plants and marine animals in the fossil record
(15). The reconstruction provided by Rothman does not resolve this
apparent paradox (5).
The correlation between marine animal genera and CO2 levels is even more puzzling. Following the simple logic invoked to relate a limitation to diversity as in terrestrial plant ecosystems, we might consider that marine animals have become increasingly limited by some resource(s) through the Phanaerozoic. We find that difficult to accept for two reasons. First, whereas the number of families (one taxonomic level higher than genera) of terrestrial plants may be a valid signature of diversity for that group of organisms, and there appears (albeit not without some disagreement) to be a relationship between diversity and productivity in terrestrial ecosystems (17), the number of genera of marine animals does not necessarily reflect diversity in the ocean, nor does it reflect productivity within that ecosystem. Consider that all flowering plants belong to one division (the Magnoliophyta) of the Viridiplantae. There are ≈250,000 species of extant Magnoliophyta; however, the genetic distance between species within this class is relatively low; all flowering plants are members of a large crown group on a short evolutionary branch of eukaryotes. In contrast, the dominant marine primary producers, the phytoplankton, are comprised of at least eight divisions yet are represented by only ≈15,000 extant species (18). Within this phylogenetically diverse group, three major classes evolved after the end-Permian extinction (251 Ma)—the dinoflagellates, the coccolithophorids, and the diatoms—and rose to ecological prominence in the Mesozoic and Cenozoic oceans. They replaced Chlorophyte and Prymnesiophytes, which dominated in the Paleozoic. By the late Cambrian, however, we can see fossil evidence of all of the major metazoan phyla. Hence, the application of number of genera in marine animals does not correlate with diversity of their ultimate food source (phytoplankton), nor does it correlate with productivity. Productivity of marine ecosystems almost certainly increased during the Mesozoic, when shallow seaways provided continentally derived nutrients (phosphate and iron) that led to the deposition and burial of massive amounts of organic matter and calcite.
Curiously, the apparent decrease in ɛtoc
in the Mesozoic and its continued steep rise in the latter portion
of the Cenozoic corresponds to the radiation of the three
aforementioned groups of eukaryotic phytoplankton taxa and,
simultaneously, the structure of marine
food webs. Whereas all
phytoplankton have the potential to discriminate against
CO2 by 25–30‰ (18), as pointed out by Rothman
(5), the actual fractionation depends on the growth rate, the
surface-to-volume ratio of the cell (i.e., cell size), and the
“permeability” of the cell membrane. In seawater, almost all
inorganic carbon is in the form of bicarbonate anion, whereas RubisCO
is highly specific for free CO2 as its substrate
(19). The concentration of free CO2 in the
surface waters of the open ocean is ≈10 μM, about an order of
magnitude lower than the half-saturation constant for RubisCO. To
compensate for low substrate concentration, phytoplankton have, to
varying degrees, evolved mechanisms to concentrate inorganic carbon;
that is, they “pump” inorganic carbon (in the form of either
CO2 or HCO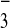 ) from the bulk
aqueous phase into the cell and thence to the site of carboxylation (in
the plastids in photoautotrophic eukaryotes). The carbon
concentrating mechanism buffers the cell from changes in free
CO2 in the aqueous phase and confounds the
interpretation of the isotopic signature in the organic matter. Hence,
to first order, unlike terrestrial plants, marine phytoplankton are not
directly limited by CO2, and consequently the
isotopic signature of organic matter does not, a priori,
reflect aqueous CO2 concentrations. This
biological “interference filter” makes the direct interpretation
of the isotopic signature, as used by Rothman in equation 2 (5),
difficult to verify experimentally; in effect, κ is highly variable
(unlike in terrestrial plants). Why then the long-term trend in
ɛtoc, especially since the Permian? And why is
ɛtoc apparently correlated with the diversity
of terrestrial plants and marine animals?
) from the bulk
aqueous phase into the cell and thence to the site of carboxylation (in
the plastids in photoautotrophic eukaryotes). The carbon
concentrating mechanism buffers the cell from changes in free
CO2 in the aqueous phase and confounds the
interpretation of the isotopic signature in the organic matter. Hence,
to first order, unlike terrestrial plants, marine phytoplankton are not
directly limited by CO2, and consequently the
isotopic signature of organic matter does not, a priori,
reflect aqueous CO2 concentrations. This
biological “interference filter” makes the direct interpretation
of the isotopic signature, as used by Rothman in equation 2 (5),
difficult to verify experimentally; in effect, κ is highly variable
(unlike in terrestrial plants). Why then the long-term trend in
ɛtoc, especially since the Permian? And why is
ɛtoc apparently correlated with the diversity
of terrestrial plants and marine animals?
When Hannibal crossed the Alps and Washington crossed the Delaware, atmospheric CO2 concentrations were about the same. From the middle of the 18th century to the present, the concentration of that molecule in Earth's atmosphere has risen exponentially.
One fundamental process, which explains the correlation among all three poxies, is the breakup of Pangea and the opening of the Atlantic ocean in mid-Mesozoic time, about 180 Ma. The tectonic processes during this period exposed large continental surfaces of silicate to the atmosphere, thereby accelerating weathering reactions that led to a long and apparently steady depletion of CO2. This process accelerated throughout the Cenozoic, presumably because orogenic (mountain-building) activity increased the rate of mantle exposure (or outgassing of CO2 declined) (20). Simultaneously, however, the same process greatly facilitated the diversification of terrestrial plants through isolation and subsequent genetic drift (the same occurred with mammals, birds, reptiles, and insects in this period). With the formation of the new ocean basin, benthic marine invertebrates became increasingly genetically isolated from the seed stock supplied from the Pan-Thalassian Ocean, such that new species (but not classes) emerged. Indeed, the tempo of evolution was accelerated by continental drift while the associated geochemical processes helped to deplete a vital resource, CO2.
The fundamental issue raised by Rothman (5), whether biological diversity can be used as a proxy for CO2, has provoked us to examine the mechanisms responsible for maintaining CO2 and diversity. We conclude that, on the time scales Rothman considers, the correlations derived are not casual but also are not causal; they are all forced by tectonics. Interestingly, the effects of human activities over the past 200 years correspond to Rothman's overall model. When Hannibal crossed the Alps and Washington crossed the Delaware, atmospheric CO2 concentrations were about the same, 280 ppm. From the middle of the 18th century to the present, the concentration of that molecule in Earth's atmosphere has risen exponentially, to the present value of ≈370 ppm, and will continue to rise through this century to approximately double that value. The rapid change is entirely a consequence of human energy-related activities, the consequences of which remain uncertain. Simultaneously, the rapid loss of habitat for both marine animals (e.g., coral reefs) and tropical rain forests is effectively the most rapid rate of extinction since the Permian. The causes for these two processes are also based on resource plunder. In conclusion, understanding the causal mechanisms controlling the global carbon cycle is the key for gaining confidence in any paleo CO2 reconstruction.
Acknowledgments
We thank the National Science Foundation and the National Aeronautics and Space Administration for supporting our research on the carbon cycle and its evolution.
Footnotes
See companion article on page 4305.
References
- 1.Falkowski P, Scholes R J, Boyle E, Canadell J, Canfield D, Elser J, Gruber N, Hibbard K, Hogberg P, Linder S, et al. Science. 2000;290:291–296. doi: 10.1126/science.290.5490.291. [DOI] [PubMed] [Google Scholar]
- 2.Reid A F, Urey H C. J Chem Phys. 1943;11:403–412. [Google Scholar]
- 3.Faure G. Principles of Isotope Geology. New York: Wiley; 1986. [Google Scholar]
- 4.Park R. Geochim Cosmochim Acta. 1961;21:110–126. [Google Scholar]
- 5.Rothman D H. Proc Natl Acad Sci USA. 2001;98:4305–4310. doi: 10.1073/pnas.071047798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Popp B N, Laws E A, Bidigare R R, Dore J E, Hanson K L, Wakeham S G. Geochim Cosmochim Acta. 1998;62:69–77. [Google Scholar]
- 7.Berner R A. Am J Sci. 1991;291:339–376. [Google Scholar]
- 8.Berner R A. Am J Sci. 1994;294:56–91. [Google Scholar]
- 9.Berner R A. Science. 1997;276:544–546. [Google Scholar]
- 10.Falkowski P G. Nature (London) 1997;387:272–275. [Google Scholar]
- 11.Tilman D, Pacala S. In: Species Diversity in Ecological Communities: Historical and Geographical Perspectives. Ricklefs R, Schluter D, editors. Chicago: Univ. of Chicago Press; 1993. pp. 13–25. [Google Scholar]
- 12.Waide R, Willig M, Steiner C, Mittelback G, Gough L, Dodson S, Juday G, Parmenter R. Annu Rev Ecol Syst. 1999;30:257–300. [Google Scholar]
- 13.Hayes J, Strauss H, Kaufman A. Chem Geol. 1999;161:103–125. [Google Scholar]
- 14.Ekart D, Cerling T, Montanez I, Tabor N. Am J Sci. 1999;299:805–827. [Google Scholar]
- 15.Berner R A, Petsch S T, Lake J A, Beerling D J, Popp B N, Lane R S, Laws E A, Westley M B, Cassar N, Woodward F I, Quick W P. Science. 2000;287:1630–1633. doi: 10.1126/science.287.5458.1630. [DOI] [PubMed] [Google Scholar]
- 16.Retallack G. Science. 1997;276:583–585. doi: 10.1126/science.276.5312.583. [DOI] [PubMed] [Google Scholar]
- 17.Morin P. Nature (London) 2000;406:463–464. doi: 10.1038/35020160. [DOI] [PubMed] [Google Scholar]
- 18.Falkowski P G, Raven J A. Aquatic Photosynthesis. Oxford: Blackwell; 1997. [Google Scholar]
- 19.Cooper T G, Filmer D, Wishnick M, Lane M D. J Biol Chem. 1969;244:1081–1083. [PubMed] [Google Scholar]
- 20.Raymo M, Ruddiman W. Nature (London) 1992;359:117–122. [Google Scholar]


