Abstract
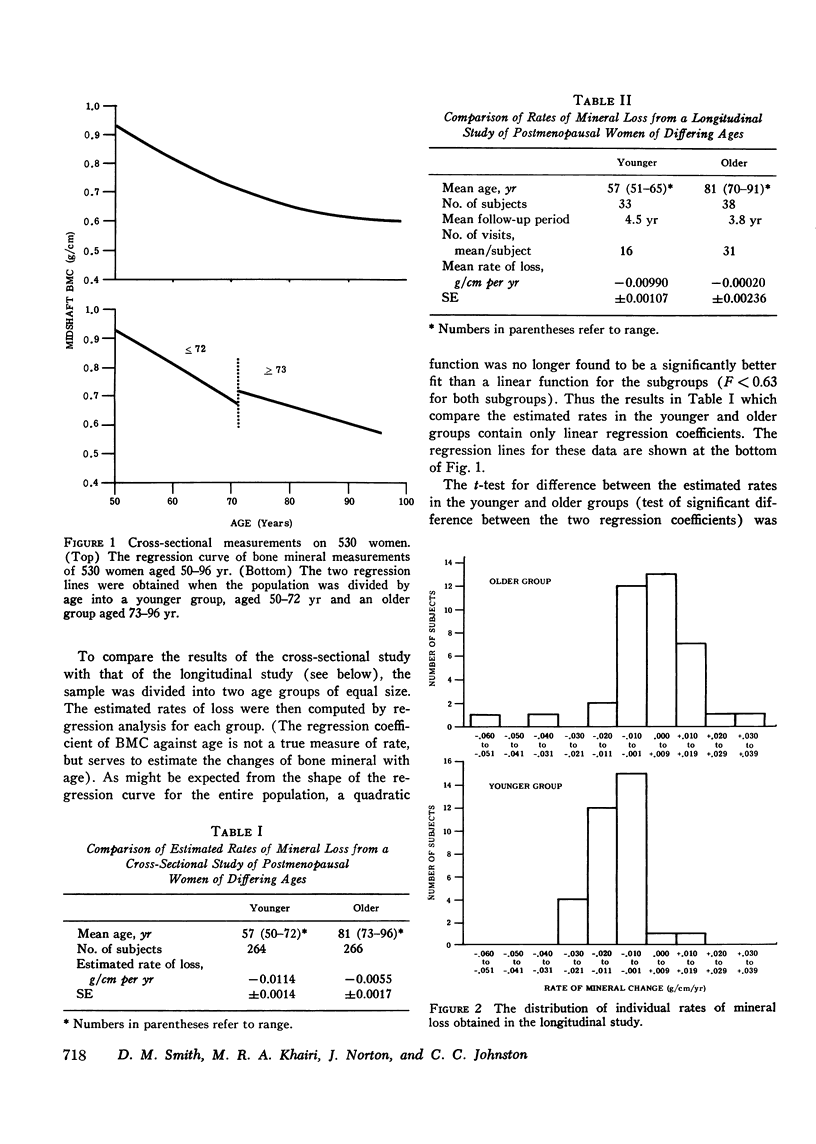
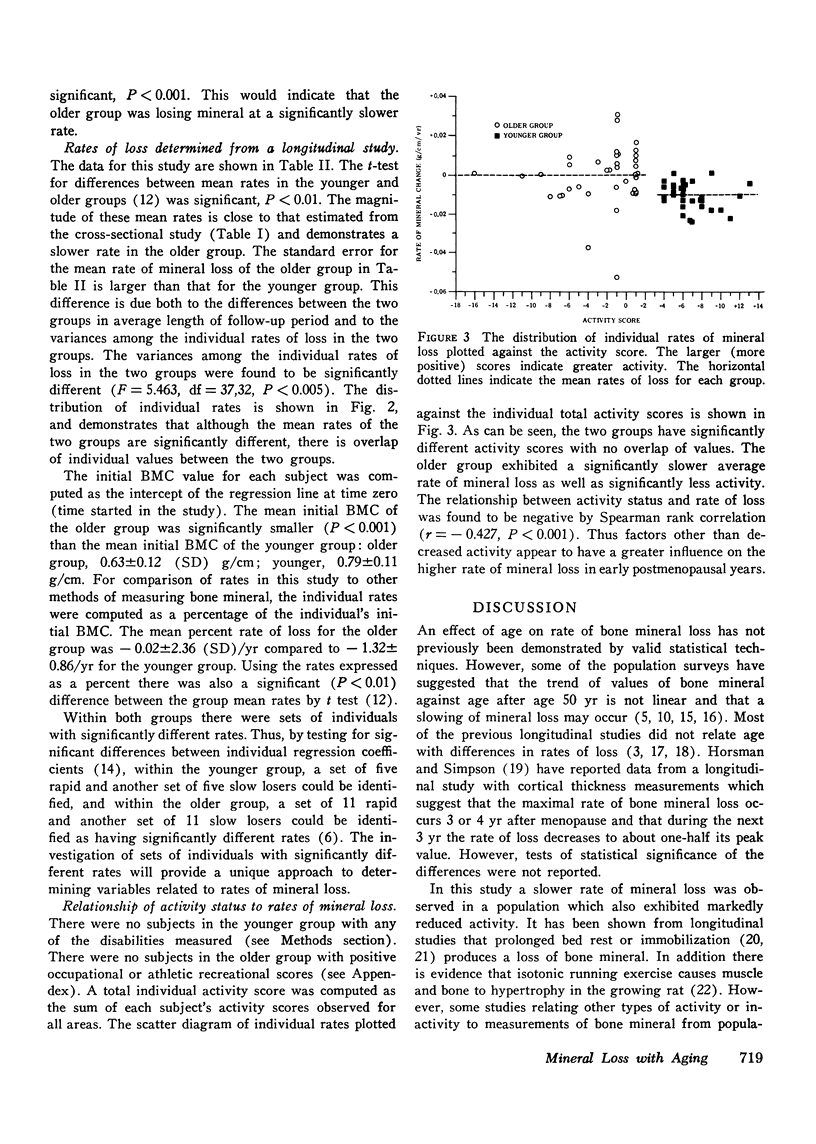
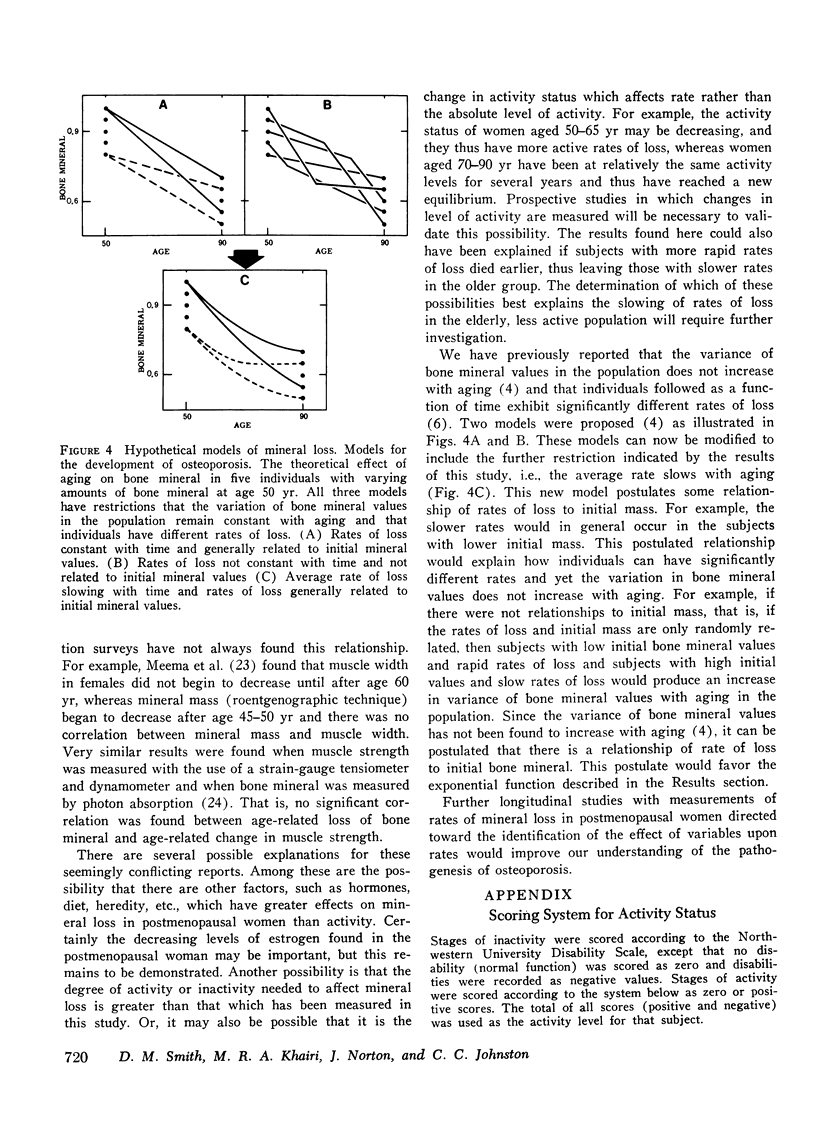
It has been postulated that the rate of mineral loss in postmenopausal women remains constant with aging and that the decreased activities of daily living associated with aging contribute to mineral loss. These hypothese were examined by measuring the bone mineral content at the midshaft of the radius with the photon absorption technique. The estimated rate of loss was calculated in a cross-sectional study as the regression coefficient of bone mineral content vs. age and in a longitudinal study as the regression coefficient of bone mineral content vs. time. In the cross-sectional study, Group A, which consisted of 264 women aged 50-72 yr, had an estimated rate of loss of -0.0114 +/- 0.0014 (SE) g/cm per yr. Group B, which consisted of 266 women aged 73-96 yr, had an estimated rate of loss of -0.0055 +/- 0.0017 g/cm per yr. In the longitudinal study, Group C consisted of 33 women aged 51-65 yr who were followed for an average of 4.5 yr with a mean number of 16 visits per subject; they were found to have a mean rate of loss of -0.00990 +/- 0.00107 g/cm per yr. Group D consisted of 38 women aged 70-91 yr who were followed for an average of 3.8 yr with a mean number of 31 visits per subjects; they were found to have a mean rate of loss of -0.00020 +/- 0.00236 g/cm per yr. The estimated and directly measured rates of loss were more rapid in the younger groups than in the older groups (A vs. B, P less than 0.001; C vs. D, P less than 0.001). These data demonstrate that the mean rate of mineral loss is not constant with aging and that in elderly subjects it is significantly slower than that of the earlier postmenopausal years. Since the elderly women were the less active, these findings suggest that factors other than decreased physical activity are more important in determining the rates of mineral loss.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P., Davies G. T., Sweetnam P. Osteoporosis and the effects of ageing on bone mass in elderly men and women. Q J Med. 1970 Oct;39(156):601–615. [PubMed] [Google Scholar]
- CANTER G. J., DE LA TORRE R., MIER M. A method for evaluating disability in patients with Parkinson's disease. J Nerv Ment Dis. 1961 Aug;133:143–147. doi: 10.1097/00005053-196108000-00010. [DOI] [PubMed] [Google Scholar]
- COCHRAN W. G. Testing a linear relation among variances. Biometrics. 1951 Mar;7(1):17–32. [PubMed] [Google Scholar]
- Garn S. M., Rohmann C. G., Wagner B. Bone loss as a general phenomenon in man. Fed Proc. 1967 Nov-Dec;26(6):1729–1736. [PubMed] [Google Scholar]
- Goldsmith N. F., Johnston J. O., Picetti G., Garcia C. Bone mineral in the radius and vertebral osteoporosis in an insured population. A correlative study using 125-I photon absorption and miniature roentgenography. J Bone Joint Surg Am. 1973 Sep;55(6):1276–1293. [PubMed] [Google Scholar]
- Horsman A., Simpson M. The measurement of sequential changes in cortical bone geometry. Br J Radiol. 1975 Jun;48(570):471–476. doi: 10.1259/0007-1285-48-570-470. [DOI] [PubMed] [Google Scholar]
- Hulley S. B., Vogel J. M., Donaldson C. L., Bayers J. H., Friedman R. J., Rosen S. N. The effect of supplemental oral phosphate on the bone mineral changes during prolonged bed rest. J Clin Invest. 1971 Dec;50(12):2506–2518. doi: 10.1172/JCI106751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzke E., Chesnut C. H., 3rd, Wergedal J. E., Baylink D. J., Nelp W. B. Relationship between local and total bone mass in osteoporosis. Metabolism. 1975 May;24(5):605–615. doi: 10.1016/0026-0495(75)90140-7. [DOI] [PubMed] [Google Scholar]
- Meema S., Reid D. B., Meema H. E. Age trends of bone mineral mass, muscle width, and subcutaneous fat in normals and osteoporotics. Calcif Tissue Res. 1973 May 9;12(2):101–112. doi: 10.1007/BF02013725. [DOI] [PubMed] [Google Scholar]
- Newton-John H. F., Morgan D. B. The loss of bone with age, osteoporosis, and fractures. Clin Orthop Relat Res. 1970;71:229–252. [PubMed] [Google Scholar]
- Nordin B. E. Clinical significance and pathogenesis of osteoporosis. Br Med J. 1971 Mar 13;1(5749):571–576. doi: 10.1136/bmj.1.5749.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saville P. D., Whyte M. P. Muscle and bone hypertrophy. Positive effect of running exercise in the rat. Clin Orthop Relat Res. 1969 Jul-Aug;65:81–88. [PubMed] [Google Scholar]
- Sinaki M., Opitz J. L., Wahner H. W. Bone mineral content: relationship to muscle strength in normal subjects. Arch Phys Med Rehabil. 1974 Nov;55(11):508–512. [PubMed] [Google Scholar]
- Smith D. M., Khairi M. R., Johnston C. C., Jr The loss of bone mineral with aging and its relationship to risk of fracture. J Clin Invest. 1975 Aug;56(2):311–318. doi: 10.1172/JCI108095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. M., Norton J. A., Jr, Khairi R., Johnston C. C., Jr The measurement of rates of mineral loss with aging. J Lab Clin Med. 1976 May;87(5):882–892. [PubMed] [Google Scholar]
- TROTTER M., BROMAN G. E., PETERSON R. R. Densites of bones of white and Negro skeletons. J Bone Joint Surg Am. 1960 Jan;42-A:50–58. [PubMed] [Google Scholar]


