Abstract
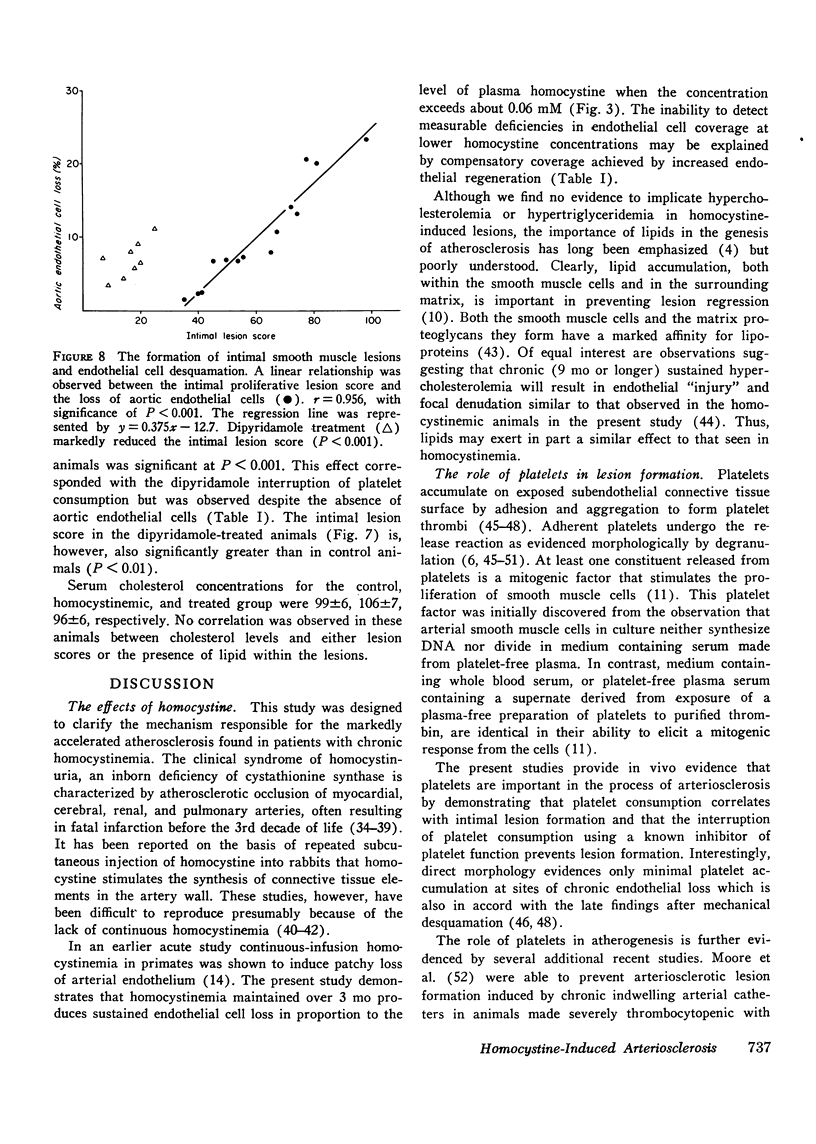
The atherogenic mechanism of homocystinemia has been defined by measuring endothelial cell loss and regeneration, platelet consumption, and intimal lesion formation in a primate model. Three groups of baboons were studied: (a) 8 control animals; (b) 15 animals after 3 mo of continuous homocystinemia; and (c) 11 animals after 3 mo of combined homocystinemia and oral treatment with dipyridamole. Experimental homocystinemia caused patchy endothelial desquamation comprising about 10% of the aortic surface despite a 25-fold increase in endothelial cell regeneration. Neither endothelial cell loss nor regeneration was changed significantly by dipyridamole. Homocystine-induced vascular deendothelialization produced a threefold increase in platelet consumption that was interrupted by dipyridamole inhibition of platelet function. All homocystinemic animals developed typical arteriosclerotic or preatherosclerotic intimal lesions composed of proliferating smooth muscle cells averaging 10-15 cell layers surrounded by large amounts of collagen, elastic fibers, glycosaminoglycans, and sometimes lipid. Intimal lesion formation was prevented by dipyridamole therapy. We conclude that homocystine-induced endothelial cell injury resulted in arteriosclerosis through platelet-mediated intimal proliferation of smooth muscle cells that can be prevented by drug-induced platelet dysfunction.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong M. L., Warner E. D., Connor W. E. Regression of coronary atheromatosis in rhesus monkeys. Circ Res. 1970 Jul;27(1):59–67. doi: 10.1161/01.res.27.1.59. [DOI] [PubMed] [Google Scholar]
- BORN G. V., CROSS M. J. THE AGGREGATION OF BLOOD PLATELETS. J Physiol. 1963 Aug;168:178–195. doi: 10.1113/jphysiol.1963.sp007185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner H. R., Haudenschild C. Adhesion of platelets to subendothelium. Ann N Y Acad Sci. 1972 Oct 27;201:22–36. doi: 10.1111/j.1749-6632.1972.tb16285.x. [DOI] [PubMed] [Google Scholar]
- Baumgartner H. R., Studer A. Folgen des Gefässkatheterismus am normo- und hypercholesterinaemischen Kaninchen. Pathol Microbiol (Basel) 1966;29(4):393–405. [PubMed] [Google Scholar]
- Björkerud S., Bondjers G. Arterial repair and atherosclerosis after mechanical injury. I. Permeability and light microscopic characteristics of endothelium in non-atherosclerotic and atherosclerotic lesions. Atherosclerosis. 1971 May-Jun;13(3):355–363. doi: 10.1016/0021-9150(71)90078-5. [DOI] [PubMed] [Google Scholar]
- Bowie E. J., Owen C. A., Jr, Thompson J. H., Jr, Didisheim P. A test of platelet adhesiveness. Mayo Clin Proc. 1969 May;44(5):306–308. [PubMed] [Google Scholar]
- Bull B. S., Schneiderman M. A., Brecher G. Platelet counts with the Coulter counter. Am J Clin Pathol. 1965 Dec;44(6):678–688. doi: 10.1093/ajcp/44.6.678. [DOI] [PubMed] [Google Scholar]
- CARSON N. A., CUSWORTH D. C., DENT C. E., FIELD C. M., NEILL D. W., WESTALL R. G. HOMOCYSTINURIA: A NEW INBORN ERROR OF METABOLISM ASSOCIATED WITH MENTAL DEFICIENCY. Arch Dis Child. 1963 Oct;38:425–436. doi: 10.1136/adc.38.201.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARSON N. A., DENT C. E., FIELD C. M., GAULL G. E. HOMOCYSTINURIA: CLINICAL AND PATHOLOGICAL REVIEW OF TEN CASES. J Pediatr. 1965 Mar;66:565–583. doi: 10.1016/s0022-3476(65)80121-4. [DOI] [PubMed] [Google Scholar]
- Christensen B. C., Garbarsch C. Repair in arterial tissue. A scanning electron microscopic (SEM) and light microscopic study on the endothelium of rabbit thoracic aorta following a single dilatation injury. Virchows Arch A Pathol Pathol Anat. 1973 Aug 9;360(2):93–106. [PubMed] [Google Scholar]
- Donahue S., Struman J. A., Gaull G. Arteriosclerosis due to homocyst (e) inemia. Failure to reproduce the model in weanling rabbits. Am J Pathol. 1974 Nov;77(2):167–163. [PMC free article] [PubMed] [Google Scholar]
- GERRITSEN T., WAISMAN H. A. HOMOCYSTINURIA: ABSENCE OF CYSTATHIONINE IN THE BRAIN. Science. 1964 Aug 7;145(3632):588–588. doi: 10.1126/science.145.3632.588. [DOI] [PubMed] [Google Scholar]
- GIBSON J. B., CARSON N. A., NEILL D. W. PATHOLOGICAL FINDINGS IN HOMOCYSTINURIA. J Clin Pathol. 1964 Jul;17:427–437. doi: 10.1136/jcp.17.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geer J. C., Catsulis C., McGill H. C., Jr, Stron J. P. Fine structure of the baboon aortic fatty streak. Am J Pathol. 1968 Feb;52(2):265–286. [PMC free article] [PubMed] [Google Scholar]
- Greenlee T. K., Jr, Ross R., Hartman J. L. The fine structure of elastic fibers. J Cell Biol. 1966 Jul;30(1):59–71. doi: 10.1083/jcb.30.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker L. A., Finch C. A. Thrombokinetics in man. J Clin Invest. 1969 Jun;48(6):963–974. doi: 10.1172/JCI106077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker L. A., Slichter S. J., Scott C. R., Ross R. Homocystinemia. Vascular injury and arterial thrombosis. N Engl J Med. 1974 Sep 12;291(11):537–543. doi: 10.1056/NEJM197409122911101. [DOI] [PubMed] [Google Scholar]
- Harker L. A., Slichter S. J. Studies of platelet and fibrinogen kinetics in patients with prosthetic heart valves. N Engl J Med. 1970 Dec 10;283(24):1302–1305. doi: 10.1056/NEJM197012102832402. [DOI] [PubMed] [Google Scholar]
- Harker L. A., Slichter S. J. The bleeding time as a screening test for evaluation of platelet function. N Engl J Med. 1972 Jul 27;287(4):155–159. doi: 10.1056/NEJM197207272870401. [DOI] [PubMed] [Google Scholar]
- Haudenschild C., Baumgartner H. R., Studer A. Significance of fixation procedure for preservation of arteries. Experientia. 1972 Jul 15;28(7):828–831. doi: 10.1007/BF01923157. [DOI] [PubMed] [Google Scholar]
- Huber J. D., Parker F., Odland G. F. A basic fuchsin and alkalinized methylene blue rapid stain for epoxy-embedded tissue. Stain Technol. 1968 Mar;43(2):83–87. doi: 10.3109/10520296809115048. [DOI] [PubMed] [Google Scholar]
- Iverius P. H. The interaction between human plasma lipoproteins and connective tissue glycosaminoglycans. J Biol Chem. 1972 Apr 25;247(8):2607–2613. [PubMed] [Google Scholar]
- JACOBSSON K. I. Studies on the determination of fibrinogen in human blood plasma. II. Studies on the trypsin and plasmin inhibitors in human blood serum. Scand J Clin Lab Invest. 1955;7 (Suppl 14):3–102. [PubMed] [Google Scholar]
- MUSTARD J. F., HEGARDT B., ROWSELL H. C., MACMILLAN R. L. EFFECT OF ADENOSINE NUCLEOTIDES ON PLATELET AGGREGATION AND CLOTTING TIME. J Lab Clin Med. 1964 Oct;64:548–559. [PubMed] [Google Scholar]
- McCully K. S. Macromolecular basis for homocystein-induced changes in proteoglycan structure in growth and arteriosclerosis. Am J Pathol. 1972 Jan;66(1):83–96. [PMC free article] [PubMed] [Google Scholar]
- McCully K. S. Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol. 1969 Jul;56(1):111–128. [PMC free article] [PubMed] [Google Scholar]
- Moore S., Friedman R. J., Singal D. P., Gauldie J., Blajchman M. A., Roberts R. S. Inhibition of injury induced thromboatherosclerotic lesions by anti-platelet serum in rabbits. Thromb Haemost. 1976 Feb 29;35(1):70–81. [PubMed] [Google Scholar]
- Murphy E. A., Francis M. E., Mustard J. F. The estimation of blood platelet survival. IV. Characteristics of the residual errors from regression. Thromb Diath Haemorrh. 1972 Dec 31;28(3):447–456. [PubMed] [Google Scholar]
- Murphy E. A., Francis M. E. The estimation of blood platelet survival. II. The multiple hit model. Thromb Diath Haemorrh. 1971;25(1):53–80. [PubMed] [Google Scholar]
- Murphy E. A. The estimation of blood platelet survival. 3. The robustness of the basic models. Thromb Diath Haemorrh. 1971 Dec 31;26(3):431–448. [PubMed] [Google Scholar]
- Mustard J. F., Packham M. A. Factors influencing platelet function: adhesion, release, and aggregation. Pharmacol Rev. 1970 Jun;22(2):97–187. [PubMed] [Google Scholar]
- POOLE J. C., SANDERS A. G., FLOREY H. W. The regeneration of aortic endothelium. J Pathol Bacteriol. 1958 Jan;75(1):133–143. doi: 10.1002/path.1700750116. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J. A. Atherosclerosis and the arterial smooth muscle cell: Proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science. 1973 Jun 29;180(4093):1332–1339. doi: 10.1126/science.180.4093.1332. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J., Kariya B., Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1207–1210. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Klebanoff S. J. The smooth muscle cell. I. In vivo synthesis of connective tissue proteins. J Cell Biol. 1971 Jul;50(1):159–171. doi: 10.1083/jcb.50.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHIMKE R. N., MCKUSICK V. A., HUANG T., POLLACK A. D. HOMOCYSTINURIA. STUDIES OF 20 FAMILIES WITH 38 AFFECTED MEMBERS. JAMA. 1965 Aug 30;193:711–719. doi: 10.1001/jama.1965.03090090017003. [DOI] [PubMed] [Google Scholar]
- Sade R. M., Folkman J. En face stripping of vascular endothelium. Microvasc Res. 1972 Jan;4(1):77–80. doi: 10.1016/0026-2862(72)90018-0. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Benditt E. P. Cell replication in the aortic endothelium: a new method for study of the problem. Lab Invest. 1973 Jun;28(6):699–707. [PubMed] [Google Scholar]
- Steele P. P., Weily H. S., Davies H., Genton E. Platelet function studies in coronary artery disease. Circulation. 1973 Dec;48(6):1194–1200. doi: 10.1161/01.cir.48.6.1194. [DOI] [PubMed] [Google Scholar]
- Stemerman M. B., Ross R. Experimental arteriosclerosis. I. Fibrous plaque formation in primates, an electron microscope study. J Exp Med. 1972 Oct 1;136(4):769–789. doi: 10.1084/jem.136.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemerman M. B. Vascular intimal components: precursors of thrombosis. Prog Hemost Thromb. 1974;2(0):1–47. [PubMed] [Google Scholar]
- Storb R., Ragde H., Thomas E. D. Extracorporeal irradiation of the blood in baboons. Radiat Res. 1969 Apr;38(1):43–54. [PubMed] [Google Scholar]
- Sullivan J. M., Harken D. E., Gorlin R. Pharmacologic control of thromboembolic complications of cardiac-valve replacement. N Engl J Med. 1971 Jun 24;284(25):1391–1394. doi: 10.1056/NEJM197106242842501. [DOI] [PubMed] [Google Scholar]
- Takeda Y. Studies of the metabolism and distribution of fibrinogen in healthy men with autologous 125-I-labeled fibrinogen. J Clin Invest. 1966 Jan;45(1):103–111. doi: 10.1172/JCI105314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlendorf B. W., Mudd S. H. Cystathionine synthase in tissue culture derived from human skin: enzyme defect in homocystinuria. Science. 1968 May 31;160(3831):1007–1009. doi: 10.1126/science.160.3831.1007. [DOI] [PubMed] [Google Scholar]
- Weiss H. J. Platelet physiology and abnormalities of platelet function (first of two parts). N Engl J Med. 1975 Sep 11;293(11):531–541. doi: 10.1056/NEJM197509112931105. [DOI] [PubMed] [Google Scholar]
- Weiss H. J., Tschopp T. B., Baumgartner H. R. Impaired interaction (adhesion-aggregation) of platelets with the subendothelium in storage-pool disease and after aspirin ingestion. A comparison with von Willebrand's disease. N Engl J Med. 1975 Sep 25;293(13):619–623. doi: 10.1056/NEJM197509252931301. [DOI] [PubMed] [Google Scholar]