Abstract
Recently we demonstrated that cessation of ovarian hormone production causes dramatic vascular remodeling in meningeal microvascular networks characterized by a significant microvessel loss. Further, even two months post ovariectomy (OVX), dura mater microvessels remain destabilized due to a decline in estrogenmediated angiopoietin-1 (Ang-1) expression and Ang-1/Tie-2 signaling. Such destabilized microvessels could be susceptible to either further regression or angiogenesis. In this study, we tested the hypothesis that initial estrogen-dependent loss of meningeal microvessels following OVX triggers stromal and vascular hypoxic responses aiming at restoring dura microvasculature. We demonstrate that two months post OVX, there is an activation of the hypoxia-inducible factor-1α (HIF-1α) and PDGF/VEGF system in the dura mater stroma and microvasculature of experimental animals accompanied by a shift in the balance between PI3K and PLCγ activity downstream of PDGF/VEGF signaling toward PI3K. It appears that the latter serves as a molecular switch favoring angiogenic responses rather than further regression of destabilized microvessels. Indeed, consistent with this idea, we have found a considerable angiogenic activity in meningeal microvascular networks that previously underwent regression. These results indicate that angioadaptation of meningeal microvessels in response to cessation of ovarian hormone production is not a unidirectional, but a very complex multi-stage process regulated on many levels. The implication of this study is that therapeutic interventions, including estrogen-based hormone replacement therapy, with physiological angioadaptation in postmenopausal or post OVX women need to be approached with the extreme caution.
Keywords: hormones, estrogen, microcirculation, remodeling, imaging, angiogenesis
Introduction
Our recent results demonstrated that, following the cessation of ovarian steroid hormone production, meningeal microvascular networks in ovariectomized (OVX) pigs undergo dramatic remodeling characterized by a 3-fold decrease in microvessel density, capillary rarefaction, and an increase in vascular permeability.1 Meningeal microvasculature remodeling of this magnitude can serve as a morphological basis for a development of multiple periand postmenopausal conditions. Further, it appears that, even two months post OVX, dura mater microvasculature remains destabilized due to an estrogen-dependent decline in angiopoietin-1 (Ang-1) production, consequent reduction in Ang-1/Tie-2 vessel-stabilizing signaling1 and therefore, susceptible to further angioadaptation.
The fate of such destabilized microvessels depends greatly on the absence or presence of angiogenic stimuli such as platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF) signaling. That is, destabilized microvessels may undergo further regression in the absence of angiogenic stimuli, or initiate a formation of new vessels, when angiogenic signals are present.2 Thus, the status of the PDGF/VEGF system could be crucial in determining the fate of the destabilized microvasculature. Importantly, angiogenic programs induced by both PDGF and VEGF via activation of their respective receptors, PDGF receptors (PDGFR) α and β and VEGF receptor-2 (Flk-1), require downstream involvement of signaling enzymes phosphatidylinositol 3′-kinase (PI3K) and phospholipase Cγ (PLCγ).3-6 Furthermore, recent results of Im and Kazlauskas indicate that the balance between PI3K and PLCγ activation could serve as a molecular switch between vessel regression and angiogenesis, whereas PLCγ competes with PI3K angiogenic stimuli by reducing the availability of their common substrate, phosphatidylinositol-4,5-bisphosphate.7,8 Therefore, knowing the status of the PDGF/VEGF system and PI3K/PLCγ signaling could be critical for assessing the state of destabilized meningeal microvasculature following OVX and foreseeing future directions of post OVX angioadaptation.
Given the magnitude of the loss of meningeal microvessels observed in post OVX animals,1 the ability to predict the fate of destabilized meningeal microvasculature could be particularly important as one can reasonably envision that any further decline in microvessels density could be catastrophic for a tissue. Here, we hypothesize that initial estrogen-dependent loss of capillaries following OVX would inevitably affect the metabolic status of the tissue and trigger hypoxic responses, including activation of the hypoxia-inducible factor-1α (HIF-1α) and PDGF/VEGF system, aiming at restoring microvasculature. To test this hypothesis, we analyzed the expression of HIF-1α, PDGF and VEGF, the status and distribution of PDGFRα, PDGFRβ and Flk-1, as well as the degree of PI3K and PLCγ activation and signs of angiogenic activity in dura mater of intact female (IF) and ovariectomized animals two months post OVX.
Results and Discussion
A significant decrease in meningeal microvessel density observed in porcine dura mater following OVX due to the estrogen-dependent loss of capillaries1 is likely to result in a reduced delivery of oxygen to the tissue and a development of hypoxic conditions. Biological responses to such conditions are often characterized by the elevated expression of the HIF-1α, which could serve as a surrogate indicator of a tissue hypoxia. Thus, to answer the question whether post OVX vascular remodeling results in a development of hypoxia in dura mater we analyzed the levels of HIF-1α expression in dura mater extracts of intact female (IF) and ovariectomized animals. The results of Western blot analysis (Fig. 1) demonstrated that post OVX loss of meningeal microvessels (Fig. 1A and B) is accompanied by a significant induction of HIF-1α expression in dura mater tissue of ovariectomized pigs compared to gonadaly intact females (Fig. 1C and D). These results indicate that indeed estrogen-dependent loss of microvessels following OVX leads to a development of hypoxic conditions. However, while being an important indicator of tissue hypoxic responses, HIF-1α is not simply a marker of a hypoxia. It has been shown in numerous studies to regulate positively a variety of angiogenic factors including PDGF, VEGF, and their related receptors.
Figure 1.
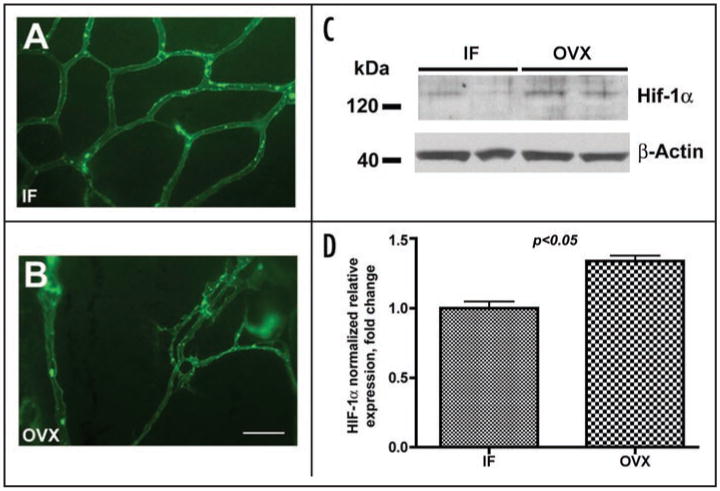
Post ovariectomy loss of meningeal microvessels is accompanied by an increase in HIF-1α expression. (A and B) Representative images of porcine dura mater microvascular networks in intact female (IF) animals (A) and ovariectomized (OVX) pigs (B) two months post OVX. Scale bar (shown in B) 100 μm. (C and D) Western blot analysis of HIF-1α expression in dura mater of IF and OVX animals. Note a significant increase in HIF-1α expression in OVX animals compared to IF.
Therefore, we investigated next the status of the PDGF/VEGF system in dura mater of gonadaly intact females and ovariectomized animals 2 months post OVX. Western blot analysis of dura mater whole tissue lysates revealed that two months post OVX there is an obvious increase in PDGF expression in OVX pigs compared to gonadaly intact females (Fig. 2A and B). This change in local PDGF expression was sufficient to increase significantly relative tyrosine phosphorylation of the PDGFRα ∼1.5-fold (p < 0.05) in post OVX animals compared to intact females, while overall PDGFRα level remained the same (Fig. 2C–E). Interestingly, immunohistochemical analysis (Fig. 2F) confirmed similar levels of PDGFRα expression in the vasculature of intact female and post OVX animals, but also showed marked redistribution of this receptor throughout dura mater stroma in ovariectomized pigs. Several groups of investigators have demonstrated that ischemia regulates positively both PDGF9 and PDGFRα10,11 expression in multiple tissues including brain10 and mesenchymal cells.11 Thus, PDGFRα redistribution and increased phosphorylation in post OVX dura likely reflects the reaction of the dura mater stroma to hypoxic conditions brought about by a significant loss of microvasculature.1 Furthermore, in this situation one can reasonably expect a concomitant increase in VEGF expression, which could be induced by hypoxia both directly12-14 and via PDGF signalling.11,15
Figure 2.
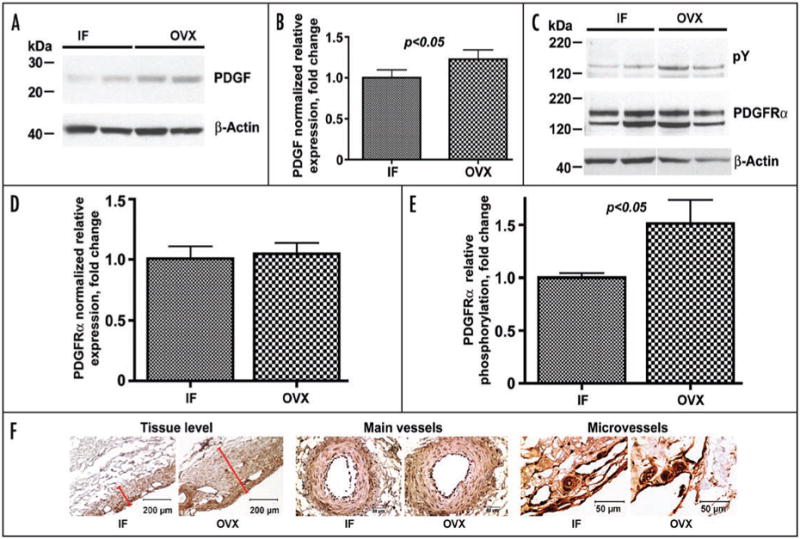
Changes in the PDGF/PDGFR system two months post OVX. (A and B) Western blot analysis of PDGF expression in dura mater of IF and OVX animals. (C–E) Western blot analysis of the relative expression and phosphorylation (pY) of PDGFRα in dura mater of IF and OVX animals. Note a significant increase in relative phosphorylation of PDGFRα in OVX animals compared to IF (E), while the relative levels of expression for this receptor remain unchanged (D). (F) Immunohistochemical analysis of PDGFRα expression in dura mater of experimental animals at the tissue level (left), in the main vessels (O.D. > 100 μm; center), and in the microvessels (O.D. < 50 μm; right). Note an apparent redistribution of PDGFRα throughout the dura mater stroma in post OVX animals compared to IF on a tissue level (red bars show the extent of PDGFRα expression in dura mater stroma of IF and OVX animals), while the relative vascular expression of the receptor remain unchanged. In (B, D and E), bar graphs show mean ± standard error of mean. In (F), scale bars are 200 μm at the Tissue level and 50 μm in the Main vessels and Microvessels.
Thus, we investigated next the levels of expression of VEGF and its receptor Flk-1 in dura mater of intact female and ovariectomized animals two months post OVX. As expected, Western blot analysis demonstrated an ∼1.5-fold increase in VEGF expression in post OVX pigs compared to intact females (Fig. 3A and B) whereas Flk-1 levels in whole tissue lysates from gonadaly intact and post OVX swine remained the same (Fig. 3C). Unlike PDGFRα expression, however, the expression of Flk-1, one of the earliest endothelial markers, is restricted to vascular tissue.16 Thus, given a 3-fold decrease in microvessel density in dura mater of post OVX animals compared to intact females,1 this suggests that relative vascular expression of Flk-1 in dura microvessels could be elevated markedly two months post OVX. Immunohistochemically, such an increase is particularly evident in microvessels with OD <50 μm (Fig. 3D).
Figure 3.
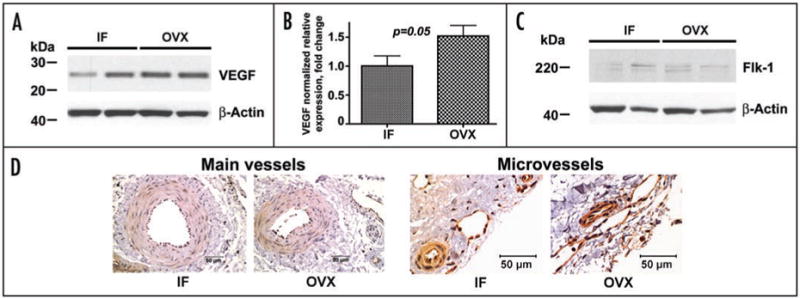
Changes in VEGF/Flk-1 system two months post OVX. (A–C) Western blot analysis of VEGF (A and B) and Flk-1 (C) expression in dura mater of IF and OVX pigs. (D) Immunohistochemical analysis of Flk-1 expression in the main vessels (O.D. > 100 μm; left), and in the microvessels (O.D. < 50 μm; right) of IF and OVX animals. Note an apparent increase in relative Flk-1 vascular expression following OVX in microvessels with OD < 50 μm. Bar graph in (B) shows mean ± standard error of mean. Scale bars, 50 μm.
Based on these results, it appears that two months post OVX there is an activation of the HIF-1α and PDGF/VEGF system in the dura mater of ovariectomized animals, apparently resulting from hypoxic stromal responses following initial post OVX loss of microvasculature. PDGF and VEGF signaling, acting upon destabilized microvasculature via PDGFRs and Flk-1, triggers complex signaling pathways involving downstream activity of PI3K and PLCγ.3-6 Importantly, the balance between PI3K and PLCγ activation was proposed recently to serve as a molecular switch between vessel regression and angiogenesis.7,8 That is, increased PLCγ activity would signal regression of destabilized microvessels, while shifting the balance toward PI3K activity will induce angiogenesis.
Therefore, our next question was whether activation of the PDGF/VEGF system in post OVX dura mater affected the balance between PI3K and PLCγ signaling. To answer this question, we analyzed changes in expression of the p85 regulatory subunit of PI3K, phosphorylation of the p85 binding motif, as well as expression and phosphorylation of PLCγ. Our results revealed no differences in p85 expression levels and phosphorylation of p85 binding motif between intact females and ovariectomized pigs (Fig. 4A and B), suggesting rather a sustained degree of PI3K activity following OVX regardless of an apparent activation of the PDGF/VEGF system. The relative phosphorylation of PLCγ, however, was moderately but highly significantly (p < 0.01) decreased in post OVX animals compared to intact females (Fig. 4C and D). Thus, two months post OVX, despite sustained levels of PI3K activation, because of a decrease in PLCγ activity, the balance between PI3K and PLCγ in dura mater of ovariectomized animals is shifting toward PI3K. This shift of the balance toward PI3K suggests that at this point in time destabilized dura microvessels are likely to initiate new vessel formation (angiogenesis) in response to enhanced PDGF/VEGF signaling, rather than undergo further regression.7,8 Indeed, indicative of active angiogenesis, we were able to observe vascular sprouts forming at multiple locations within microvascular networks, which previously underwent regression (Fig. 4E).
Figure 4.
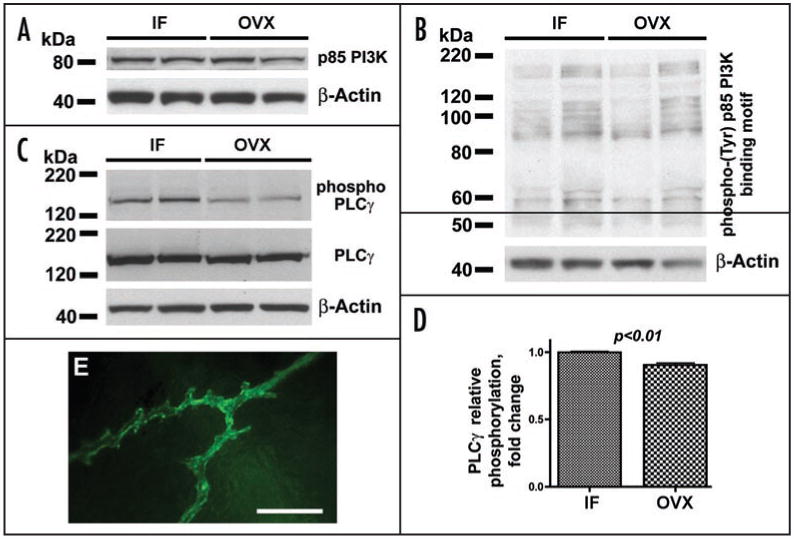
Western blot analysis of the expression of p85 PI3K regulatory subunit (A), phosphorylation of p85-binding motif (B), and expression and phosphorylation of PLCγ in dura mater of IF and OVX Yucatan miniature pigs 2 months post OVX. Note a moderate, but highly significant, decrease in PLCγ relative phosphorylation (C and D) in OVX animals compared to intact females, while p85 expression (A) and phosphorylation of p85-binding motif (B) remain unchanged. (E) Vascular sprouts in dura mater microvascular networks previously undergoing regression in ovariectomized animals 2 months post OVX. Bar graph in (D) shows mean ± standard error of mean. Scale bar in (E), 100 μm.
It appears that post OVX remodeling of meningeal microvascular networks is regulated by a complex interplay of stromal and vascular responses. Initial estrogen-dependent loss of capillaries following OVX1 affects the metabolic status of dura mater stroma and triggers hypoxic responses activating HIF-1α and the PDGF/VEGF system (Fig. 5). At this point in time, the shift of the balance between PI3K and PLCγ in destabilized microvessels downstream of PDGF/VEGF signaling toward the PI3K activity favors angiogenic responses rather than further microvessel regression (Fig. 5). Apparently, post OVX angioadaptation is not a unidirectional, but an extremely complex multi-stage process regulated on many levels. The initial stages of this process, brought about by a cessation of ovarian steroid hormone production, are estrogen-dependent and characterized by a significant microvessel loss.1 In contrast, later stages, described by the activation of HIF-1α and the PDGF/VEGF system and initiation of angiogenic responses, are not necessarily estrogen-dependent. They are likely regulated by the reaction of the dura mater stroma to hypoxic conditions and may as well reflect tissue and vascular system adaptation to reduced systemic E2 levels. At present, we do not know whether an apparent shift in post OVX remodeling of meningeal microvascular networks toward angiogenesis two months post OVX will result in a complete or only a partial restoration of meningeal microvasculature. What is clear, though, that our current knowledge of fine molecular and cellular mechanisms governing angioadaptation initiated by a cessation of ovarian hormone production is not sufficient to predict how various therapeutic interventions, including estrogen-based hormone replacement therapy, will affect this dynamic process. There is an obvious possibility that, depending on the time and regimens of hormone replacement therapy, it may interfere with physiological adaptational responses and cause negative rather than positive effects, suggesting that hormone replacement therapy in postmenopausal or post OVX women should be approached with the extreme caution.
Figure 5.
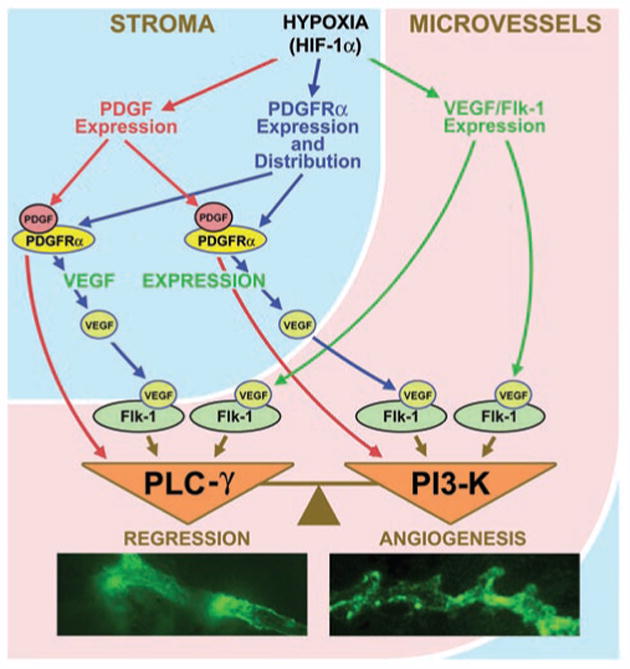
Schematic representation of the PDGF/VEGF-dependent control of vascular remodeling in hypoxic dura mater of post OVX animals. Hypoxia-induced activation of PDGF/VEGF system in dura mater stroma and microvessels controls remodeling of destabilized microvessels, while the balance between PI3K and PLCγ activity downstream of PDGF/VEGF signaling serves as a molecular switch between microvessel regression and angiogenesis.
Methods
Animals
Mature 9–12 month-old female Yucatan miniature swine were used in this study in accordance to the University of Missouri ACUC approved protocol. Bilateral OVX was performed under general anesthesia by a team of surgeons at the National Center for Gender Physiology Swine Hormone Core. Animals were sacrificed at indicated time points, and dura mater was acquired and processed as described previously.17,18 The body mass at sacrifice was 38.9 ± 2.2 kg (n = 9) and 35.5 ± 3.9 kg (n = 5) for IF and OVX animals respectively.
Imaging
Alexa Fluor 488-conjugated soybean agglutinin (SBA) at 30 μg/ml (final concentration) was used for visualizing microvasculature as described previously.1,17,18 Briefly, the dura mater corresponding to one hemisphere was collected within 15–30 min after animal's sacrifice, and placed immediately on ice in a porcine Krebs solution (PKS, Krebs physiological salt supplemented with 1.0 mg/ml porcine albumin). Special attention was paid to minimize dura mater damage during its separation from the skull. The collected dura was rinsed twice with ice-cold PKS, and transferred to a refrigerated tissue dissection dish filled with PKS and connected to a circulating bath to maintain the sample temperature from 0°C to 4°C during the dissection procedure. Next, the dura was dissected and trimmed under control of a low-power stereomicroscope SMZ-2B (Nikon, Japan) to remove arachnoid granulations and release the dura's folds to flatten the tissue onto Sylgard-coated 100 mm tissue culture dish. During the trimming, particular care was taken not to damage the major blood vessels detectable under stereomicroscope. Next, an approximately 5 mm long segment of the Median Meningeal artery was separated carefully from surrounding tissues, the sample was transferred onto the fluorescent microscope stage, and the artery was cannulated using a pipette manufactured from 1 mm O.D. borosilicate glass capillary with 90 μm opening at the tip. To remove blood, the dura mater vasculature was perfused for 20 min with PKS using a precision syringe infusion/withdrawal pump KDS210 (KD Scientific, USA) at physiological rate (15 μl/min). To visualize the perfused vascular tree, microvessels were perfused for an additional 30 min with PKS containing SBA lectin (30 μg/ml) conjugated with Alexa Fluor 488, followed by 30 min perfusion with PKS to remove unbound dye before imaging. Terminal dura mater microvascular networks were imaged first using a fluorescence video microscopy system [Laborlux 8 microscope (Leitz Wetzlar, Germany) equipped with 75-watt xenon lamp and QICAM high performance digital CCD camera (Quantitative Imaging Corporation, Burnaby, Canada)] with controlled gain, offset and exposure time; and then a confocal system [IX-70 Olympus Microscope equipped with Solamere confocal unit with dual laser line capabilities (50 mW 488 nm & 514 nm, 10 mW 457) and acoustic coupler].
Western blot analysis
The whole tissue lysates prepared from dura mater of IF and OVX animals (30 μg of total protein per lane) were resolved on NuPAGE 4–12% gradient Bis-Tris gels (Invitrogen, Carlsbad, California, USA), transferred to nitrocellulose membranes, and analyzed using rabbit polyclonal antibodies against HIF-1α, PDGF, PDGFRα, PDGFRβ and VEGF, mouse monoclonal antibody against Flk-1 (all Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit polyclonal antibodies against PI3K p85 subunit, phospho p85-binding motif, PLCγ and phospho PLCγ (all Cell Signaling Technology, Danvers, MA, USA) in conjunction with corresponding HRP-labeled secondary antibodies and enhanced chemiluminescence (ECL Plus) detection system (Amersham, UK). Equal loading and transfer was controlled by Ponceau S staining. Subsequently, each membrane was striped and reprobed with anti-β-Actin loading control (Abcam, Cambridge, MA, USA), which was used for normalizing relative expression of target proteins.
Immunohistochemistry
Rabbit polyclonal antibodies against PDGFRα, PDGFRβ and VEGF, as well as mouse monoclonal antibody against Flk-1 (all Santa Cruz Biotechnology, Santa Cruz, CA, USA) were used for immunohistochemical analysis of formaldehydefixed, paraffin embedded serial sections of porcine dura mater in conjunction with corresponding HRP-labeled secondary antibodies.
Statistical analysis
The statistical analysis of data was performed using GraphPad Prism version 4 software (GraphPad Software, Inc., San Diego, CA, USA). Two-tailed t test was used to assess statistical significance of data. Bar graphs represent mean ± standard error of mean.
Acknowledgments
We thank National Center for Gender Physiology and Environmental Adaptation Swine Hormone Core for technical assistance. This research was supported by the Merit Review Award from the VA Biomedical Laboratory Research and Development Service (to V.V.G.), and grants from the American Heart Association 0830287N (to O.V.G.), NIH 5RO1 HL078816-03 (to V.H.H.), and NIH HL-52490 (to J.R.T.).
References
- 1.Glinskii OV, Abraha TW, Turk JR, Rubin LJ, Huxley VH, Glinsky VV. Microvascular Network Remodeling in Dura Mater of Ovariectomized Pigs: Role for Angiopietin-1 in Estrogen-Dependent Control of Vascular Stability. Am J Physiol Heart Circ Physiol. 2007;293:1131–7. doi: 10.1152/ajpheart.01156.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zakrzewicz A, Secomb TW, Pries AR. Angioadaptation: keeping the vascular system in shape. News Physiol Sci. 2002;17:197–201. doi: 10.1152/nips.01395.2001. [DOI] [PubMed] [Google Scholar]
- 3.Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336–43. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 4.Gille H, Kowalski J, Yu L, Chen H, Pisabarro MT, Davis-Smyth T, Ferrara N. A repressor sequence in the juxtamembrane domain of Flt-1 (VEGFR-1) constitutively inhibits vascular endothelial growth factor-dependent phosphatidylinositol 3-kinase activation and endothelial cell migration. EMBO J. 2000;19:4064–73. doi: 10.1093/emboj/19.15.4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawson ND, Mugford JW, Diamond BA, Weinstein BM. Phospholipase C gamma-1 is required downstream of vascular endothelial growth factor during arterial development. Genes Dev. 2003;17:1346–51. doi: 10.1101/gad.1072203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi T, Yamaguchi S, Chida K, Shibuya M. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-gamma and DNA synthesis in vascular endothelial cells. EMBO J. 2001;20:2768–78. doi: 10.1093/emboj/20.11.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Im E, Kazlauskas A. New insights regarding vessel regression. Cell Cycle. 2006;5:2057–9. doi: 10.4161/cc.5.18.3210. [DOI] [PubMed] [Google Scholar]
- 8.Im E, Kazlauskas A. Regulating angiogenesis at the level of PtdIns-4,5-P2. EMBO J. 2006;25:2075–82. doi: 10.1038/sj.emboj.7601100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eng E, Holgren C, Hubchak S, Naaz P, Schnaper HW. Hypoxia regulates PDGF-B interactions between glomerular capillary endothelial and mesangial cells. Kidney Int. 2005;68:695–703. doi: 10.1111/j.1523-1755.2005.00448.x. [DOI] [PubMed] [Google Scholar]
- 10.Morioka I, Tsuneishi S, Takada S, Matsuo M. PDGF-alpha receptor expression following hypoxic-ischemic injury in the neonatal rat brain. Kobe J Med Sci. 2004;50:21–30. [PubMed] [Google Scholar]
- 11.Tsutsumi N, Yonemitsu Y, Shikada Y, Onimaru M, Tanii M, Okano S, Kaneko K, Hasegawa M, Hashizume M, Maehara Y, Sueishi K. Essential role of PDGFRalpha-p70S6K signaling in mesenchymal cells during therapeutic and tumor angiogenesis in vivo: role of PDGFRalpha during angiogenesis. Circ Res. 2004;94:1186–94. doi: 10.1161/01.RES.0000126925.66005.39. [DOI] [PubMed] [Google Scholar]
- 12.Fukumura D, Xu L, Chen Y, Gohongi T, Seed B, Jain RK. Hypoxia and acidosis independently upregulate vascular endothelial growth factor transcription in brain tumors in vivo. Cancer Res. 2001;61:6020–4. [PubMed] [Google Scholar]
- 13.Levy AP, Levy NS, Wegner S, Goldberg MA. Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia. J Biol Chem. 1995;270:13333–40. doi: 10.1074/jbc.270.22.13333. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Cox SR, Morita T, Kourembanas S. Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5′ enhancer. Circ Res. 1995;77:638–43. doi: 10.1161/01.res.77.3.638. [DOI] [PubMed] [Google Scholar]
- 15.Shikada Y, Yonemitsu Y, Koga T, Onimaru M, Nakano T, Okano S, Sata S, Nakagawa K, Yoshino I, Maehara Y, Sueishi K. Platelet-derived growth factor-AA is an essential and autocrine regulator of vascular endothelial growth factor expression in non-small cell lung carcinomas. Cancer Res. 2005;65:7241–8. doi: 10.1158/0008-5472.CAN-04-4171. [DOI] [PubMed] [Google Scholar]
- 16.Flamme I, Breier G, Risau W. Vascular endothelial growth factor (VEGF) and VEGF receptor 2 (flk-1) are expressed during vasculogenesis and vascular differentiation in the quail embryo. Dev Biol. 1995;169:699–712. doi: 10.1006/dbio.1995.1180. [DOI] [PubMed] [Google Scholar]
- 17.Glinskii OV, Huxley VH, Turk JR, Deutscher SL, Quinn TP, Pienta KJ, Glinsky VV. Continuous Real Time Ex Vivo Epifluorescent Video Microscopy for Studying Cancer Cell Interactions with Dura Mater Microvasculature. Clin Exp Metastasis. 2003;20:451–8. doi: 10.1023/a:1025449031136. [DOI] [PubMed] [Google Scholar]
- 18.Glinskii OV, Turk JR, Pienta KJ, Huxley VH, Glinsky VV. Evidence of Porcine and Human Endothelium Activation by Cancer-Associated Carbohydrates Expressed on Glycoproteins and Tumor Cells. J Physiol (London) 2004;554:89–99. doi: 10.1113/jphysiol.2003.054783. [DOI] [PMC free article] [PubMed] [Google Scholar]


