Abstract
Whether insulin influences microvascular exchange is important in understanding its specific role in insulin resistance and the treatment of diabetes. We investigated whether insulin could induce changes in the microvascular flux of albumin from the mesenteric venules of anesthetized male and female Sprague-Dawley rats (n = 11). After catheterization for monitoring of mean arterial pressure (MAP), a loop of small intestine was exteriorized. The mesentery was draped over a coverslip for observation and suffused continuously with bicarbonate-buffered solution (BBS) (pH 7.4, 37°C). After intravenous injection of Alexa 594™ labeled bovine serum albumin (BSA, 8 mg/kg), fluorescence intensity (If) was recorded on videotape for 30 minutes BBS suffusion and 75 minutes suffusion of BBS plus 0.02 U/ml porcine insulin. Microvascular flux of BSA was measured as a leak index (LI) of If in a 10 × 30 μm window over a postcapillary venule relative to If of the adjoining tissue. Insulin induced a rapid 34% decrease in LI within 5 minutes (p < 0.05) that was sustained for the next 30 minutes. We also observed gender and age differences in the permeability response to insulin, as there was a sustained ∼59% decrease in LI in adult females (n = 5) after 25 minutes, whereas there was an acute, transient (15 min) 45% decrease in LI in juvenile males (n = 6). We conclude that insulin reduces mesenteric venule permeability differently in males and females. Further studies are needed to differentiate the permeability responses with respect to age and gender.
Keywords: Permeability, microcirculation, endothelium, insulin, diabetes
1. Introduction
Insulin resistance is the decreased ability of insulin to promote glucose uptake by its target tissues. In turn, insulin resistance leads to hyperinsulinemia where circulating levels of insulin are elevated. Hyperinsulinemia is associated with endothelial dysfunction (decreased endothelial reactivity) [2,5], and is a precursor to type 2 diabetes [4]. One manifestation of diabetes-related endothelial dysfunction is an alteration in the exchange barrier characterized by a change in microvascular permeability [1]. We investigated whether insulin could alter microvascular permeability to albumin from measures of the flux of dye-labeled albumin from venules in the intact autoperfused rat mesenteric microvasculature.
2. Materials and methods
2.1. Animal preparation
Sprague-Dawley rats (Hilltop Labs, Scottsdale, USA), juvenile males (30–45 days) weighing 256–352 g (n = 6) and adult females (75–120 days) weighing 240–274 g (n = 5) were used for this study. The methods used in this study were performed according to those described previously by Rumbaut et al. [6]. The animals were anesthetized by intraperitoneal injection with 125 mg/kg thiobutabarbital (Inactin®; Sigma, St. Louis, USA). Tracheotomy was performed to facilitate the breathing, and the internal carotid artery and external jugular vein were catheterized for monitoring mean arterial pressure, and for injecting Alexa 594™ (Molecular Probes, Eugene, USA) labeled bovine serum albumin (BSA; Sigma, St. Louis, MO, 8 mg/kg), respectively. The labeling of BSA with Alexa 594™ were performed by methods outlined by Huxley et al. [3]. The animal was placed on a plastic tray and the mesentery was exteriorized and draped over a coverslip for observation. The mesentery was suffused continuously with bicarbonate-buffered solution (BBS; composition: 132 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 20 mM NaHCO3 and 2.0 mM CaCl2 at pH 7.4, 37°C) that was bubbled with a N2/CO2 gas mixture. Unless specified otherwise all chemicals were purchased from Sigma, St. Louis, USA.
2.2. Intravital microscope observation
The mesentery was placed over a Nikon Diaphot 200 inverted microscope (Nikon USA, Melville, USA) and viewed with ×32 objective lens (Leica Microsystems Inc, Bannockburn, USA) and images captured on videotape. A Plunix CA-7CN camera (Plunix America, Sunnyvale, USA) was used to capture bright field image and fluorescence observed using a PTI IC-100 intensified charge-coupled device camera (Photon Technology International, Monmouth Junction, USA) using a filter cube for the red spectra (EX/EM 595/615 nm).
2.3. Assessment of albumin leak
Postcapillary venule of a diameter of 30–40 μm diameter with less than 3 adhering leukocytes over a 100 μm length of the vessel was selected and videotaped throughout the experiment [6]. Alexa 594™ labeled BSA (8 mg/kg) was injected intravenously and fluorescence intensity was recorded on videotape every 10 minutes for 30 minutes of BBS suffusion and 75 minutes suffusion of BBS plus 10−7 M (0.02 U/ml) insulin. The fluorescence intensity was measured from the video screen by playing back the videotape and using NIH Image® video analysis software. Leak index (LI) was calculated as fluorescence intensity in a 10 × 30 μm window over a postcapillary venule (Iv) relative to fluorescence intensity of the adjoining tissue (It): LI = (Iv − background)/(It − background).
2.4. Data analysis
Statistical analysis was performed using one-sample t-test with StatView® software. Results were expressed as mean ± standard error of mean (SEM) and a probability (p) of ⩽0.05 was considered significant.
3. Results
In the total animals (n = 11, except at time point 75 minutes where n = 10), insulin treatment induced a 34% decrease in permeability at 5 minutes which was sustained for 30 minutes (Fig. 1). Within those animals, the sexually immature (juvenile) male group (n = 6, except at time point 75 minutes where n = 5) had an acute, transient 45% decrease in permeability at 15 minutes (Fig. 2). The adult female group (n = 5) had a sustained, >51% depression in permeability in response to insulin starting at 25 minutes (Fig. 3).
Fig. 1.
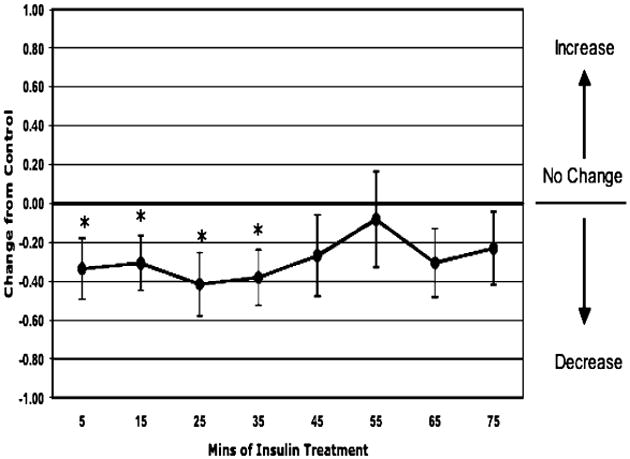
Permeability response of in situ autoperfused rat mesenteric venules to suffusate insulin (0.02 U/ml). The data are given as the difference from control values as a function of time in minutes for a mixed population of 11 rats. Insulin treatment resulted in a significant decrease in venular permeability at 5 minutes, which was sustained for the subsequent 30 minutes (*p < 0.05 vs. control).
Fig. 2.
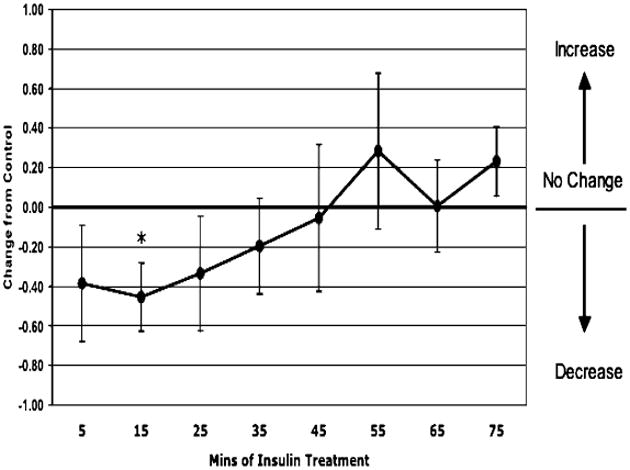
Permeability response of in situ autoperfused juvenile male rat mesenteric venules to suffusate insulin (0.02 U/ml) in 6 rats. Insulin produced an acute decrease in permeability at 15 minutes (*p = 0.05 vs. control).
Fig. 3.
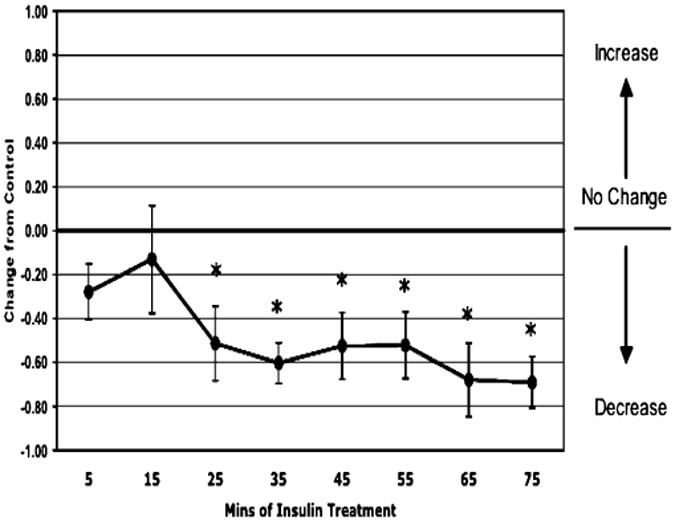
Permeability response of in situ autoperfused adult female rat mesenteric venules to suffusate insulin (0.02 U/ml) in 5 rats. Insulin induced a sustained depression in permeability starting at 25 minutes (*p < 0.05 vs. control).
4. Discussion
Consistent with our hypothesis, suffusion of insulin resulted in a net reduction of venular permeability to albumin. This decrease in microvascular permeability may indicate an overall phenomenon that is occurring in the endothelium in the hyperinsulinemic state. Insulin resistance has been shown to be present for many years prior to the diagnosis of type 2 diabetes [4]. Thus insulin resistance, and subsequently hyperinsulinemia, may cause endothelial dysfunction, altering the endothelial exchange function of the microvessels, creating an environment where insulin is sequestered in the microvessels and less able to reach its target tissues. This may, in turn, create a positive-feedback system, further stimulating the production of insulin by the pancreas and exacerbating the detrimental effect of hyperinsulinemia on the endothelium. Further studies, including the measurement of the capillary permeability of insulin, are required to elucidate the mechanism of insulin's action on the barrier properties of the microvascular wall.
Interestingly, our results also demonstrated that the permeability responses differed with respect to gender and age. The venules of juvenile males had a short lived response whereas those of adult female rats had a much larger response that was sustained during the time of the experiment. Gender differences have been shown in clinical cases of type 2 diabetes [7]. Diabetic women have shown to have greater risks for coronary artery diseases and ketoacidosis than men, while diabetic men have greater number of limb amputations and more cases of retinopathy than women. Clearly, given these results, additional studies are required to further clarify the specific changes in capillary permeability responses among the sexes and age.
Acknowledgments
We thank James Wenzel for technical assistance in performing the experiments and Susan Bingaman for the dye-labeling of BSA. This work was supported by the NIH grant R37-HL-42528 (V.H. Huxley).
Footnotes
Copyright of Clinical Hemorheology & Microcirculation is the property of IOS Press and its content may not be copied or emailed to multiple sites or posted to a listserv without the copyright holder's express written permission. However, users may print, download, or email articles for individual use.
References
- 1.Antonetti D, Barber A, Khin S, Lieth E, Tarbell J, Gardner T Penn State Retina Research Group. Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: vascular endothelial growth factor decreases occludin in retinal endothelial cells. Diabetes. 1998;47:1953–1959. doi: 10.2337/diabetes.47.12.1953. [DOI] [PubMed] [Google Scholar]
- 2.Arcaro G, Cretti A, Balzano S, Lechi A, Muggeo M, Bonora E, Bonadonna R. Insulin causes endothelial dysfunction in humans: sites and mechanisms. Circulation. 2002;105:576–582. doi: 10.1161/hc0502.103333. [DOI] [PubMed] [Google Scholar]
- 3.Bingaman S, Huxley V, Rumbaut R. Fluorescent dyes modify properties of proteins used in microvascular research. Microcirculation. 2003;10:21–231. doi: 10.1038/sj.mn.7800186. [DOI] [PubMed] [Google Scholar]
- 4.Caballero A. Endothelial dysfunction in obesity and insulin resistance: a road to diabetes and heart disease. Obes Res. 2003;11:1278–1289. doi: 10.1038/oby.2003.174. [DOI] [PubMed] [Google Scholar]
- 5.Campia U, Sullivan G, Bryant M, Waclawiw M, Quon M, Panza J. Insulin impairs endothelium-dependent vasodilation independent of insulin sensitivity or lipid profile. Am J Physiol. 2004;286:H76–H82. doi: 10.1152/ajpheart.00539.2003. [DOI] [PubMed] [Google Scholar]
- 6.Rumbaut R, Harris N, Sial A, Huxley V, Granger N. Leakage responses to L-NAME differ with the fluorescent dye used to label albumin. Am J Physiol. 1999;276:H333–H339. doi: 10.1152/ajpheart.1999.276.1.H333. [DOI] [PubMed] [Google Scholar]
- 7.American Diabetes Association. 2002 http://www.diabetes.org/statistics.


