Abstract
Gender influences volume regulation via several mechanisms; whether these include microvascular exchange, especially in the heart, is not known. In response to adenosine (Ado), permeability (Ps)to protein of coronary arterioles of female pigs decreases acutely. Whether Ado induces similar Ps changes in arterioles from males or whether equivalent responses occur in coronary venules of either sex has not been determined. Hypotheses that 1) basal Ps properties and 2) Ps responses to vasoactive stimuli are sex independent were evaluated from measures of Ps to two hydrophilic proteins, α-lactalbumin and porcine serum albumin (PSA), in arterioles and venules isolated from hearts of adult male and female pigs. Consistent with hypothesis 1, basal Ps values of both microvessel types were independent of sex. Contrary to hypothesis 2, Ps responses to Ado varied with sex, protein, and vessel type. Confirming earlier studies, Ado induced a ~20% decrease in Ps to both proteins in coronary arterioles from females. In arterioles from males, Ado did not change Ps for α-lactalbumin (, 3 ± 13%) whereas Ps for PSA () decreased by 27 ± 8% (P < 0.005). In venules from females, Ado elevated by 44 ± 20% (P < 0.05), whereas in those from males, Ado reduced by 24 ± 5% (P < 0.05). The variety of outcomes is consistent with transvascular protein and protein-carried solute flux being regulated by multiple sex-dependent mechanisms in the heart and provides evidence of differences in exchange homeostasis of males and females in health and, likely, disease.
Keywords: albumin, α-lactalbumin, protein flux, porcine, heart, microvascular exchange, arteriole, venule, transvascular flux, male, female
SEX-RELATED DIFFERENCES have been documented extensively with respect to vascular tone and coronary and peripheral vascular blood vessel responsiveness to a variety of stimuli (13, 15, 45, 49). Less is known about the relationship between gender and vascular permeability, especially as it relates to substrate delivery and volume regulation. There are data demonstrating sex-specific differences in vascular volume (17) and volume regulation, specifically following perturbations such as hemorrhage (2, 53), recovery from exercise (44), or changes in position (14, 54), but not fundamental differences in microvascular permeability properties. Sex-specific differences have been shown in several studies of porcine coronary artery and arteriole basal tone and reactivity. In this model, for example, Laughlin et al. (40) assessed vascular reactivity of skeletal muscle conduit arteries and found that basal reactivity depended on both the animal’s sex and anatomic origin of the artery. Miller and colleagues demonstrated sex differences in basal vasomotor tone and responses to a variety of vasoactive agents in addition to sex-related differences in a variety of factors such as platelet products (46), phosphodiesterase activity (3), and K+/Ca2+ channel activity (3).
In response to adenosine (Ado), a cell-permeant breakdown product from ATP metabolism, permeability (Ps) of coronary arterioles isolated from the hearts of sexually mature female pigs decreased from baseline levels (29). In a limited study of arterioles (24) and venules (27) isolated from the hearts of Yucatan miniature swine, the data were consistent with gender- and exercise training-related differences for basal arteriolar permeability for a small protein, α-lactalbumin, but not the for a larger plasma protein, porcine serum albumin (PSA). Similarly, there appeared to be differences in the permeability response to adenosine with the animal’s sex (26, 30). The outcome of these studies thus raised the question of whether it is correct to assume that permeability properties are the same in both adult male and female mammals. Thus the purpose of the present study was to determine whether the sex of an animal influenced 1) the basal barrier properties of coronary microvessels to macromolecules or 2) changes in barrier selectivity after exposure to Ado. Our hypothesis was that both basal Ps and permeability responses (ΔPs) would be independent of sex. To test the hypothesis, paired measures of Ps to α-lactalbumin () and porcine serum albumin () were made in arterioles and venules isolated from the hearts of male and female pigs before and after suffusion with 10−5 M Ado. There were two basic findings: in arterioles, whereas basal Ps was independent of the sex of the animal, the response to Ado differed with both sex and the protein used to probe the barrier; in venules, again, basal was sex independent, whereas the response to Ado was sex dependent and varied from that seen in the arterioles. This mixture of results is consistent with the interpretation that the transvascular flux of protein and solutes carried by proteins (such as free fatty acids on albumin) in coronary microvessels is regulated by several mechanisms, that the contribution of these mechanisms to net flux involves gender, and that the mechanisms defining basal properties differ from those regulating acute permeability responses to a stimulus such as Ado. The implication of the study is that male and female animals regulate volume and exchange homeostasis by different mechanisms that are sensitive, at least in part, to the reproductive hormones. Preliminary results of these studies have been reported previously (24, 26, 30).
MATERIALS AND METHODS
Animals
All animal care and research was conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals under the supervision of the Office of Laboratory Medicine at the University of Missouri, Columbia, MO.
Studies were carried out on 74 sexually mature Yucatan miniature swine [20- to 40-kg females (N = 52) and 23- to 45-kg males (N = 27) aged 13–16 mo]. On the day of experimentation, the animals were sedated with ketamine (25 mg/kg im) and Rompun (2.25 mg/kg im), anesthetized with pentobarbital sodium (20 mg/kg iv), intubated, and then ventilated with room air. After placement of a catheter into an ear vein, heparin was administered (1,000 U/kg), and a left thoracotomy was performed. The heart was excised, and, after the wet weight was determined, it was immersed into cold (4°C) mammalian Krebs solution (32) until the right ventricular wall (5–7 × 2–3 cm) was removed and placed into fresh Krebs solution containing 10 mg/ml PSA (4°C; Sigma, St. Louis, MO) for transport to the laboratory. The pieces of heart were submerged in Krebs-PSA solution and pinned onto a closed cell foam pad with minuten pins (Carolina Biological, Burlington, NC) to maintain the tissue at a constant length.
Arterioles
The epicardial surface of the myocardium was reflected to reveal the arterial branches embedded in connective tissue. A plexus consisting of interconnected arterioles was removed from the surrounding myocytes. In the plexus, arterioles of <100 μm in diameter and ~1,000 μm in length branched from larger vessels (>250 μm in diameter), which, in turn, had originated from the right coronary artery. The arterioles on which permeability measurements were made ranged from 13 to 109 μm in internal diameter (ID), representing A2 through A6 order vessels as defined by Kassab et al. (37), and the majority (90%) were from orders A4 and A5. The arteriolar plexus could contain microvessels that, in situ, spanned the width of the ventricular wall from epicardium to endocardium.
Venules
When both venules and arterioles were harvested from the same tissue segment, the venules were isolated first because of their proximity to the epicardial surface and their distribution into the myocardium at the epicardial surface away from the arterial tree. In the case of the venules, the plexus contained vessels of irregular, noncylindrical shapes as described previously by Kassab et al. (36). In the present studies, the venules ranged in diameter from 34 to 126 μm (ID), representing orders V3 through V5 [Kassab −3 to −5 (36), with 92% from orders V4 and V5], with an average diameter of 73 ± 5 μm.
The excised microvascular plexus was moved from the deep culture dish by using a transfer pipette filled with Krebs-PSA. Minuten pins (0.1 or 0.2 mm; FST or Carolina Biological) were used to secure the plexus to a 3-mm-deep Sylgard pad (Dow Corning, Midland, MI) that was set on an inverted 5-cm-diameter organ culture dish (Falcon 1008) at approximately its in vivo length. One segment of the microvascular plexus was cannulated with a beveled glass theta micropipette (WPI, Sarasota, FL) (32) by using a single-dimension hydraulic microdrive (Frederick Haer, Brunswick, ME) mounted on a Prior micromanipulator (Stoelting, Chicago, IL) with fine adjustment in the z-axis. Microvascular plexus perfusion was established through several branches immediately upon cannulation as evidenced by displacement of red blood cells by the perfusion solution.
The perfused plexus was transilluminated and viewed at ×10 magnification with a fixed-stage inverted microscope (Diavert, Leica, NJ or Olympus IX70) equipped with an adjustable magnifier for placement of the perfusion micropipette. The light path of the microscope was split 50/50 and projected simultaneously to a video system and to an analog microscope photometer (PTI, Brunswick, NJ, or Solamere, Deer Valley, UT). Vessels were imaged using a black and white charge-coupled device camera (Dage-MTI 72; Geni-Sys, Michigan City, IN) fitted with an image intensifier (Dage-MTI) or a low-light camera (PTI) and displayed on two video monitors (projecting a field of view of 0.65 × 0.78 to 1.30 × 1.56 mm; Sony). A pseudocolor picture was generated using NIH Image. Output from the photometer was displayed on a strip chart recorder (Hewlett-Packard, Dallas, TX or Cole Parmer, Vernon Hills, IL) and used in the determination of solute flux.
Solutions
Mammalian Krebs
All perfusion and suffusion solutions were mixed fresh daily. The Krebs base consisted of (in mM) 141.4 NaCl, 4.7 KCl, 2.0 CaCl2·2H2O, 1.2 MgSO4, 1.2 NaH2PO4, 5.0 glucose, 3.0 NaHCO3, and 1.5 Na-HEPES. The pH of the solution was 7.37 and 7.41 ± 0.01 at 4 and 37°C, respectively.
Krebs-PSA
PSA was used as the colloid in all solutions. Before being used in any of the subsequent experiments, the protein was dissolved at a concentration of 60 mg/ml and dialyzed in 12,000 – 14,000 molecular weight (MW) cutoff dialysis tubing (SpectroPor; Spectrum, Houston, TX) against 2 liters of Krebs solution in three sessions over 72 h. The dialysis procedure equilibrated the plasma protein solutions with the Krebs, removed small, water-soluble vasoactive contaminants, and set the pH of the protein solution (28, 29). The protein content of the final dialysate was determined using absorbance spectroscopy and was adjusted so that the perfusate and suffusate solutions contained 20 and 10 mg/ml, respectively.
Labeled test solutes: α-lactalbumin and PSA
Two globular proteins, α-lactalbumin (14,000 MW; Sigma) and PSA (65,000 MW), were used to probe the selectivity characteristics of the arteriolar wall. Depending on the experiment, α-lactalbumin and/or PSA was present as an unlabeled solute (washout) in one-half of the theta pipette and as a dye-labeled solute [fluorescently tagged with tetramethyl rhodamine isothiocyanate (TRITC) isomer L (Calbiochem, La Jolla, CA or Molecular Probes, Eugene, OR) or 2′,4,5,6,7,7′-hexafluorfluorescein (Oregon Green 514; Molecular Probes)] in the second half of the theta pipette (29). Concentrated stock solutions of the dye-labeled proteins were stored frozen in small quantities until the day of the experiment, when a defrosted vial was then diluted with fresh PSA and filtered through 0.45-μm cellulose filter cartridges (μStar LB; CoStar, Pleasanton, CA) before use. The final protein content of the probe solutions was 23.5 (0.56 mM) and 20 mg/ml for α-lactalbumin and PSA, respectively. Because the TRITC-PSA concentration was 0.17 mM, the total PSA concentration was 0.33 mM. The net oncotic pressure of these protein solutions ranged between 6 and 8 cmH2O.
Adenosine
Ado was dissolved in Krebs (10−3 M stock) and prepared in a 1:100 dilution into PSA. The final concentration was 10−5 M Ado in 10 mg/ml protein.
Measurement of Microvessel Protein Flux
The methods for assessing Ps to proteins in isolated microvessels and its limitations are described elsewhere (27, 29, 32). Briefly, to control the choice of solution and the hydrostatic pressure in the vessel segment, the microvessels were perfused with either washout or dye solution using a system of manometers connected by hydraulic switches. By switching between the two pairs of manometers, the perfusate in the vessel could contain just nonfluorescent solution, could be changed rapidly to the dye solution for the time necessary to measure solute flux, or could be changed back to the clear washout to reestablish the baseline within seconds. A relatively low perfusion pressure (15.8 ± 0.5 cmH2O) was used in these experiments to minimize the contribution of convective coupling, or solvent drag, to the estimates of apparent permeability (29). The perfused plexus was transilluminated and viewed at ×10 or ×20 magnification (Leitz UM 10, numerical aperture 0.32, or Olympus) with a fixed-stage inverted microscope (Diavert; Leica) equipped with an adjustable magnifier (up to ×2, providing up to ×20 real magnification). The light path of the microscope was split 50/50 and projected simultaneously to a video system and to an analog microscope photometer (Leitz or PTI). Light intensity at the focal plane of the vessel in this system ranged between 5 and 11 mW/cm2 when the N2 filter cube (Leitz) for the red fluorescent dye (TRITC) or the H2 filter cube (Leitz) for the green fluorescent dye (Oregon Green 514) was positioned in the light path. At these light intensities, fading of the fluorescent signal and/or light-induced changes in function are either absent or minimized (50, 51).
When the dye solution perfused the microvessel, fluorescence intensity (If) emanated from a section prescribed by a rectangular window (≥3 vessel diameters wide) located in the light path between the vessel and the photometer that defined the area over which apparent solute permeability (Ps) was measured. Solute flux per unit surface area and constant concentration gradient (Js/SΔC, cm/s) was determined from the relation
| (1) |
where ΔI0 is the step change of fluorescence when the dye replaces the nonfluorescent washout solution in the vessel lumen, (dIf/dt)i is the initial change in fluorescence intensity as solute moves across the vessel wall, and d is the microvessel internal diameter (μm). The vessels were assumed to be circular in cross section and to have a volume-to-surface area ratio of 0.25d. All experimental protocols were performed at 15°C to minimize changes in d (39).
Experimental Protocols
Flux was determined twice in all vessels. First, Ps to α-lactalbumin (in arterioles) or PSA (in arterioles and venules), two proteins of similar charge and shape but of dissimilar size, was assessed during suffusion with mammalian Krebs and protein to determine whether properties of the microvessel barrier differed with sex. Second, the microvessels were then exposed to 10−5 M Ado in Krebs-PSA in the suffusion to test whether a metabolic vasodilator known to alter microvascular permeability influenced movement of the protein probes across the wall. Measures of flux commenced after a minimum of 5 min of suffusion with Ado and continued for up to 90 min, depending on the rate of dye flux from the microvessels (e.g., “high”-permeability vessels were exposed to basal and Ado perfusions for shorter times than “low”-permeability vessels). Diameter was assessed under both conditions.
Statistical Analysis
Data were derived over a 7-yr period from 5 groups of 8 –16 cage-sedentary males and 10 groups of 8 –16 cage-sedentary females that were part of a program project grant (NIH P01-HL-52490). Only data from arterioles and venules in which measures of both basal permeability and permeability after exposure to Ado were obtained have been analyzed and reported in this study. A minimum average five measures of Js/SΔC at a single pressure was used to represent apparent Ps for an individual vessel. Unlike in previous studies (25, 29, 34), in this study with the large number of vessels (n = 161), we found that only the data for the from female pigs were skewed (e.g., not distributed normally using the Kolmogorov-Smirnov test for normality, P < 0.05); therefore, for clarity, means ± SE are reported, and in the case of α-lactalbumin, medians ± median absolute deviation (MAD) are reported in parentheses for the females and combined sexes (StatView 5.0; SAS Institute, Cary, NC). Because of the nonparametric distribution of control values of α-lactalbumin, both the Games-Howell (nonparametric) and Tukey-Kramer honestly significant difference (parametric) (10, 11, 16) post hoc tests were used to test for differences in value of Ps with sex and solute by factorial ANOVA. The response to Ado was calculated as the ratio of the permeability under test conditions relative to that under control conditions. In all cases, the responses were normally distributed; thus comparisons were made by factorial ANOVA. Multivariate ANOVA confirmed the analyses for basal Ps and the response of Ps to Ado with vessel type and sex. A significant change in diameter or permeability from basal levels was determined using paired Student’s t-test or the Wilcoxon signed rank test, as appropriate. A power analysis (10, 11, 48) indicated that a minimum of seven animals per group was required per solute to minimize both type I and type II errors. A significance level of P < 0.05 was set before the power analysis was calculated and before the experiments were performed.
RESULTS
Influence of Sex on Basal Arteriole Permeability to Proteins
Solute permeability was measured under basal conditions on 137 coronary arterioles measuring 43.4 ± 1.8 μm in diameter (mean ± SE; range 13–126 μm). When Ps values are examined without regard to sex, basal is 12.1 ± 1.4 × 10−7 cm/s (mean ± SE; median 7.1 × 10−7 cm/s ± MADof3.6 × 10−7 cm/s; n = 76) and basal is 6.3 ± 0.6 × 10−7 cm/s (mean ± SE; n = 61). was significantly greater than (P < 0.01). No differences are observed for either basal or when the data are split by sex (Fig. 1); of arterioles remains significantly greater than from female and male pigs, respectively (P < 0.05 in each case).
Fig. 1.
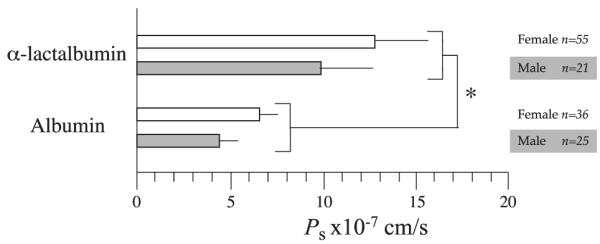
Basal coronary arteriole permeability (Ps)to α-lactalbumin and to porcine serum albumin (PSA) is shown for microvessels isolated from hearts of female and male pigs. Data are means ± SE. There is no statistical difference in Ps to either solute with sex (P = 0.36 and 0.40 for α-lactalbumin and PSA, respectively). Whether the data are combined or split by sex, permeability to α-lactalbumin is greater than that to PSA (*P < 0.05).
Influence of Sex on Basal Venule Permeability to Proteins
was assessed under basal conditions on 24 coronary venules measuring 71 ± 4.9 μm in diameter (mean ± SE; range 34 –104 μm). When permeability values are examined without regard to sex, basal is 22.2 ± 3.5 × 10−7 cm/s. No differences are observed for basal when the data are split by sex (P = 0.69). Basal of venules was significantly greater than of arterioles (P < 0.001, Fig. 2).
Fig. 2.
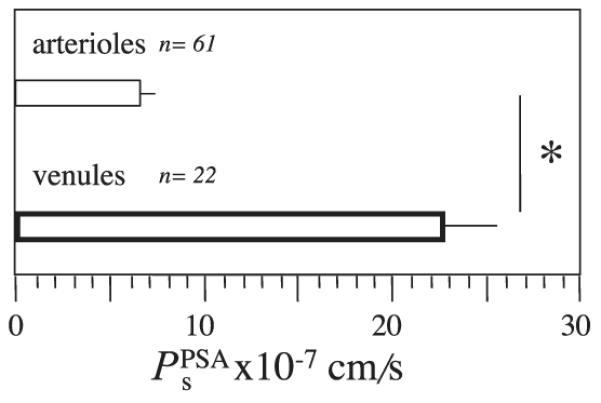
Under basal conditions, a gradient in permeability exists between arterioles and venules isolated from male and female pig hearts. Mean Ps to PSA () is shown for arterioles and venules isolated from hearts of 52 female and 27 male pigs. Data are means ± SE and are grouped because there are no differences in Ps in either vessel type with sex. *P < 0.05.
Influence of Sex on Arteriole Permeability Response to Adenosine
After 5 min of suffusion of the arteriolar plexus with 10−5 M Ado, independently of sex, diameter increased by 9 ± 4% (P < 0.001). When neither sex nor solute is considered, the data show that suffusion of arterioles with Ado resulted in a significant 15 ± 5% decrease in permeability from basal levels. The overall response (a decrease in Ps) is maintained whether the data are split by protein probe (−13 ± 5% and −22 ± 4% for α-lactalbumin and PSA, respectively) or by sex (−15 ± 7% and −13 ± 4% for males and females, respectively; Fig. 3).
Fig. 3.
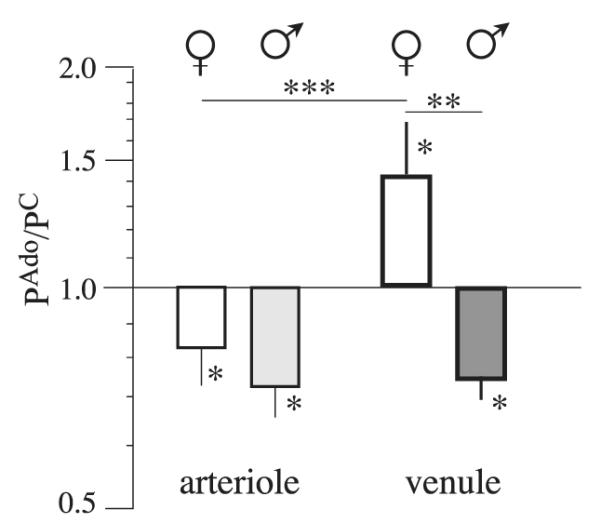
Sex- and microvessel-sensitive changes in Ps to PSA in response to adenosine (Ado, 10−5 M). The paired mean permeability response (, where C is control) to a minimum of 5 min of suffusion with Ado is shown for PSA in arterioles (left) and venules (right) isolated from hearts of female (open bars) and male (shaded bars) pigs. Data are means ± SE. *P < 0.05, significant changes in permeability from basal levels. Significant differences also exist in direction and magnitude of responses with sex (**P < 0.01) and vessel type in females (***P < 0.01).
As shown in Fig. 4, when the data are split by sex and solute, it becomes apparent that, except in the case of males when the solute is α-lactalbumin, Ado reduced Ps of arterioles from basal levels. In arterioles isolated from the hearts of male pigs, did not change on exposure to Ado (3 ± 13%, n = 21, P = 0.84 for males), whereas there was a significant decrease in in arterioles from female pig hearts (−20 ± 5%, n = 54, P < 0.001). Despite the lack of response to Ado with respect to , there was a robust decrease in PPSAs in arterioles from the same population of male pigs (−27 ± 8%, n = 24, P < 0.001 for males) that was somewhat greater in the magnitude of the response than that of arterioles from female pigs (−18 ± 4%, n = 35, P = 0.03 for females). The result is that only arterioles from male pigs in the presence of Ado demonstrated a greater selectivity for macromolecules than that predicted by simple molecular diffusion (Fig. 4).
Fig. 4.
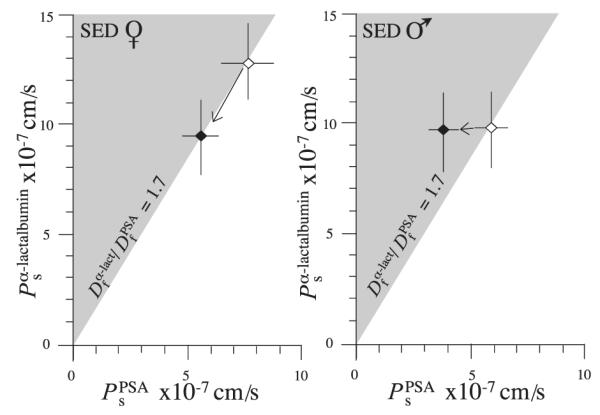
Comparison of distribution of coronary arteriolar permeabilities to PSA and α-lactalbumin before (◇) and after (◆) exposure to Ado. Data are means ± SE for arterioles isolated from female (A) and male (B) hearts, respectively. On the abscissa is Ps for the larger protein, PSA (), and on the ordinate is Ps for the smaller protein, α-lactalbumin . A solid line is drawn with a slope of 1.7 indicating where the data would lie if the solutes were to cross the arteriolar wall by free diffusion (Df, free diffusion coefficient). The shaded area is where the data would be expected to fall if the arteriolar barrier contained structures that further restricted the passage of large relative to small macromolecules (ultrafiltration). SED, sedentary animals.
Influence of Sex on Venule Permeability Response to Adenosine
The diameter of the venules from male and female pigs did not change with 10−5 M Ado (2 ± 2%). When neither sex nor solute is considered, the data show that suffusion with Ado resulted in no change in Ps from basal levels (15 ± 15%). As shown in Fig. 3, however, when the data are split by sex, they show that exposure to Ado resulted in a significant increase in venules from female pig hearts (41 ± 20% n = 15, P <0.05) and a significant decrease in venules isolated from male hearts (−21 ± 6%, n = 13, P <0.01). Neither venule diameter or Strahler classification influenced the basal venule permeability or venule permeability responses to Ado for either sex (P = 0.32 and 0.83 for males and females for the slope of the relationship between diameter and , and P = 0.49; P = 0.61 and 0.68 when comparing basal permeability for V4 vs. V5 in male and females; P = 0.30 and 0.82 when comparing permeability responses for males and females, respectively). The difference in the direction of the responses differs significantly with the sex of the animal (P = 0.02). There is no significant difference in the magnitude of the decrease in Ps between arterioles and venules isolated from male hearts, whereas the increase in of venules on exposure to Ado differs between arterioles and venules isolated from the female pigs (P = 0.05).
DISCUSSION
The data in the present study represent the test of two hypotheses: first, that the sex of intact, sexually mature animals is without influence on the basal barrier properties of coronary arterioles and venules to macromolecules, and second, that the acute change in barrier properties of these microvessels in response to Ado is also independent of sex.
The most significant findings of this study are that while the sex of the animal is without influence on the basal permeability of coronary arterioles to macromolecules, the permeability responses of coronary arterioles to Ado are functions of both the sex of the animal and the protein chosen to probe the barrier properties of the microvessel wall. Furthermore, in females, but not in males, the responses of the arteriolar vessels differ from those of venules. These outcomes are important because they illustrate not only the nonuniformity of microvascular barrier within a single organ to macromolecules and that the flux of large solutes under stimulated conditions is governed by multiple mechanisms but also that exchange homeostasis is achieved differently by sexually mature females and males.
Pathways for Transvascular Macromolecule Exchange
Occam’s razor, which states that assumptions introduced to explain a process must not be multiplied beyond necessity, has prevailed in the formulation of models of microvascular exchange. Consequently, it has long been held that common pathways conduct probes with similar properties (in this case, two monomeric, water-soluble proteins of similar charge but different masses) across the walls of exchange vessels. Furthermore, it is assumed that these pathways do not differ among vertebrates or between the sexes of these species. Consequently, a common set of cellular and molecular mechanisms are thought to govern transvascular movement of solutes within specific organs under most physiological conditions.
The outcomes of the present study fail to fit any of the existing models of microvascular permeability. In the “simplest case,” water-soluble solutes, including proteins, traverse the microvascular barrier via water-filled pathways. These pathways could exist between the cells constituting the microvascular barrier (e.g., the junctional regions), and/or they could be pathways through the cell [e.g., “pores” or fused vesicle chains or vesiculovacuolar organelles (see Ref. 43 for a recent review)]. In this scenario, protein passage through these pathways would be characterized by “passive” free diffusive transport. The predicted behavior of two solutes traversing a barrier of thickness Δx would be that the ratio of the permeabilities equals the ratio of the free diffusion coefficients (Df):
| (2) |
or
In the frog mesenteric model, the ratio was on the order of 8 to 10 (31), the behavior expected if there were structures that further restricted the passage of larger substances, such as albumin (31, 32). In endothelial cell monolayers, with the use of dextrans of molecular weights similar to that of α-lactalbumin and albumin, the ratio of permeabilities was close to the ratio of the free diffusion coefficients, the behavior expected for barriers that offer no ultrafiltration (43). Either selective restriction of the smaller protein, α-lactalbumin, or flux augmentation of the larger protein, albumin, would result in having values less than the ratio of the free diffusion coefficients. In the present study, without regard to sex, of arterioles was 12.1 × 10−7 cm/s ÷ 6.3 × 10−7 cm/s = 1.92. This value, close to the ratio of the free diffusion coefficients, is a result similar to that found in the endothelial monolayer models. In fact, when the data are examined by sex, the ratios follow even more closely the behavior expected from a passive diffusive mechanism with no ultrafiltration:
A graphical representation of these data is given in Fig. 4. Of interest, the absolute values of are from 2 to 10 times lower than those reported in cultured cell models with continuous endothelium (8, 9, 12, 18, 61).
Addition of Ado failed to change the relationship while decreasing permeability to both solutes (Fig. 4) in the case of the coronary arterioles from the female pigs. Of interest, the difference in permeability responses with sex is illustrated by the arterioles from the male pigs, in which suffusion with Ado resulted in a marked increase in to 2.67, a value more consistent with, yet still different from, the behavior of exchange microvessels of the frog mesentery.
It could be argued that the ratios of the permeabilities are artifactual because, perhaps, the fluorescent probes on the proteins may not remain bound during the time of measurement or because they reflect charge differences between TRITC-PSA and Oregon Green 514-α-lactalbumin. With regard to the first assertion, while it is possible that the dyes do not remain covalently bound to the proteins, our experience has been that less than 1% of the total fluorescent signal can be detected after extensive centrifugation in low-molecular-mass cutoff filters (<5 kDa for α-lactalbumin and <30 kDa for PSA), and the measured permeability to free dyes is an order of magnitude higher than that measured to the dye-protein construct (unpublished data). Furthermore, this argument does not account for the results shown in Fig. 3, where increases in venules from female hearts while it decreases in venules isolated from male hearts and/or in Fig. 4, where the ratios are sensitive to both the sex of the animal and the exposure to Ado. Finally, there is no evidence of retention of either of the dyes by the microvessels, a behavior consistent with the presence of unconjugated dyes (4). With respect to the notion that perhaps a large charge difference exists between Oregon Green 514-α-lactalbumin and TRITC-PSA, no such behavior has been recorded with the use of native gel electrophoresis (4), and we did not appear to get different values of Ps when the dyes on the proteins were switched (unpublished data). Finally, as referenced above, in a different in vivo animal model, the frog mesentery, we and others have observed permeability ratios well in excess of the ratio of the free diffusion coefficients (6, 7, 19, 20, 29).
The data of the present study, both under basal conditions and after Ado suffusion, illustrate clearly that a model of the barrier to macromolecules being regulated by purely diffusive mechanisms is too limited and will not account for the observed behavior in either this study or previous studies of coronary arteriolar or venular permeability (24, 25, 27, 29, 30, 32, 34). Furthermore, the acute permeability responses to Ado provide evidence that it is inappropriate to consider the barrier as a static entity under normal physiological conditions. The mechanisms responsible for the transvascular movement of α-lactalbumin and albumin appear to differ with sex, resulting in males shifting barrier properties from one that favors the flux of albumin in the absence of Ado to one that does not (Fig. 4). In contrast, the selectivity of arterioles from female hearts is not changed by the presence of Ado in the face of changing barrier properties (Figs. 3 and 4). A fundamental inference from the present study is that although volume status and solute distributions are obviously regulated to maintain balance, the selection of mechanisms by which adult males and females achieve homeostasis differs.
Ado-Sensitive Mechanisms
The Ado receptor subtype(s) and the mechanisms linking Ado receptor activation to changes in intact microvascular permeability have yet to be determined definitively. Some data exist with regard to the receptor subtypes present in porcine coronary arterioles. Pharmacological, functional, and molecular data are consistent with the absence of Ado A1 and the presence of message and/or Ado A2A receptors in juvenile porcine coronary arterioles from both sexes (50 –100 μm; 21). We also found message for A1 receptors and the presence of message for A2A and A2B receptors in arterioles from a mixed sex subpopulation of the adult Yucatan miniature swine used for the present study (55). Unfortunately, the microvessel samples from males and females were pooled so that it was not possible to determine whether sex differences exist. In that study, the functional responses in arterioles and venules also were pooled, resulting in an overall decrease in arteriolar Ps and no change in venular Ps on exposure to 10−5 M Ado. Pooling the data in the present study yields identical results, thereby masking the sex difference in permeability function in the venules. While it is not yet known whether message and/or receptor distribution differs with sex, it can be postulated that in coronary arterioles from males, where only the flux of PSA was reduced during Ado perfusion, Ado A2 receptor-linked mechanisms mediate albumin flux. It is interesting to note that in the study by Hein et al. (21) on porcine coronary arteriole dilation, whereas Ado A2A receptor activation was involved in the relaxation, it appeared to be independent of cAMP signaling pathways.
Furthermore, in the same study in which we found message for A2A and A2B in arterioles, we found message for A3 receptors. Furthermore, in venules pooled from both sexes, the same mRNA profile was observed. Of interest, although message for A3 was present in arterioles and venules, we could not detect expression of the receptor protein. Offhand, these observations fail to provide the basis for an explanation for the differences in Ado-based permeability responses of arterioles and venules.
How the cyclic nucleotides are involved ultimately in the regulation of permeability remains to be determined, because the existing data are contradictory. Activation of cAMP results in a reduction in volume flux in intact, perfused frog mesenteric microvessels (1, 20, 21, 47) and a decrease in solute flux in isolated perfused porcine coronary arterioles (33). Conversely, direct and indirect activation of the cGMP signaling pathways in intact isolated (57–60) and in situ microvessels elicits loss of barrier function to solutes and volume (27, 28, 42, 50–52). Consistent with in vivo data, direct activation of cAMP-dependent pathways in some endothelial culture systems results in an “enhancement of barrier function” (5, 38, 41), whereas in many cases in which endothelial monolayers were used, contradictory results were observed (22, 23, 35). Furthermore, the case of the permeability response to Ado differs with the origin of the cells. In monolayers of rat coronary microvessel endothelium, stimulation with Ado resulted in an increase in permeability to FITC-labeled albumin, whereas in monolayers of endothelium derived from porcine aorta, Ado reduced permeability (56). The difficulty with formulating a comprehensive picture of the mechanisms involved in the regulation of vascular permeability is illustrated by the comprehensive study of Ikeda et al. (35). In that study, a variety of responses to histamine were observed that changed with tissue origin and species when permeability to FITC-Dextran 70, intracellular calcium, and F-actin content were measured in endothelial monolayers from aorta and vein of cows and humans. Obviously, the regulation of the barrier function of endothelium and intact blood vessels involves multiple mechanisms, requiring further study.
Implications Concerning Exchange Function in Males and Females
Although further work with the use of both in vivo and in vitro models is required to fully understand the mechanisms regulating the barrier separating circulating blood from the working myocytes of the heart, the results of this study demonstrate several important features. In coronary arterioles, the finding that Ado decreases permeability to both solutes similarly in arterioles from females and not in arterioles from males demonstrates, first, that the mechanisms regulating basal permeability differ from those that determine acute changes in exchange; second, that the acute regulatory mechanisms differ with sex; third, that albumin and a similarly shaped but smaller protein, α-lactalbumin, do not traverse the barrier by the same route; and fourth, that sex influences the route used by α-lactalbumin to cross the wall of intact coronary arterioles differently from that used by albumin. In coronary venules, although the basal permeability to PSA was indeed greater than that of arterioles, Ado induced opposite permeability responses in females and males. One physiological outcome is that during Ado stimulation, the permeability gradient between arterioles and venules is enhanced in females relative to that of the males, favoring increased albumin flux (and albumin-associated substrates such as free fatty acids, drugs, hormones) independent of Ado’s effects on vascular tone and blood flow to metabolizing tissue.
In the aggregate, with respect to volume balance and solute exchange, the net consequence of these differences may only slightly influence the distribution of water, proteins, and free fatty acids under basal conditions but may substantially influence their relative distributions under conditions in which Ado is formed, as occurs during conditions that increase myocardial metabolism, such as exercise, inflammation, or stress.
ACKNOWLEDGMENTS
Dr. Li Ping Ji, Susan Bingaman, Steve Sieveking, Pam Thorne, Chris Samples Aaker, Tammy Strawn, and Denise Stowers contributed extensive technical assistance in all aspects of these studies, and Dr. Donna A. Williams contributed expertise in the conduct of many of the experiments reported in this article. We thank Drs. Rolando E. Rumbaut, Leona J. Rubin, and Allan W. Jones for extended, on-going discussions on the content.
GRANTS This work was supported by National Heart, Lung, and Blood Institute Grants P01-HL-52490, R37-HL-42528, R01-HL-34872, R01-HL-36088, and T32-HL-07094 and by a National Aeronautics and Space Administration-Missouri University partnership for Understanding Sex Differences in Physiology.
REFERENCES
- 1.Adamson RH, Liu B, Fry GN, Rubin LL, Curry FE. Microvascular permeability and number of tight junctions are modulated by cAMP. Am J Physiol Heart Circ Physiol. 1998;274:H1885–H1894. doi: 10.1152/ajpheart.1998.274.6.H1885. [DOI] [PubMed] [Google Scholar]
- 2.Ba ZF, Kuebler JF, Rue IIILW, Bland KI, Wang P, Chaudry IH. Gender dimorphic tissue perfusion response after acute hemorrhage and resuscitation: role of vascular endothelial cell function. Am J Physiol Heart Circ Physiol. 2003;284:H2162–H2169. doi: 10.1152/ajpheart.00724.2002. [DOI] [PubMed] [Google Scholar]
- 3.Barber DA, Burnett JC, Jr, Fitzpatrick LA, Sieck GC, Miller VM. Gender and relaxation to C-type natriuretic peptide in porcine coronary arteries. J Cardiovasc Pharmacol. 1998;32:5–11. doi: 10.1097/00005344-199807000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Bingaman S, Huxley VH, Rumbaut RE. Fluorescent dyes modify properties of proteins used in microvascular research. Microcirculation. 2003;10:221–231. doi: 10.1038/sj.mn.7800186. [DOI] [PubMed] [Google Scholar]
- 5.Bottaro D, Shepro D, Peterson S, Hechtman HB. Serotonin, histamine, and norepinephrine mediation of endothelial and vascular smooth muscle cell movement. Am J Physiol Cell Physiol. 1985;248:C252–C257. doi: 10.1152/ajpcell.1985.248.3.C252. [DOI] [PubMed] [Google Scholar]
- 6.Curry FE. Permeability coefficients of the capillary wall to low molecular weight hydrophilic solutes measured in single perfused capillaries of frog mesentery. Microvasc Res. 1979;17:290–308. doi: 10.1016/s0026-2862(79)80005-9. [DOI] [PubMed] [Google Scholar]
- 7.Curry FE, Huxley VH, Adamson RH. Permeability of single capillaries to intermediate-sized colored solutes. Am J Physiol Heart Circ Physiol. 1983;245:H495–H505. doi: 10.1152/ajpheart.1983.245.3.H495. [DOI] [PubMed] [Google Scholar]
- 8.DeMaio L, Tarbell JM, Scaduto RC, Jr, Gardner TW, Antonetti DA. A transmural pressure gradient induces mechanical and biological adaptive responses in endothelial cells. Am J Physiol Heart Circ Physiol. 2004;286:H731–H741. doi: 10.1152/ajpheart.00427.2003. [DOI] [PubMed] [Google Scholar]
- 9.Dull RO, Jo H, Sill H, Hollis TM, Tarbell JM. The effect of varying albumin concentration and hydrostatic pressure on hydraulic conductivity and albumin permeability of cultured endothelial monolayers. Microvasc Res. 1991;41:390–407. doi: 10.1016/0026-2862(91)90037-c. [DOI] [PubMed] [Google Scholar]
- 10.Dunnet CW. Pairwise multiple comparisons in the homogeneous variance, unequal sample size case. J Am Stat Assoc. 1980;75:789–795. [Google Scholar]
- 11.Dunnet CW. Pairwise multiple comparisons in the unequal variance case. J Am Stat Assoc. 1980;75:796–800. [Google Scholar]
- 12.Ehringer WD, Edwards MJ, Miller FN. Mechanisms of α-thrombin, histamine, and bradykinin induced endothelial permeability. J Cell Physiol. 1996;167:562–569. doi: 10.1002/(SICI)1097-4652(199606)167:3<562::AID-JCP20>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 13.English KM, Jones RD, Jones TH, Morice AH, Channer KS. Gender differences in the vasomotor effects of different steroid hormones in rat pulmonary and coronary arteries. Horm Metab Res. 2001;33:645–652. doi: 10.1055/s-2001-18689. [DOI] [PubMed] [Google Scholar]
- 14.Fu Q, Arbab-Zadeh A, Perhohen MA, Zhang R, Zuckerman JH, Levine BD. Hemodynamics of orthostatic intolerance: implications for gender differences. Am J Physiol Heart Circ Physiol. 2004;286:H449–H457. doi: 10.1152/ajpheart.00735.2002. [DOI] [PubMed] [Google Scholar]
- 15.Fulton CT, Stallone JN. Sexual dimorphism in prostanoid-potentiated vascular contraction: roles of endothelium and ovarian steroids. Am J Physiol Heart Circ Physiol. 2002;283:H2062–H2073. doi: 10.1152/ajpheart.00099.2002. [DOI] [PubMed] [Google Scholar]
- 16.Games PA, Howell JF. Pairwise multiple comparison procedures with unequal n’s and/or variances: a Monte Carlo study. J Educ Stat. 1976;1:113–125. [Google Scholar]
- 17.Green HJ, Carter S, Grant S, Tupling R, Coates G, Ali M. Vascular volumes and hematology in male and female runners and cyclists. Eur J Appl Physiol Occup Physiol. 1999;79:244–250. doi: 10.1007/s004210050502. [DOI] [PubMed] [Google Scholar]
- 18.Gunduz D, Hirche F, Hartel FV, Rodewald CW, Schafer M, Pfitzer G, Piper HM, Noll T. ATP antagonism of thrombin-induced endothelial barrier permeability. Cardiovasc Res. 2003;59:470–478. doi: 10.1016/s0008-6363(03)00427-9. [DOI] [PubMed] [Google Scholar]
- 19.He P, Pagakis SN, Curry FE. Measurement of cytoplasmic calcium in single microvessels with increased permeability. Am J Physiol Heart Circ Physiol. 1990;258:H1366–H1374. doi: 10.1152/ajpheart.1990.258.5.H1366. [DOI] [PubMed] [Google Scholar]
- 20.He P, Zeng M, Curry FE. Dominant role of cAMP in regulation of microvessel permeability. Am J Physiol Heart Circ Physiol. 2000;278:H1124–H1133. doi: 10.1152/ajpheart.2000.278.4.H1124. [DOI] [PubMed] [Google Scholar]
- 21.Hein TW, Wang W, Zoghi B, Muthuchamy M, Kuo L. Functional and molecular characterization of receptor subtypes mediating coronary microvascular dilation to adenosine. J Mol Cell Cardiol. 2001;33:271–282. doi: 10.1006/jmcc.2000.1298. [DOI] [PubMed] [Google Scholar]
- 22.Hempel A, Noll T, Muhs A, Piper HM. Functional antagonism between cAMP and cGMP on permeability of coronary endothelial monolayers. Am J Physiol Heart Circ Physiol. 1996;270:H1264–H1271. doi: 10.1152/ajpheart.1996.270.4.H1264. [DOI] [PubMed] [Google Scholar]
- 23.Holschermann H, Noll T, Hempel A, Piper HM. Dual role of cGMP in modulation of macromolecule permeability of aortic endothelial cells. Am J Physiol Heart Circ Physiol. 1997;272:H91–H98. doi: 10.1152/ajpheart.1997.272.1.H91. [DOI] [PubMed] [Google Scholar]
- 24.Huxley VH. Differences in basal arteriolar permeability to protein in arterioles isolated from the hearts of male versus female pigs. Third Asian Congress for Microcirculation; Bangkok, Thailand. Bologna, Italy: Monduzzi Editore; 1997. p. 23. [Google Scholar]
- 25.Huxley VH. Evidence for augmented albumin transport in coronary and skeletal muscle arterioles (Abstract) FASEB J. 2001;15:A45. [Google Scholar]
- 26.Huxley VH. Influences of gender and training on coronary vascular permeability (Ps) to proteins. XXXIV IUPS Congress Christ Church; New Zealand. 2001. [Google Scholar]
- 27.Huxley VH. What do measures of flux tell us about vascular wall biology? Microcirculation. 1998;5:109–116. [PubMed] [Google Scholar]
- 28.Huxley VH, Rumbaut RE. The microvasculature as a dynamic regulator of volume and solute exchange. Clin Exp Pharmacol Physiol. 2000;27:847–854. doi: 10.1046/j.1440-1681.2000.03344.x. [DOI] [PubMed] [Google Scholar]
- 29.Huxley VH, Williams DA. Basal and adenosine-mediated protein flux from isolated coronary arterioles. Am J Physiol Heart Circ Physiol. 1996;271:H1099–H1108. doi: 10.1152/ajpheart.1996.271.3.H1099. [DOI] [PubMed] [Google Scholar]
- 30.Huxley VH, Williams DA. Coronary microvessel permeability to proteins of different size: influences of exercise training, gender and adenosine. XXXIII IUPS Congress St Petersburg; Russia. 1997. [Google Scholar]
- 31.Huxley VH, Williams DA. Microcirculation—Flow and Transport. In: Callow AD, Ernst CB, editors. Vascular Surgery: Theory and Practice. Appleton & Lange; Stamford, CA: 1995. pp. 49–78. [Google Scholar]
- 32.Huxley VH, Williams DA. Role of a glycocalyx on coronary arteriole permeability to proteins: evidence from enzyme treatments. Am J Physiol Heart Circ Physiol. 2000;278:H1177–H1185. doi: 10.1152/ajpheart.2000.278.4.H1177. [DOI] [PubMed] [Google Scholar]
- 33.Huxley VH, Williams DA, Ji LP. Reduction of α-lactalbumin flux from porcine coronary arterioles by isoproterenol (Abstract) FASEB J. 1999;13:A4. [Google Scholar]
- 34.Huxley VH, Williams DA, Meyer DJ, Jr, Laughlin MH. Altered basal and adenosine-mediated protein flux from coronary arterioles isolated from exercise-trained pigs. Acta Physiol Scand. 1997;160:315–325. doi: 10.1046/j.1365-201X.1997.00171.x. [DOI] [PubMed] [Google Scholar]
- 35.Ikeda K, Utoguchi N, Makimoto H, Mizuguchi H, Nakagawa S, Mayumi T. Different reactions of aortic and venular endothelial cell monolayers to histamine on macromolecular permeability: role of cAMP, cytosolic Ca2+ and F-actin. Inflammation. 1999;23:87–97. doi: 10.1023/a:1020295718728. [DOI] [PubMed] [Google Scholar]
- 36.Kassab GS, Lin DH, Fung YC. Morphometry of pig coronary venous system. Am J Physiol Heart Circ Physiol. 1994;267:H2100–H2113. doi: 10.1152/ajpheart.1994.267.6.H2100. [DOI] [PubMed] [Google Scholar]
- 37.Kassab GS, Rider CA, Tang NJ, Fung YC. Morphometry of pig coronary arterial trees. Am J Physiol Heart Circ Physiol. 1993;265:H350–H365. doi: 10.1152/ajpheart.1993.265.1.H350. [DOI] [PubMed] [Google Scholar]
- 38.Kelley C, D’Amore P, Hechtman HB, Shepro D. Vasoactive hormones and cAMP affect pericyte contraction and stress fibres in vitro. J Muscle Res Cell Motil. 1988;9:184–194. doi: 10.1007/BF01773740. [DOI] [PubMed] [Google Scholar]
- 39.Kimura M, Dietrich HH, Huxley VH, Reichner DR, Dacey RG., Jr Measurement of hydraulic conductivity in isolated arterioles of rat brain cortex. Am J Physiol Heart Circ Physiol. 1993;264:H1788–H1797. doi: 10.1152/ajpheart.1993.264.6.H1788. [DOI] [PubMed] [Google Scholar]
- 40.Laughlin MH, Schrage WG, McAllister RM, Garverick HA, Jones AW. Interaction of gender and exercise training: vasomotor reactivity of porcine skeletal muscle arteries. J Appl Physiol. 2001;90:216–227. doi: 10.1152/jappl.2001.90.1.216. [DOI] [PubMed] [Google Scholar]
- 41.Lum H, Jaffe HA, Schulz IT, Masood A, RayChaudhury A, Green RD. Expression of PKA inhibitor (PKI) gene abolishes cAMP-mediated protection to endothelial barrier dysfunction. Am J Physiol Cell Physiol. 1999;277:C580–C588. doi: 10.1152/ajpcell.1999.277.3.C580. [DOI] [PubMed] [Google Scholar]
- 42.Meyer DJ, Jr, Huxley VH. Capillary hydraulic conductivity is elevated by cGMP-dependent vasodilators. Circ Res. 1992;70:382–391. doi: 10.1161/01.res.70.2.382. [DOI] [PubMed] [Google Scholar]
- 43.Michel CC, Curry FE. Microvascular permeability. Physiol Rev. 1999;79:703–761. doi: 10.1152/physrev.1999.79.3.703. [DOI] [PubMed] [Google Scholar]
- 44.Mier CM, Domenick MA, Turner NS, Wilmore JH. Changes in stroke volume and maximal aerobic capacity with increased blood volume in men women. J Appl Physiol. 1996;80:1180–1186. doi: 10.1152/jappl.1996.80.4.1180. [DOI] [PubMed] [Google Scholar]
- 45.Miller VM. Gender and vascular reactivity. Lupus. 1999;8:409–415. doi: 10.1177/096120339900800516. [DOI] [PubMed] [Google Scholar]
- 46.Miller VM, Lewis DA, Barber DA. Gender differences and endothelium- and platelet-derived factors in the coronary circulation. Clin Exp Pharmacol Physiol. 1999;26:132–136. doi: 10.1046/j.1440-1681.1999.02997.x. [DOI] [PubMed] [Google Scholar]
- 47.Mitchell AL, Huxley VH, Meyer DJ., Jr Topical isoproterenol decreases mesenteric capillary water permeability (Abstract) FASEB J. 1997;11:A202. [Google Scholar]
- 48.Neter J. Applied Linear Statistical Models: Regression, Analysis of Variance, and Experimental Designs. Irwin; Homewood, IL: 1990. [Google Scholar]
- 49.Orshal J, Khalil R. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol. 2004;286:R233–R249. doi: 10.1152/ajpregu.00338.2003. [DOI] [PubMed] [Google Scholar]
- 50.Rumbaut RE, Harris NR, Sial AJ, Huxley VH, Granger DN. Leakage responses to L-NAME differ with the fluorescent dye used to label albumin. Am J Physiol Heart Circ Physiol. 1999;276:H333–H339. doi: 10.1152/ajpheart.1999.276.1.H333. [DOI] [PubMed] [Google Scholar]
- 51.Rumbaut RE, Sial AJ. Differential phototoxicity of fluorescent dye-labeled albumin conjugates. Microcirculation. 1999;6:205–213. [PubMed] [Google Scholar]
- 52.Rumbaut RE, Wang J, Huxley VH. Differential effects of L-NAME on rat venular hydraulic conductivity. Am J Physiol Heart Circ Physiol. 2000;279:H2017–H2023. doi: 10.1152/ajpheart.2000.279.4.H2017. [DOI] [PubMed] [Google Scholar]
- 53.Slimmer LM, Blair ML. Female reproductive cycle influences plasma volume and protein restitution after hemorrhage in the conscious rat. Am J Physiol Regul Integr Comp Physiol. 1996;271:R626–R633. doi: 10.1152/ajpregu.1996.271.3.R626. [DOI] [PubMed] [Google Scholar]
- 54.Vernikos J, Dallman MF, Keil LC, O’Hara D, Convertino VA. Gender differences in endocrine responses to posture and 7 days of −6 degrees head-down bed rest. Am J Physiol Endocrinol Metab. 1993;265:E153–E161. doi: 10.1152/ajpendo.1993.265.1.E153. [DOI] [PubMed] [Google Scholar]
- 55.Wang J, Whitt SP, Rubin LJ, Huxley VH. Differential coronary microvascular exchange responses to adenosine: roles of receptor and microvessel subtypes. Microcirculation. doi: 10.1080/10739680590934736. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watanabe H, Kuhne W, Schwartz P, Piper HM. A2 adenosine receptor stimulation increases macromolecule permeability of coronary endothelial cells. Am J Physiol Heart Circ Physiol. 1992;262:H1174–H1181. doi: 10.1152/ajpheart.1992.262.4.H1174. [DOI] [PubMed] [Google Scholar]
- 57.Wu HM, Huang Q, Yuan Y, Granger HJ. VEGF induces NO-dependent hyperpermeability in coronary venules. Am J Physiol Heart Circ Physiol. 1996;271:H2735–H2739. doi: 10.1152/ajpheart.1996.271.6.H2735. [DOI] [PubMed] [Google Scholar]
- 58.Yuan Y, Chilian WM, Granger HJ, Zawieja DC. Permeability to albumin in isolated coronary venules. Am J Physiol Heart Circ Physiol. 1993;265:H543–H552. doi: 10.1152/ajpheart.1993.265.2.H543. [DOI] [PubMed] [Google Scholar]
- 59.Yuan Y, Granger HJ, Zawieja DC, Chilian WM. Flow modulates coronary venular permeability by a nitric oxide-related mechanism. Am J Physiol Heart Circ Physiol. 1992;263:H641–H646. doi: 10.1152/ajpheart.1992.263.2.H641. [DOI] [PubMed] [Google Scholar]
- 60.Yuan Y, Granger HJ, Zawieja DC, DeFily DV, Chilian WM. Histamine increases venular permeability via a phospholipase C-NO synthase-guanylate cyclase cascade. Am J Physiol Heart Circ Physiol. 1993;264:H1734–H1739. doi: 10.1152/ajpheart.1993.264.5.H1734. [DOI] [PubMed] [Google Scholar]
- 61.Zoukourian C, Wautier MP, Chappey O, Dosquet C, Rohban T, Schmidt AM, Stern D, Wautier JL. Endothelial cell dysfunction secondary to the adhesion of diabetic erythrocytes. Modulation by iloprost. Int Angiol. 1996;15:195–200. [PubMed] [Google Scholar]


