Abstract
To evaluate the effects of physiologic hyperglucagonemia on splanchnic glucose output, glucagon was infused in a dose of 3 ng/kg per min to healthy subjects in the basal state and after splanchnic glucose output had been inhibited by an infusion of glucose (2 mg/kg per min). In the basal state, infusion of glucagon causing a 309 +/- 25 pg/ml rise in plasma concentration was accompanied by a rapid increase in splanchnic glucose output to values two to three times basal by 7-15 min. The rise in arterial blood glucose (0.5-1.5 mM) correlated directly with the increment in splanchnic glucose output. Despite continued glucagon infusion, and in the face of stable insulin levels, splanchnic glucose output declined after 22 min, returning to basal levels by 30-45 min. In the subjects initially receiving the glucose infusion, arterial insulin concentration rose by 5-12 muU/ml, while splanchnic glucose output fell by 85-100%. Infusion of glucagon causing an increment in plasma glucagon concentration of 272 +/- 30 pg/ml reversed the inhibition in splanchnic glucose production within 5 min. Splanchnic glucose output reached a peak increment 60% above basal levels at 10 min, and subsequently declined to levels 20-25% below basal at 30-45 min. These findings provide direct evidence that physiologic increments in plasma glucagon stimulate splanchnic glucose output in the basal state and reverse insulin-mediated inhibition of splanchnic glucose production in normal man. The transient nature of the stimulatory effect of glucagon on splanchnic glucose output suggests the rapid development of inhibition or reversal of glucagon action. This inhibition does not appear to depend on increased insulin secretio.
Full text
PDF
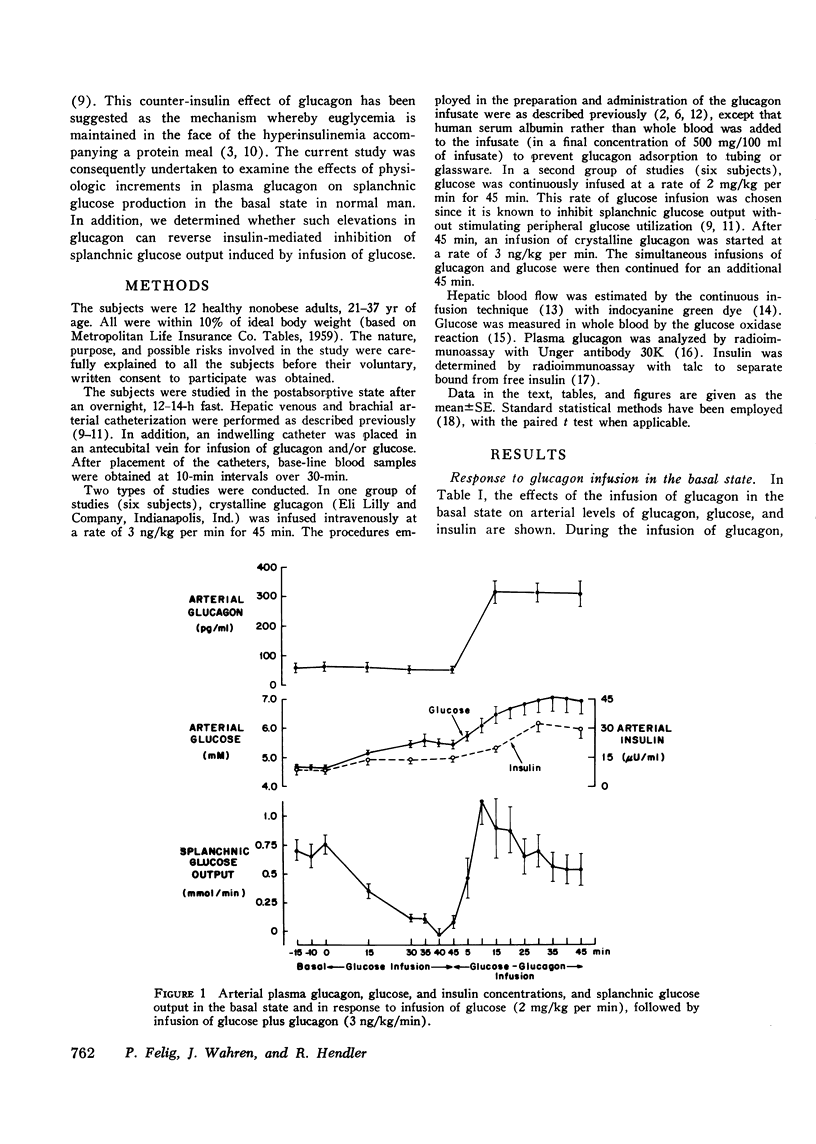
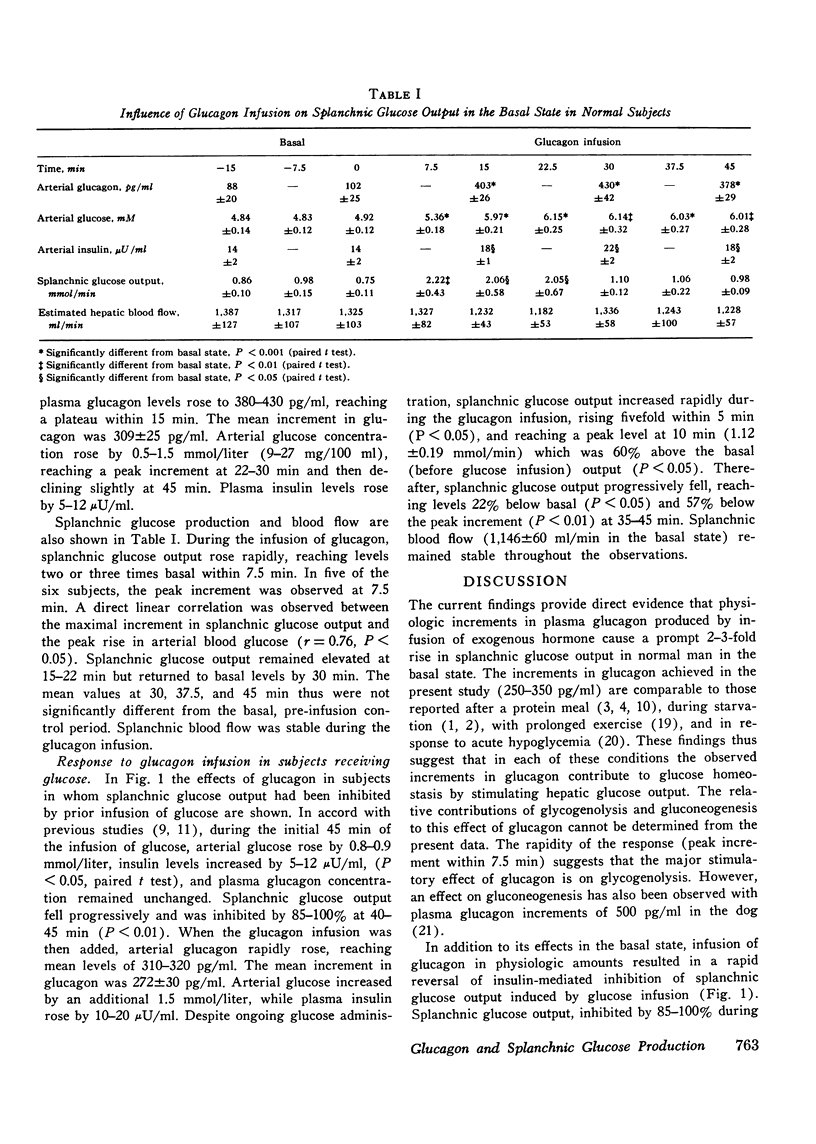


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilar-Parada E., Eisentraut A. M., Unger R. H. Effects of starvation on plasma pancreatic glucagon in normal man. Diabetes. 1969 Nov;18(11):717–723. doi: 10.2337/diab.18.11.717. [DOI] [PubMed] [Google Scholar]
- Aguilar-Parada E., Eisentraut A. M., Unger R. H. Pancreatic glucagon secretion in normal and diabetic subjects. Am J Med Sci. 1969 Jun;257(6):415–419. doi: 10.1097/00000441-196906000-00008. [DOI] [PubMed] [Google Scholar]
- Ahlborg G., Felig P., Hagenfeldt L., Hendler R., Wahren J. Substrate turnover during prolonged exercise in man. Splanchnic and leg metabolism of glucose, free fatty acids, and amino acids. J Clin Invest. 1974 Apr;53(4):1080–1090. doi: 10.1172/JCI107645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes A. J., Bloom A., Crowley M. F., Tuttlebee J. W., Bloom S. R., Alberti K. G., Smythe P., Turnell D. Is glucagon important in stable insulin-dependent diabetics? Lancet. 1975 Oct 18;2(7938):734–737. doi: 10.1016/s0140-6736(75)90722-9. [DOI] [PubMed] [Google Scholar]
- Cherrington A. D., Vranic M. Effect of interaction between insulin and glucagon on glucose turnover and FFA concentration in normal and depancreatized dogs. Metabolism. 1974 Aug;23(8):729–744. doi: 10.1016/0026-0495(74)90005-5. [DOI] [PubMed] [Google Scholar]
- Chiasson J. L., Cook J., Liljenquist J. E., Lacy W. W. Glucagon stimulation of gluconeogenesis from alanine in the intact dog. Am J Physiol. 1974 Jul;227(1):19–23. doi: 10.1152/ajplegacy.1974.227.1.19. [DOI] [PubMed] [Google Scholar]
- Chiasson J. L., Liljenquist J. E., Sinclair-Smith B. C., Lacy W. W. Gluconeogenesis from alanine in normal postabsorptive man. Intrahepatic stimulatory effect of glucagon. Diabetes. 1975 Jun;24(6):574–584. doi: 10.2337/diab.24.6.574. [DOI] [PubMed] [Google Scholar]
- Felig P., Wahren J., Hendler R., Brundin T. Splanchnic glucose and amino acid metabolism in obesity. J Clin Invest. 1974 Feb;53(2):582–590. doi: 10.1172/JCI107593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felig P., Wahren J. Influence of endogenous insulin secretion on splanchnic glucose and amino acid metabolism in man. J Clin Invest. 1971 Aug;50(8):1702–1711. doi: 10.1172/JCI106659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerich J. E., Schneider V., Dippe S. E., Langlois M., Noacco C., Karam J. H., Forsham P. H. Characterization of the glucagon response to hypoglycemia in man. J Clin Endocrinol Metab. 1974 Jan;38(1):77–82. doi: 10.1210/jcem-38-1-77. [DOI] [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- Insel P. A., Liljenquist J. E., Tobin J. D., Sherwin R. S., Watkins P., Andres R., Berman M. Insulin control of glucose metabolism in man: a new kinetic analysis. J Clin Invest. 1975 May;55(5):1057–1066. doi: 10.1172/JCI108006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljenquist J. E., Bomboy J. D., Lewis S. B., Sinclair-Smith B. C., Felts P. W., Lacy W. W., Crofford O. B., Liddle G. W. Effect of glucagon on net splanchnic cyclic AMP production in normal and diabetic men. J Clin Invest. 1974 Jan;53(1):198–204. doi: 10.1172/JCI107538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosselin G., Assan R., Yalow R. S., Berson S. A. Separation of antibody-bound and unbound peptide hormones labelled with iodine-131 by talcum powder and precipitated silica. Nature. 1966 Oct 22;212(5060):355–357. doi: 10.1038/212355a0. [DOI] [PubMed] [Google Scholar]
- Rowell L. B., Kraning K. K., 2nd, Evans T. O., Kennedy J. W., Blackmon J. R., Kusumi F. Splanchnic removal of lactate and pyruvate during prolonged exercise in man. J Appl Physiol. 1966 Nov;21(6):1773–1783. doi: 10.1152/jappl.1966.21.6.1773. [DOI] [PubMed] [Google Scholar]
- Sherwin R. S., Bastl C., Finkelstein F. O., Fisher M., Black H., Hendler R., Felig P. Influence of uremia and hemodialysis on the turnover and metabolic effects of glucagon. J Clin Invest. 1976 Mar;57(3):722–731. doi: 10.1172/JCI108330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin R. S., Fisher M., Hendler R., Felig P. Hyperglucagonemia and blood glucose regulation in normal, obese and diabetic subjects. N Engl J Med. 1976 Feb 26;294(9):455–461. doi: 10.1056/NEJM197602262940901. [DOI] [PubMed] [Google Scholar]
- Unger R. H. Alpha- and beta-cell interrelationships in health and disease. Metabolism. 1974 Jun;23(6):581–593. doi: 10.1016/0026-0495(74)90086-9. [DOI] [PubMed] [Google Scholar]
- Unger R. H., Ohneda A., Aguilar-Parada E., Eisentraut A. M. The role of aminogenic glucagon secretion in blood glucose homeostasis. J Clin Invest. 1969 May;48(5):810–822. doi: 10.1172/JCI106039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren J., Felig P., Hagenfeldt L. Effect of protein ingestion on splanchnic and leg metabolism in normal man and in patients with diabetes mellitus. J Clin Invest. 1976 Apr;57(4):987–999. doi: 10.1172/JCI108375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise J. K., Hendler R., Felig P. Influence of glucocorticoids on glucagon secretion and plasma amino acid concentrations in man. J Clin Invest. 1973 Nov;52(11):2774–2782. doi: 10.1172/JCI107473. [DOI] [PMC free article] [PubMed] [Google Scholar]



