Abstract
The mechanisms for translocation of heavy metals from soil to epigeal mosses were investigated. The first mechanism was demonstrated for 137Cs and involved the uplifting of the pollutant-containing dust from the soil, followed by the local secondary deposition on surfaces of epigeal mosses and epiphytic lichens. The second mechanism involved the diffusion of metal cations from the soil through water wetting the moss into the gametophyte. The mechanism was demonstrated by measuring the electric conductance of wetted gametophytes with single ends immersed in solutions of Cu and Na salts. In addition, the concentrations of Cu and Cd were compared in moss samples exposed to the natural soil and to the soil contaminated with the metals. The exposition to the contaminated soil resulted in the statistically significant increase of metal concentrations in the gametophytes.
Keywords: Heavy metals, Soil, Moss, Translocation, Diffusion, Secondary emission
Introduction
Bryophytes are the descendants of Paleozoic Rhyniopsida which exhibited the gametophyte-dominated life cycles. Historically, bryophytes were considered a taxonomic division that included three classes: hornworts, liverworts and mosses. Recently, the name Bryophyta has been assigned to the proper mosses (Shaw and Goffinet 2000). In 1947, Buch suggested to divide Bryophyta into endo-, ecto- and mixohydric species, depending on their water relations (Markert and Weckert 1989). Endohydric species, such as Polytrichastrum formosum, have internally diversified systems of water conduction which transport water in a way similar to that in higher plants. Their leaves have epidermis and cuticula, which hamper the water intake through surfaces. Ectohydric species, such as Pleurozium schreberi, have no vascular bundles, so they absorb nutrients directly from the wet and dry deposition. The species which fall between the ecto- and endohydric mosses are called mixohydric (Schofield 1981). They have less diversified and less efficient vascular bundles than the endohydric species.
The methods using plant and animal organisms to assess the environmental pollution and the mechanisms of pollutant translocation developed rapidly in the second half of the twentieth century. A significant example of such approach was the application of mosses and lichens to evaluate the distribution of radionuclides released to the atmosphere from the trial nuclear blasts in the 1950s and 1960s (Kłos et al. 2004). Every 5 years since 1990, many European countries carry out the cyclic studies of trace elements accumulated in mosses. The studies belong to the International Cooperative Programme on Effects of Air Pollution on Natural Vegetation and Crops (Harmens et al. 2010a). The programme is coordinated by the ICP Vegetation Coordination Centre, the Centre for Ecology and Hydrology in Bangor, UK. In 2005, it gathered 28 countries, including Poland (Harmens et al. 2010a, b). Similar studies were carried out in 2000 by the Vysehrad Group countries—Hungary, Czech Republic, Poland and Slovakia (Suchara et al. 2007). Biomonitoring studies utilising mosses include also the local cases, such as a pilot study comparing the sorption properties of various moss species in Latvia 1993 (Ceburnis et al. 1997); determination of the concentrations of heavy metals in moss samples collected around the industrial facilities in Estonia, in 1992, 1997 and 2002, carried out to estimate the distribution of the deposition of these metals in the vicinity of their sources (Liiv and Kaasik 2004); studies in the Romanian city of Baia Mare hosting several copper, zinc and lead works (Culicov et al. 2002); studies in Bulgaria and the European part of Turkey (Coşkun et al. 2009); studies in Greece (Yurukova et al. 2009) and studies in Belgrade, where moss samples collected in autumn 2004 were analysed using the neutron activation analysis (INAA) to determine 36 elements and to indicate their main sources—fuel burning for energy production and vehicle emissions (Aničić et al. 2007). Determination of heavy metals accumulated in mosses were also carried out in Vietnam (Nguyen-Viet et al. 2007), Pakistan (Rahman et al. 2000) and in the South Ural (Smirnov et al. 2004).
The biomonitoring methods usually utilise the ectohydric mosses such as P. schreberi (Zechmeister et al. 2005), Hylocomium splenders (Grünfeld 2005), Hypnum cupressiforme (Coşkun et al. 2005), Scleropodium purum (Couto et al. 2005) and Dicranella heteromalla (Astel et al. 2008), while the endohydric mosses are used rarely. It is assumed that the ectohydric mosses uptake the mineral components mainly from the wet and dry deposition, so they are not much influenced by the soil composition (Fernández et al. 1999; Gjengedal and Steinnes 1990). Some authors disagree with that and claim that the ectohydric mosses also can translocate the elements from the soil and conduct them internally (Eckstein and Karlsson 1999; Okland et al. 1999). Within the structures of the ectohydric mosses, water is transported due to the capillary forces (Dilks and Proctor 1979; Proctol and Tuba 2002). Mosses which lack the conducting system have elongated cells, which promote the transport of water driven by the surface tension.
The presented study was aimed at the qualitative evaluation of the translocation of metals from the soil to the green parts of the ectohydric and epigeal moss P. schreberi. Two mechanisms of the process were considered, one based on the transfer of metals with dusts uplifted from the soil and the other based on the diffusion of metal ions in aqueous solution wetting a moss. The results of the study should help to interpret the data from the biomonitoring studies utilising mosses to evaluate the deposition of pollutants.
Materials and Methods
The research was arranged into two stages. The first stage was carried out to evaluate the translocation of pollutants with dusts uplifted from the soil. The marker used was 137Cs, which had no inflowing character at the time of the study. The second stage dealt with the translocation of pollutants by diffusion in aqueous solutions wetting the mosses. Sodium, copper or cadmium ions were used as markers in three various experimental setups.
Translocation of Pollutants with Dusts Uplifted from the Soil
Kinetics of 137Cs translocation with dusts uplifted from the soil was evaluated using the epigeal moss P. schreberi. The results were compared to those published earlier for the epiphytic lichen Hypogymnia physodes (Kłos et al. 2009). Samples of the moss and the lichen including their natural substrates were taken from the forest area in which the 137Cs activity in soil was lower than 100 Bq kg−1 and exposed in another forest area, where the activity exceeded 1,400 Bq kg−1. The areas were located within the Opole Anomaly—the region in which the activity of 137Cs recorded in 1994 in the surface layer of soil 10 cm thick locally exceeded the country mean value by a factor of hundred (Jagielak et al. 1997). The radioactive caesium has been originally released by the Chernobyl disaster and is still deposited in the forested areas and wastelands of the Opole Anomaly.
The moss samples including the substrate were placed directly on the soil, while the lichen-covered branches were placed 1.0 to 1.5 m above the ground, on the heaps of dry spruce branches arranged horizontally in places unshaded by large tree crowns. The control samples of the moss and the lichen were transplanted accordingly within the areas with low soil activity of 137Cs. All transplants were exposed for 220 days and sampled three times for the analyses. After the exposition, the 14-g samples of all transplants were cleaned of mechanical impurities, dried at 363 K and analysed for the 137Cs activity.
Diffusion in Aqueous Solution Wetting the Moss
Translocation of pollutants by diffusion in solutions was examined both in the laboratory and in the field experiments.
Laboratory Experiments
Samples of the moss for the laboratory experiments were collected from a single place 1 m2 large. The samples were moved to the laboratory, dried without prior washing and cleaned of mechanical impurities.
Figure 1 shows two laboratory setups used for two different sets of experiments. In the first set of experiments, the electric conductance of a wetted gametophyte was measured in time. First, the gametophyte was connected to platinum electrodes, wetted and immersed with one end in 20 cm3 of demineralised water in a closed container equipped with a pipette (Fig. 1a). The conductance of the gametophyte changed due to salts released from the extracellular spaces and, probably, also from the intracellular spaces. After about 20 h, when the conductometer readings stabilised, 2 cm3 of solution was removed from the container using the built-in pipette and replaced with 2 cm3 of a 1-mmol dm−3 (63.6 mg dm−3) solution of copper sulphate. The changes of electric conductance in time, which indicated the diffusion of ions in the solution, were recorded. The experiment was repeated for sodium ions (c Na = 2 mmol dm−3 or 46.0 mg dm−3), which have much lower affinity to the sorption structures of mosses than the copper ions. The gram-equivalent concentrations (val per cubic decimeter) of copper and sodium ions were identical.
Fig. 1.
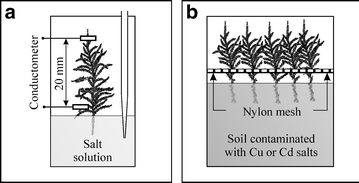
a, b Setups for the laboratory experiments
The second set of experiment consisted in placing the ends of several gametophytes in 200-g portions of clean soil (a control sample) and soil artificially contaminated with solutions of Cu or Cd salts (Fig. 1b). The samples were placed in glass cylinders 20 cm high and 20 cm in diameter. Each experiment lasted for 10 days. Twice a day, the moss samples were wetted with about 5 cm3 of demineralised water.
After 10 days of exposition, the upper parts of the moss samples were dried at 363 K, homogenised and mineralised in a microwave mineraliser. Copper and cadmium were determined using the flame version of the atomic absorption spectrometry (F-AAS).
Field Experiments
Field experiments were carried out in a forest located 20 km away from the city of Opole. Figure 2 shows the arrangement of the experimental site. Mosses and several genetic layers of soil were removed from a rectangular plot of soil about 0.5 m2 large. Then, a box was placed in the hole and filled with removed layers of soil carefully arranged in the proper order. The soil in the box was wetted with 5 dm3 of a 70-mg dm−3 solution of Cd salt. Finally, the removed moss was carefully placed atop the soil in the box. The area around the box was restored to the original condition.
Fig. 2.
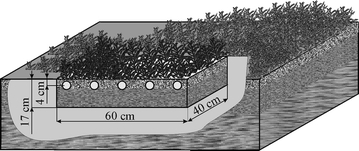
The site for the field experiments
The site was left unattended for 2 months, from January to February 2011. Then, samples of the moss and soil were taken from the box and from six places in the distance of 1–5 m from the box. The samples were homogenised, dried at 363 K and mineralised in a microwave mineraliser. Copper and cadmium were determined using the F-AAS.
Analysis
The electric conductance of gametophytes was measured with a CC-551 conductometer from Elmetron, Poland. The cell with platinum electrodes supplied with the alternating current of 10 kHz was shown in Fig. 1a.
The 137Cs activity in samples was measured with a gamma spectrometer fitted with a high-resolution germanium detector HPGe (Canberra) characterised by a 1.29-keV FWHM at 662 and 1.70 keV FWHM at 1,332 keV, as well as a relative efficiency of 21.7%. Both the measurements and the analyses of spectra were computer controlled with the GENIE 2000 software.
Concentrations of copper and cadmium in solutions after mineralisation were determined with an atomic absorption spectrometer SOLAAR 969 from UNICAM/Thermo Electron Corporation, USA. The spectrometer was calibrated against standards available from ANALTYIKA Ltd., Czech Republic. The upper limits of the linear relations between the concentrations of the analytes and the instrument signal were assumed equal to the highest concentrations of the calibration standards: 5 mg dm−3 for Cu and 2 mg dm−3 for Cd.
Quality Assessment/Quality Control
The energy and efficiency calibration of the gamma spectrometer was performed against standard solutions type MBSS 2 from the Czech Metrological Institute in Prague, which covered the energy range from 59.54 keV (241Am) to 1,836.06 keV (88Y). Geometry of the calibration source and samples was a 450-cm3 Marinelli container. The minimum detectable activity was lower than 2.0 Bq kg−1. The quality control of the Cu and Cd analyses was assured by determination of the method quantification limits (MQLs), test analyses of the BCR-482 lichen reference materials from the Institute for Reference Materials and Measurements in Belgium and the comparative interlaboratory analyses carried out in cooperation with the Health Institute in Hradec Králové, Czech Republic (ZÚHK).
The MQL values obtained in both laboratories for the determination of Cu and Cd in mosses and lichens were 0.5 μg g−1. The results of test analyses of the BCR-482 reference material are summarized in Table 1. The maximal standard deviations of the mean, characterising the results obtained for the homogenised moss samples, were 3.9% for Cu and 10.3% for Cd.
Table 1.
Measured and certified values of Cu and Cd concentration in BCR 482 lichen reference material
| Element | Certified value | ±Uncertainty | Mean | ±SD | D a (%) |
|---|---|---|---|---|---|
| μg g−1 d.m. | |||||
| Cu | 7.03 | 0.19 | 6.54 | 0.18 | −7.0 |
| Cd | 0.56 | 0.02 | 0.50 | 0.04 | −6.3 |
aDeviation—a difference between a measured value and a certified value, divided by the certified value
d.m. dry mass
Results and Discussion
Translocation of 137Cs from Soil to Mosses and Lichens
Samples of the moss and the lichen including their natural substrates were taken from the forest area in which the 137Cs was low and transplanted for exposition in another forest area, where the 137Cs activity in soil exceeded 1,400 Bq kg−1. The transplanted mosses and lichens were sampled for analyses on the 54th, 157th and 220th day of the exposition. Samples after the 137Cs analyses were not used again. Figure 3 shows the kinetics of the 137Cs translocation from the soil to the epiphytic lichen (Kłos et al. 2009) and to the epigeal moss. The uncertainty of the 137Cs determination has not exceeded ±6%.
Fig. 3.
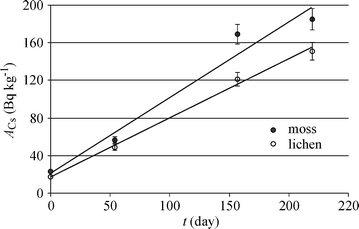
Kinetics of 137Cs translocation from soil to mosses and lichens
For many years, the radioactive 137Cs occurring in the area selected for the present study has not had the influent character. For instance, the mean 137Cs activity in air determined for Poland was 1.4 μBq/m3 in the third quarter of 2006 (Message 2006) and 1.0 μBq/m3 in the third quarter of 2010 (Message 2010). Therefore, the radioactive caesium accumulated in mosses and lichens exposed in this study originated mainly from the soil. Its main source was the plant fragments uplifted from the soil by winds and deposited to the moss and lichen surfaces. The activity of 137Cs in control samples exposed over the soil with low 137Cs activity exhibited no statistically significant variation. The moss/lichen values in becquerels per kilogram were 21.2/17.4 initially, 26.4/20.8 after 54 days and 25.3/19.1 after 220 days. Larger increase of the 137Cs concentration in mosses than in lichens could result from a relatively higher deposition of the local dust on mosses exposed directly on the soil than on lichen exposed at 1.0 to 1.5 m above the soil, as well as from the diffusional processes discussed in the following section.
Translocation of pollutants with dusts uplifted from the soil is always considered in the biomonitoring studies. The share of the analytes originating from the soil is evaluated using the enrichment factors which compare the relative concentrations of analytes in mosses (Bargagli et al. 1995) or in lichens (Bergamaschi et al. 2005) to these in the soil. The relative concentrations are usually calculated against aluminium or scandium, the elements that rarely enter the atmospheric aerosol from the anthropogenic sources. If the shares of an analyte in the soil and in a moss or lichen are proportional, the soil can be indicated as a source of this analyte. Sometimes the scandium and aluminium reference elements are replaced with the sum of the concentrations of the rare earth elements (Chiarenzelli et al. 2001).
Diffusion in Aqueous Solutions Wetting the Mosses
Figure 4 shows the changes of the relative electric conductance of a gametophyte, G/G 0, in time. The variable G 0 is the stable conductance of water after the first stage of the experiment (∼20 h), while G is the electric conductance measured after the addition of the CuSO4 solution (Fig. 4a) or NaCl solution (Fig. 4b). The choice of the relative conductance was justified by the undefined geometry of the measured system. The measurements were taken either continuously, with the conductometer permanently switched on, or at the beginning and at the end of the experiment only (after 480 min for CuSO4 and 240 min for NaCl), to check up on the effect of the diffusion forced by the electrical field generated by the conductometer.
Fig. 4.
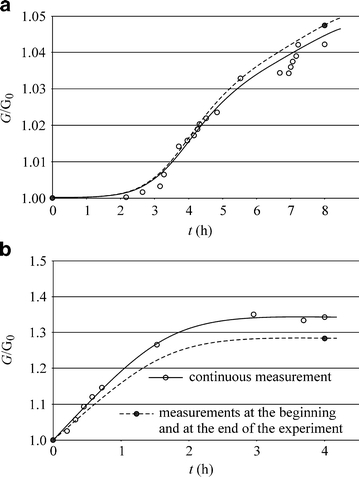
Changes in time of the relative electric conductance G/G 0 of a wetted gametophyte with one end immersed in aqueous solution of a CuSO4 and b NaCl
Data presented in Fig. 4 prove the diffusion of ions takes place in the solution wetting a gametophyte. Although the concentrations of Na and Cu ions in the experiments were higher than their concentrations in natural soil solutions, the experiments clearly indicated the plausibility of the diffusional mechanism of ion transport from the soil to mosses. Copper diffused much slower than sodium, since it was much better sorbed by the moss. The presented results are qualitative only because the gametophytes used had different sizes. Table 2 shows the results of the laboratory experiments carried out to evaluate the increasing concentrations of Cu and Cd in gametophytes with ends rooted in contaminated soil (Fig. 1b).
Table 2.
Concentrations of Cu and Cd accumulated in gametophytes exposed for 10 days to soil contaminated with these metals (displayed errors are standard deviations)
| Metal | Metal concentration (μg g−1 d.m.) | |||
|---|---|---|---|---|
| Soil | Moss | |||
| Control sample | Sample | Control sample | Sample | |
| Cu | 4.32 ± 0.18 | 120 ± 12 | 12.62 ± 0.78 | 19.3 ± 7.0 |
| Cd | 1.35 ± 0.18 | 13.1 ± 1.1 | 1.75 ± 0.16 | 2.35 ± 0.08 |
| 26.20 ± 0.96 | 4.25 ± 0.18 | |||
Data in Table 2 show the concentrations of metals in gametophyte and in the soil are correlated. The observation confirms the thesis that metal cations can diffuse from the soil up to the green parts of mosses. Again, the concentrations of Cu and Cd in the experiments were higher than in the forest soil under the natural conditions. However, only under such conditions, the statistically significant changes of metal concentrations in the gametophytes could be followed.
Table 3 shows the results of the experiment carried out in the field (Fig. 3). Cadmium was used as a model pollutant because it has higher mobility in soil and thence higher bioavailability for plants than copper (Brallier et al. 1996).
Table 3.
Concentrations of Cd accumulated in gametophytes exposed for 2 months under field conditions (displayed errors are standard deviations)
| Sample | Concentration of Cd (μg g−1 d.m.) |
|---|---|
| Forest soil | 1.49 ± 0.28 |
| Contaminated soil | 7.11 ± 0.44 |
| Moss from the forest | 1.80 ± 0.22 |
| Moss from the box | 2.98 ± 0.18 |
The results of the field experiment also show the statistically significant increase of cadmium concentration (65%) in mosses growing on the contaminated soil. Translocation of Cd with dusts uplifted from the soil was avoided, as the experiment was carried out in wet winter period (January and February).
In the natural habitat of the mosses, translocation of heavy metals from soil is influenced by many abiotic factors either enhancing or hampering the process. For instance, the chemical composition and sorption properties of the soil influence the mobility and bioavailability of metals. The climatic conditions influence the soil wetness, which reduces the translocation of metals with dust but favours their diffusional transport through the solutions wetting the mosses. The aforementioned mechanisms of translocation are also valid for macroelements necessary for the plants, such as Na, K, Mg and Ca cations. Under the natural conditions, macroelements reduce the sorption of heavy-metal cations, as shown for lichens (Kłos et al. 2007).
Conclusions
The presented results indicate two different mechanisms controlling the translocation of heavy metals from soil to the epigeal mosses. The first mechanism consists in transporting the metals with the dusts uplifted from the soil, while the second one employs diffusion of metal cations through the aqueous solutions wetting the mosses. The shares of each mechanism in the translocation depend on the local climatic conditions. Detail study of the factors influencing both mechanisms can by advantageous for the interpretation of the biomonitoring studies based on mosses.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Aničić M, Frontasyeva MV, Tomašević M, Popović A. Assessment of atmospheric deposition of heavy metals and other elements in Belgrade using the moss biomonitoring technique and neutron activation analysis. Environmental Monitoring and Assessment. 2007;129:207–219. doi: 10.1007/s10661-006-9354-y. [DOI] [PubMed] [Google Scholar]
- Astel A, Astel K, Biziuk M. PCA and multidimensional visualization techniques united to aid in the bioindication of elements from transplanted Sphagnum palustre moss exposed in the Gdańsk City area. Environmental Science and Pollution Research. 2008;15(1):41–50. doi: 10.1065/espr2007.05.422. [DOI] [PubMed] [Google Scholar]
- Bargagli R, Brown DH, Nelli L. Metal biomonitoring with mosses: procedures for correcting for soil contamination. Environmental Pollution. 1995;89:169–175. doi: 10.1016/0269-7491(94)00055-I. [DOI] [PubMed] [Google Scholar]
- Bergamaschi L, Rizzio E, Giaveri G, Giordani L, Profumo A, Gallorini M. INAA for the determination of trace elements and evaluation of their enrichment factors in lichens of high altitude areas. Journal of Radioanalytical and Nuclear Chemistry. 2005;263(3):721–724. doi: 10.1007/s10967-005-0648-2. [DOI] [Google Scholar]
- Brallier S, Harrison RB, Henry ChL, Dongsen X. Liming effects on availability of Cd, Cu, Ni and Zn in a soil amended with sewage sludge 16 years previously. Water, Air, and Soil Pollution. 1996;86:195–206. doi: 10.1007/BF00279156. [DOI] [Google Scholar]
- Ceburnis D, Rühling Å, Kvietkus K. Extended study of atmospheric heavy metal deposition in Lithuania based on moss analysis. Environmental Monitoring and Assessment. 1997;47:135–152. doi: 10.1023/A:1005779101732. [DOI] [Google Scholar]
- Chiarenzelli JR, Aspler LB, Dunn C, Cousens B, Ozarko DL, Powis KB. Multi-element and rare earth element composition of lichens, mosses, and vascular plants from the Central Barrenlands, Nunavut, Canada. Applied Geochemistry. 2001;16:245–270. doi: 10.1016/S0883-2927(00)00027-5. [DOI] [Google Scholar]
- Coşkun M, Frontasyeva MV, Steiness E, Cotuk AY, Pavlov SS, Coskun M, et al. Atmospheric deposition of heavy metals in Thrace studies by analysis of moss (Hypnum cupressiforme) Bulletin of Environmental Contamination and Toxicology. 2005;74:201–209. doi: 10.1007/s00128-004-0569-8. [DOI] [PubMed] [Google Scholar]
- Coşkun M, Yurukova L, Çayir A, Coşkun M, Gecheva G. Cross-border response of mosses to heavy metal atmospheric deposition in Southeastern Bulgaria and European Turkey. Environmental Monitoring and Assessment. 2009;157:529–537. doi: 10.1007/s10661-008-0553-6. [DOI] [PubMed] [Google Scholar]
- Couto JA, Fernández JA, Aboal JR, Carballeira A. Active biomonitoring of element with terrestrial mosses: a comparison of bulk and dry deposition. Science of the Total Environment. 2005;324:211–222. doi: 10.1016/j.scitotenv.2003.10.024. [DOI] [PubMed] [Google Scholar]
- Culicov OA, Frontasyeva MV, Steinnes E, Okina OS, Santa Z, Todoran R. Atmospheric deposition of heavy metals around the lead and copper–zinc smelters in Baia Mare, Romania, studied by the moss biomonitoring technique, neutron activation analysis and flame atomic absorption spectrometry. Journal of Radioanalytical and Nuclear Chemistry. 2002;254(1):109–115. doi: 10.1023/A:1020853800937. [DOI] [Google Scholar]
- Dilks TJK, Proctor MCF. Photosynthesis, respiration and water content in bryophytes. New Phytologist. 1979;82:97–114. doi: 10.1111/j.1469-8137.1979.tb07564.x. [DOI] [Google Scholar]
- Eckstein RL, Karlsson PS. Recycling of nitrogen among segments of Hylocomnium splenders as compared with Polytrichum commune: implications for clonal integration in an ectohydric bryophyte. Oikos. 1999;86:87–96. doi: 10.2307/3546572. [DOI] [Google Scholar]
- Fernández JA, Puche F, Gimeno C, Carballeira A. Primeros datos sobre el biocontrol de la deposición atmosférica de metais pesados en las provincias de Valencia, Castellón y Teruel mediante musgos terrestres. Ecología. 1999;13:83–91. [Google Scholar]
- Gjengedal E, Steinnes E. Uptake of metal ions in moss from artificial precipitation. Environmental Monitoring and Assessment. 1990;14:77–87. doi: 10.1007/BF00394359. [DOI] [PubMed] [Google Scholar]
- Grünfeld K. Integrating spatio-temporal information in environmental monitoring data—a visualization approach applied to moss data. Science of the Total Environment. 2005;347:1–20. doi: 10.1016/j.scitotenv.2004.12.054. [DOI] [PubMed] [Google Scholar]
- Harmens, H., Mills, G., Hayes, F., Jones, L., Norris, D., Cooper, D. (2010a). ICP vegetation annual report 2008/2009. http://icpvegetation.ceh.ac.uk. Accessed 20 Feb 2011.
- Harmens H, Norris DA, Steinnes E, Kubin E, Piispanen J, Alber R, et al. Mosses as biomonitors of atmospheric heavy metal deposition: spatial patterns and temporal trends in Europe. Environmental Pollution. 2010;158:3144–3156. doi: 10.1016/j.envpol.2010.06.039. [DOI] [PubMed] [Google Scholar]
- Jagielak J, Biernacka M, Henschke J, Sosińska A. Radiological atlas of Poland (Radiologiczny Atlas Polski) Warsaw: Biblioteka Monitoringu Środowiska, PIOŚ; 1997. [Google Scholar]
- Kłos A, Rajfur M, Wacławek M, Wacławek W. Lichen application for assessing environmental pollution with radionuclides. Chemia i Inżynieria Ekologiczna. 2004;11(12):1323–1332. [Google Scholar]
- Kłos A, Rajfur M, Wacławek M, Wacławek W. Heavy metal sorption in the lichen cationactive layer. Bioelectrochemistry. 2007;71:60–65. doi: 10.1016/j.bioelechem.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Kłos A, Rajfur M, Wacławek M, Wacławek W. 137Cs transfer from local particulate matter to lichens and mosses. Nukleonika. 2009;54(4):297–303. [Google Scholar]
- Liiv S, Kaasik M. Trace metals in mosses in the Estonian oil shale processing region. Journal of Atmospheric Chemistry. 2004;49:563–578. doi: 10.1007/s10874-004-1266-z. [DOI] [Google Scholar]
- Markert B, Weckert V. Fluctuations of element concentrations during the growing season of Polytrichum formosum. Water, Air, and Soil Pollution. 1989;43:177–189. doi: 10.1007/BF00175592. [DOI] [Google Scholar]
- Message. (2006). Komunikat Prezesa Państwowej Agencji Atomistyki z dnia 16 października 2006 r. w sprawie sytuacji radiacyjnej kraju w III kwartale 2006 r. (The announcement of the President of the National Atomic Energy Agency, 16th October 2006 on the matter of the radiational situation of the country in the 3rd quarter of 2006).
- Message. (2010). Komunikat Prezesa Państwowej Agencji Atomistyki z dnia 15 października 2010 r. w sprawie sytuacji radiacyjnej kraju w III kwartale 2010 r. (The announcement of the President of the National Atomic Energy Agency, 15th October 2010 on the matter of the radiational situation of the country in the 3 rd quarter of 2010).
- Nguyen-Viet H, Bernard N, Mitchell EAD, Cortet J, Badot P-M, Gilbert D. Relationship between testate amoeba (protist) communities and atmospheric heavy metals accumulated in Barbula indica (Bryophyta) in Vietnam. Microbial Ecology. 2007;53:53–65. doi: 10.1007/s00248-006-9108-y. [DOI] [PubMed] [Google Scholar]
- Okland T, Okland RH, Steinnes E. Element concentration in the boreal forest moss Hylocomnium splenders: variation related to gradients in vegetation and local environmental factors. Plant and Soil. 1999;209:71–83. doi: 10.1023/A:1004524017264. [DOI] [Google Scholar]
- Proctol MCF, Tuba Z. Poikilohydry and homoihydry: antithesis or spectrum of possibilities? New Phytologist. 2002;156(3):327–349. doi: 10.1046/j.1469-8137.2002.00526.x. [DOI] [PubMed] [Google Scholar]
- Rahman U, Awan MA, Hassan ST, Khattak MM. Mosses as indicators of atmospheric pollution of trace metals (Cd, Cu, Mn, Pb and Zn) in the vicinity of coal-fired brick kilns in north-eastern suburbs of Islamabad, Pakistan. Journal of Radioanalytical and Nuclear Chemistry. 2000;246(2):331–336. doi: 10.1023/A:1006782710160. [DOI] [Google Scholar]
- Schofield WB. Ecological significance of morphological characters in the moss gametophyte. Bryologist. 1981;84(2):149–165. doi: 10.2307/3242819. [DOI] [Google Scholar]
- Shaw AJ, Goffinet B. Bryophyte biology. Cambridge: Cambridge University Press; 2000. [Google Scholar]
- Smirnov LI, Frontasyeva MV, Steinnes E, Lyapunov SM, Cherchintsev VD, Romanov SA, Samosadnyi VT. Multidimensional statistical analysis of the concentration of heavy metals and radionuclides in moss and soil in Southern Urals. Atomic Energy. 2004;97(1):510–515. doi: 10.1023/B:ATEN.0000045705.55947.41. [DOI] [Google Scholar]
- Suchara I, Maňkovská B, Sucharová J, Florek M, Godzik B, Rabnecz G, et al. Mapping of main sources of pollutants and their transport in the Visegrad space. Part II: fifty three elements. Project 11007-2006-IVF. Zvolen: KLEMO spol. s r.o., (Ltd.); 2007. [Google Scholar]
- Yurukova L, Tsakiri E, Cayir A. Cross-border response of moss, Hypnum cupressiforme Hedw., to atmospheric deposition in southern Bulgaria and northeastern Greece. Bulletin of Environmental Contamination and Toxicology. 2009;83:174–179. doi: 10.1007/s00128-008-9601-8. [DOI] [PubMed] [Google Scholar]
- Zechmeister HG, Hohenwallner D, Riss A, Hanus-Iilnar A. Estimation of element deposition derived from road traffic sources by using mosses. Environmental Pollution. 2005;138:238–249. doi: 10.1016/j.envpol.2005.04.005. [DOI] [PubMed] [Google Scholar]


