Abstract
Cognitive impairment remains prevalent in the era of combination antiretroviral therapy (cART) and may be partially due to comorbidities. We postulated that insulin resistance (IR) is negatively associated with cognitive performance. We completed a cross-sectional analysis among 1547 (1201 HIV+) women enrolled in the Women's Interagency HIV Study (WIHS). We evaluated the association of IR with cognitive measures among all WIHS women with concurrent fasting bloods and cognitive testing [Trails A, Trails B, and Symbol Digit Modalities Test (SDMT)] using multiple linear regression models. A smaller subgroup also completed the Stroop test (n=1036). IR was estimated using the Homeostasis Model Assessment (HOMA). Higher HOMA was associated with poorer performance on the SDMT, Stroop Color-Naming (SCN) trial, and Stroop interference trial, but remained statistically significant only for the SCN in models adjusting for important factors [β=3.78 s (95% CI: 0.48–7.08), p=0.025, for highest vs. lowest quartile of HOMA]. HIV status did not appear to substantially impact the relationship of HOMA with SCN. There was a small but statistically significant association of HOMA and reduced neuropsychological performance on the SCN test in this cohort of women.
Introduction
Insulin resistance (IR) represents the syndrome whereby body tissue becomes increasingly unresponsive to insulin in a setting of increased glucose loads, a process that is generally considered to be a precursor to diabetes mellitus (DM). Both IR and DM are linked to obesity, which has become a major health issue, affecting 36% of women in the United States.1 HIV-specific factors such as immune activation and specific antiretroviral drugs have been associated with IR.2–5
A recent meta-analysis demonstrated that DM is associated with a 54% increased risk of Alzheimer's disease and abnormal glucose or insulin levels with a 63% increased risk.6 In several large studies of HIV-uninfected patients, IR itself has been linked to poorer cognition, with some suggestion that this relationship was more evident in women.7–9 In a separate study of postmenopausal women on hormone replacement therapy and at risk for Alzheimer's disease (AD), IR was associated with smaller hippocampal volumes.7,10 Together, these studies identify potential risk for cognitive impairment associated with IR and hint to a potential vulnerability in women. Examining associations between IR and cognitive function in HIV-infected women could be substantially informative.
We have previously theorized that comorbidity contributes to cognitive impairment in the era of combination antiretroviral therapy (cART) and could partially account for some of the impaired neuropsychological testing performance seen despite suppression of HIV RNA.11 In the Hawaii Aging with HIV Cohort study (HAHC), DM was more frequent in patients with HIV-associated dementia (HAD) and a pattern of higher fasting glucose levels was noted with worsening cognition in participants without DM.12 A second analysis in this cohort confirmed an association between IR estimated by the Homeostasis Model Assessment of IR (HOMA) and global neuropsychological performance with emphasis noted on tests of psychomotor speed but not memory.13,14 Others have noted a relationship between components of the metabolic syndrome and stroke in HIV, a condition likely to impact neuropsychological performance,15 and a recent analysis in the Multicenter AIDS Cohort Study (MACS) identified a relationship between carotid intima media thickness and cognitive performance, raising concerns that the mechanism of injury may relate to cerebrovascular damage rather than direct effects of IR as postulated in HIV-uninfected populations.16,17
In this study, we sought to determine if IR was associated with impaired neuropsychological performance in the Women's Interagency HIV Study (WIHS). We also sought to determine if HIV status influenced this relationship.
Materials and Methods
The WIHS is a multicenter longitudinal observational study of HIV-infected and HIV-uninfected women. Subjects were enrolled at six sites in New York (Bronx and Brooklyn), California (Los Angeles and San Francisco), Washington, D.C., and Chicago. Details of the design are described elsewhere.18 Between October 2004 and March 2007 most women completed a brief neuropsychological testing battery consisting of the Trail Making Test (Parts A and B), a measure of processing speed and cognitive flexibility, and the Symbol Digit Modalities Test (SDMT), a measure of speed of information processing and perceptual motor ability (Analysis 1).19–21 Trail Making and SDMT were examined in relation to concurrent fasting insulin and glucose measures for 503 HIV-uninfected and 1201 HIV-infected subjects with those cognitive measures. Separately, the Comalli version of the Stroop task was completed between October 2006 and September 2008.22 Stroop performance was examined with concurrent fasting insulin and glucose levels available for 347 HIV-negative and 689 HIV-positive participants (Analysis 2).
The parent WIHS selection criteria exclude subjects with clinical dementia at the time of enrollment. All subjects were ambulatory, able to attend an outpatient study visit, and signed Institutional Review Board-approved consent forms. Subjects selected for these analyses met the following additional criteria: no history of diabetes, use of diabetes medication or current fasting glucose over 125 mg/dl, and education level greater than 6 years. Since some subjects had concurrent neuropsychological and fasting insulin/glucose testing done on more than one occasion, we used the first visit in this longitudinal study in which a subject had concurrent fasting insulin levels and cognitive testing. For many subjects, a different visit had to be used for Analysis 2 (Stroop measurements).
Standard detailed medication and medical history were obtained to capture information related to HIV disease stage and metabolic risk factors. Concurrent fasting lipid panels and blood pressures were obtained.
In Part A of the Trail Making Test, subjects were shown a paper displaying individual encircled numbers in a visual array, and they were instructed to rapidly connect the numbers in numerical order. In Part B, the paper displays an array of encircled numbers and letters. Subjects were instructed to draw a line alternating between numbers and letters in alphabetical and numerical order as quickly as possible (e.g., connect “1” to “A” to “2” to “B”). For both Parts, the outcome measure was the number of seconds necessary to complete the test. For the SDMT, subjects were shown a piece of paper displaying a key of nine symbols each paired with a number and below, a rows of numbers on top of empty boxes. Subjects were instructed to fill in the empty boxes with the symbols that correspond to the numbers. The outcome measure was the number of correctly transcribed symbol/number pairs in 90 s (maximum=110).
Trials 1 and 2 of the Stroop served as measures of psychomotor speed and attention. Subjects were instructed to name the color that matches the color within a displayed rectangle (trial 1) and to name the word that matches the displayed word (trial 2). The outcome measure was the number of seconds it takes to complete each trial. Trial 3 of the Stroop served as a measure of executive function, specifically inhibition. Subjects were instructed to name the print color of a series of color names while inhibiting the tendency to read the word (e.g., to say “red” when the word “blue” is shown in red-colored print). The outcome measure was the number of seconds needed to complete the trial.
All neuropsychological test measures were completed in English or Spanish, based on primary language of the subject (95% completed in English). Educational attainment was measured in years of formal education and further estimated IQ by the Wide Range Achievement Test (WRAT).23 The WRAT involves reading recognition, spelling, and arithmetic computation. The WRAT was included as a measure of educational attainment based on evidence that the WRAT and other literacy measures are a more valid index of educational experience than years of school among African Americans.24 HIV status was confirmed by FDA-approved enzyme-linked immunosorbent assay and confirmed with Western blot. Plasma HIV RNA levels and CD4 counts were completed using standard techniques. Insulin resistance was estimated using HOMA, which is defined as (insulin×glucose)/405 with insulin measured in μU/ml and glucose measured in mg/dl.13 Fasting specimens for glucose determination were collected in tubes with glycolytic inhibitors. Serum for insulin determination was obtained at the same time and all specimens were stored (−70°C) until the day of assay. Plasma glucose was measured using the hexokinase method and insulin was measured using the IMMULITE 2000 assay at a central laboratory (Quest Diagnostics, Baltimore, MD).
Statistical approach
We used Chi-squared and Mann–Whitney tests to compare variables among participants categorized by HOMA quartiles. For the primary analyses, we utilized raw neuropsychological data without z-transformation, with quartile of HOMA-IR as the primary independent variable of interest. We used quartiles rather than modeling HOMA as a continuous variable because of concerns about nonlinearity and because results would be more easily understandable than using HOMA-IR or a transformation of it as a continuous predictor. Because different visits were used for Analysis 1 and 2, we used different HOMA quartile categories for those analyses in order to keep group sizes approximately equal. Potential confounding variables were analyzed to determine the independent contribution of HOMA to each neuropsychological testing outcome in multivariate linear regression models. We included HOMA in all models and selected additional covariates forward step-wise with p<0.10 required for entry, and then evaluated each remaining unselected covariate as a single addition to the resulting multivariate models. Candidate variables included HIV status, suppression of plasma virus (<1000 copies/ml), hypertension and mean arterial blood pressure, smoking (both current and total pack-years), education, risk for acquiring HIV, current use of cocaine or heroin, language, study site, AIDS diagnosis, hepatitis C RNA positive, on HAART, fasting cholesterol, waist-hip ratio, weight, body mass index, and estimated IQ.
Results
One thousand five hundred and forty-seven subjects met inclusion criteria for Analysis 1 (Trails A, Trails B, SDMT). The mean age of the HIV-infected group was higher than that of the HIV-uninfected group (42±8.9 vs. 37±9.8 years, p<0.0001). As expected, increasing severity of IR correlated with higher frequency of other metabolic abnormalities (Table 1). cART use was more highly prevalent and mean CD4 lymphocyte counts were higher with increasing IR severity. Insulin resistance was also associated with hepatitis C. The sample size for Analysis 2 (Stroop) was smaller than that of Analysis 1; however, demographic and clinical variables were similar with women approximately 2 years older, as expected based on timing of these tests (data not shown).
Table 1.
Baseline Demographic and Clinical Constitution of the Women from Analysis 1 by HOMA-IR Quartile
| Lowest quartile | Second quartile | Third quartile | Highest quartile | p-value | |
|---|---|---|---|---|---|
| HOMA-IR range | 0.29–1.29 | 1.30–2.12 | 2.13–3.61 | 4.30–49.70 | |
| Sample size | 388 | 386 | 390 | 383 | |
| Age, mean±SD | 40.1±9.7 | 39.6±9.4 | 41.1±8.5 | 42.3±9.9 | 0.00031 |
| Education | 0.00072 | ||||
| Grades 7–11 | 34% | 35% | 33% | 37% | |
| Completed high school | 30% | 30% | 31% | 37% | |
| Some college | 25% | 30% | 26% | 23% | |
| Completed 4 years of college | 8% | 4% | 9% | 3% | |
| Attended/completed graduate | 4% | 1% | 2% | 1% | |
| Race | |||||
| White (non-Hispanic) | 51 (13.1%) | 41 (10.6%) | 52 (13.3%) | 29 (7.6%) | 0.0112 |
| White (Hispanic) | 29 (7.5%) | 45 (11.7%) | 38 (9.7%) | 36 (9.4%) | |
| African-American (non-Hispanic) | 248 (63.9%) | 227 (58.8%) | 235 (60.3%) | 223 (58.2%) | |
| African-American (Hispanic) | 16 (4.1%) | 11 (2.8%) | 8 (2.1%) | 16 (4.2%) | |
| Other (Hispanic) | 35 (9.0%) | 47 (12.2%) | 48 (12.3%) | 66 (17.2%) | |
| API | 5 (1.3%) | 4 (1.0%) | 0 (0.0%) | 2 (0.5%) | |
| Native American/Alaskan | 1 (0.3%) | 2 (0.5%) | 2 (0.5%) | 5 (1.3%) | |
| Other | 3 (0.8%) | 9 (2.3%) | 7 (1.8%) | 6 (1.6%) | |
| Primary language, % English | 98% | 93% | 94% | 94% | 0.0162 |
| HIV status, % HIV+ | 67% | 70% | 73% | 74% | 0.00252 |
| Primary HIV risk factor | 0.0972 | ||||
| Intravenous drug use | 18% | 17% | 23% | 25% | |
| Heterosexual risk | 36% | 40% | 37% | 37% | |
| Transfusion risk | 2% | 2% | 2% | 2% | |
| No identified risk | 44% | 41% | 39% | 35% | |
| Current use of heroin or cocaine | 15% | 11% | 8% | 9% | 0.00742 |
| Metabolic parameters | |||||
| BMI, mean±SD | 25.0±5.42 | 27.4±6.1 | 30.8±7.4 | 32.5±8.81 | <0.00011 |
| Hypertension | 33% | 33% | 39% | 54% | <0.00012 |
| Current smoker | 51% | 49% | 39% | 43% | 0.0292 |
| Fasting cholesterol (mg/dl), mean±SD | 173±40 | 173±38 | 176±42 | 178±42 | 0.0201 |
| HIV parameters | |||||
| On antiretroviral therapy | 59% | 61% | 64% | 70% | 0.0492 |
| CD4 count, mean±SD | 467±309 | 500±325 | 526±322 | 554±332 | 0.00741 |
| Plasma HIV RNA, mean±SD | 28,300±155,000 | 18,600±65,900 | 17,800±61,400 | 22,500±136,000 | 0.00751 |
| Hepatitis C positive RNA | 14% | 16% | 18% | 24% | 0.00112 |
p-values for differences among groups by 1Mann–Whitney test or 2Chi-square test.
HOMA-IR, homeostasis model assessment-insulin resistance;
API, Asian and Pacific Islander; BMI, body mass index.
In univariate linear models, increasing HOMA quartiles were statistically associated with poorer neuropsychological test performance on the SDMT, Stroop color-naming, and Stroop interference trials. In multivariate models, only the Stroop color-naming trial met statistical significance for the two highest quartiles of HOMA (72.0 s for highest and 70.9 s for the second highest compared to 64.8 s for the lowest quartile, Table 2). In secondary confirmatory analyses, we examined HOMA as a continuous predictor in univariate models. Here, the Stroop color-naming task [β=0.68 per 2-fold increase in HOMA (0.32 to 1.04, p=0.0002)] and the Stroop interference trial [β=0.86 (0.08 to 1.65, p=0.031)] reached statistical significance.
Table 2.
Univariate and Multivariate Models for Group Means on Cognitive Tests Associated with Quartile of HOMA Compared to the Lowest Quartile
| |
Estimated effect (95% confidence interval) p-value |
|
|---|---|---|
| Univariate model | Multivariate model | |
| Trails A | ||
| Second quartile | −2.79 (−5.01 to −0.57), 0.014 | −3.10 (−5.98 to −0.22), 0.035 |
| Third quartile | −1.52 (−3.74 to 0.70), 0.18 | − 1.77 (−4.59 to1.04), 0.22 |
| Highest quartile | −0.89 (−3.11 to 1.33), 0.43 | −0.72 (−3.30 to 1.86), 0.58 |
| Trails B | ||
| Second quartile | 0.90 (−5.96 to 7.76), 0.80 | 2.64 (−5.46 to 10.73), 0.52 |
| Third quartile | 1.79 (−5.07 to 8.64), 0.61 | 0.88 (−7.19 to 8.94), 0.83 |
| Highest quartile | 4.19 (−2.69 to 11.07), 0.23 | 2.80 (−5.83 to 11.43), 0.52 |
| Symbol-Digit Modalities Test | ||
| Second quartile | −1.73 (−3.48 to 0.03), 0.054 | −1.53 (−3.42 to 0.35), 0.11 |
| Third quartile | −0.53 (−2.28 to 1.23), 0.55 | 0.60 (−1.20 to 2.41), 0.51 |
| Highest quartile | −2.56 (−4.32 to −0.80), 0.004 | − 0.76 (−2.63 to 1.10), 0.42 |
| Stroop Color-Naming | ||
| Second quartile** | 4.26 (1.33 to 7.19), 0.0045 | 2.34 (−0.73 to 5.42), 0.13 |
| Third quartile | 6.04 (3.09 to 9.00), <0.0001 | 3.24 (0.05 to 6.44), 0.047 |
| Highest quartile | 7.17 (4.22 to 10.11), <0.0001 | 3.78 (0.48 to 7.08), 0.025 |
| Stroop Interference | ||
| Second quartile** | 7.67 (1.28 to 14.06), 0.019 | 4.35 (−2.88 to 11.57), 0.24 |
| Third quartile | 14.43 (8.00 to 20.87), <0.0001 | 4.98 (−2.49 to 12.45), 0.19 |
| Highest quartile | 13.12 (6.70 to 19.54), <0.0001 | 3.73 (−3.98 to 11.43), 0.34 |
Quartile categories for Stroop differ from those of Trails A, B, and SDMT (see Materials and Methods).
Variables included in each model: Trails A [estimated IQ, age, mean arterial blood pressure, body mass index (BMI), current smoking status], Trails B (estimated IQ, age, mean arterial blood pressure, weight, education, hepatitis C virus (HCV), research study site), Symbol Digit Modalities Test (SDMT) (estimated IQ, age, education, HCV, current use of cocaine or heroin, HIV status, research study site), Stroop color-naming (estimated IQ, age, waist-hip ratio, language of testing, HCV, research study site), Stroop interference (estimated IQ, age, pack-years of smoking, waist-hip ratio, research site).
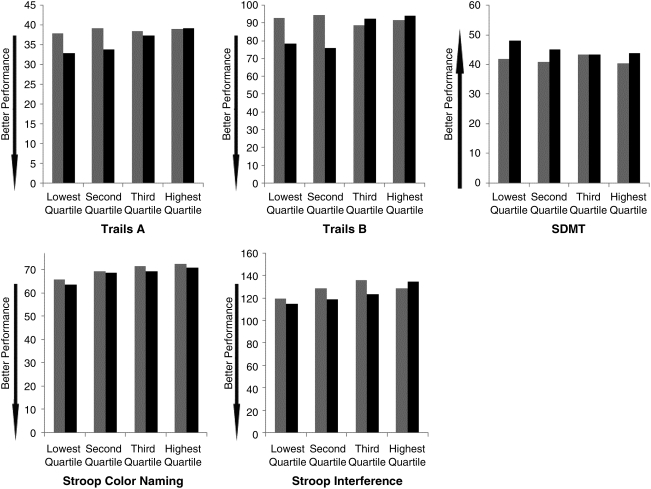
We investigated the possibility that the impact of IR differed by HIV status by adding an HIV*HOMA interaction variable to the final multivariate models. HIV status did not substantially modify the relationship for Stroop color-naming in models that included an interaction variable, although there was substantial uncertainty in the interaction estimates. A statistically significant interaction (p=0.025) was noted for the Stroop interference trial (compared to lowest quartile, second quartile effect 0.6 among HIV-uninfected compared to 5.7 among HIV-infected women; third quartile: −2.0 in HIV-uninfected compared to 7.6 among HIV-infected women; highest quartile: 12.7 among HIV-uninfected compared to −0.3 among HIV-infected women). On other models, no interaction effects were statistically significant, although confidence intervals did include substantial values in all cases. Examining unadjusted raw neuropsychological data, we noted a pattern suggestive of poorer performance on most tests with increasing quartile of HOMA among HIV-uninfected women but only on the SDMT, Stroop color-naming, and interference trial for HIV-infected women (Fig. 1).
FIG. 1.
Mean raw neuropsychological testing scores by quartile of insulin resistance (HOMA) and HIV status. Better performance represented by lower scores on Trails A, Trails B, and Stroop (time to complete task) and by higher scores on the Symbol Digit Modalities Test (tasks completed in 90 s). HIV infected in gray bars, HIV uninfected in black bars.
Discussion
This cross-sectional analysis of a large group of women at risk for IR identified an association between IR and neuropsychological testing performance; however, the effects are diminished substantially when controlling for other factors. Only the color-naming trial of the Stroop test retained statistical significance in adjusted models. While we limited our number of comparisons and included measures of cognitive domains shown in previous studies to be related to IR, we are not aware of any a priori reason why the color-naming test would be more influenced by HOMA than the other measures, so this could be a random occurrence despite having p<0.05. Our analyses could not rule out HIV*HOMA interaction effects on the Stoop color-naming trial as there was substantial uncertainty in the estimates. Interaction effects were suggested in other models, particularly the Stroop interference trial.
These effects are small; however, the study is limited in scope of neuropsychological testing performed. The mechanism by which peripheral IR may impact brain function is unclear. Numerous mechanisms have been postulated including the facilitative impact of insulin on memory performance and hindrance in the face of IR, the influence of glycosylated end products, concurrent inflammation, and competitive interactions with insulin degradation enzyme, an enzyme also involved in brain amyloid processing.17 Our study did not directly investigate the etiology of insulin resistance in this population, a topic previously addressed in the WIHS.5
In HIV-uninfected individuals, involvement of frontal and hippocampal circuits appears to be important. Peripheral IR correlates to a decreased global metabolic rate measured by [18F]fluorodeoxyglucose positron emission tomography with more dramatic effects noted in the amygdala and hippocampus.25 Congruent with this finding, neuropsychological deficits often note abnormalities in declarative memory tasks and therapeutic interventions manipulating plasma insulin levels result in enhanced memory performance.26 In women at risk for AD, IR correlates to lower hippocampal volumes.10 Regional atrophy of the medial temporal lobes has been described in DM.27 Knowledge that hippocampal and limbic structures are highly concentrated with insulin receptors provides added basis for physiological–anatomical correlations to these findings.28
In this retrospective analysis of mostly HIV-infected women, our limited battery did not include tests of memory. It is somewhat unexpected that correlations between IR and executive functioning were not noted. Our battery included several tests of psychomotor speed, an area of function previously thought to be associated with IR in HIV patients14; however, only the color-naming trial showed a statistically significant relationship with IR. Although this test would be expected to represent psychomotor speed capabilities, a lack of a substantial relationship in other tests that would be more specific to this domain (SDMT, Trails A) brings this finding into question. A study within this cohort to examine IR with a broader cognitive battery has been initiated. It is possible that the impact of IR is modulated in HIV by coexisting morbidity. The group with the worst IR also appeared to have higher CD4 counts and was more commonly on cART; however, supplemental evaluations of these factors did not substantially alter our main findings concerning HOMA -IR.
In HIV, it remains possible that the relationship between IR and cognitive performance is largely due to associated risk for cerebrovascular injury. An evaluation from the MACS supports this assertion with correlations to cognition noted in relation to carotid intima-media thickness.16 The tightly bound relationship of cerebrovascular injury and aspects of the metabolic syndrome has been noted in HIV-negative populations; however, statistically significant associations to cognitive performance are generally identified only for hypertension and IR.29 Hypertension was introduced into our analysis; however, it did not meet criteria for inclusion in the final models, although mean arterial blood pressure was included as a numeric predictor in final models for Trails A and Trails B tests, with higher values associated with worse scores. Given that these women are relatively younger, it remains possible that more robust findings will be found in populations of older women. The cooccurrence of IR and other metabolic derangements in HIV raises added concerns for this vulnerable population.11 A follow-up study within this cohort that will examine these issues with an extended battery is underway.
Acknowledgments
We thank the WIHS participants, K23AG032872 (V.V.), The Larry L. Hillblom Foundation (V.V.), The UCSF Alzheimer's Disease Research Center (3 P50 AG023501, Bruce Miller), and RR024131 (CTSA). Data in this paper were collected by the Women's Interagency HIV Study (WIHS) Collaborative Study Group with centers (Principal Investigators) at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff); Washington DC, Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); and Data Coordinating Center (Stephen Gange). The WIHS is funded by the National Institute of Allergy and Infectious Diseases (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590) and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (UO1-HD-32632). The study is cofunded by the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Deafness and Other Communication Disorders. Funding is also provided by the National Center for Research Resources (UCSF-CTSI Grant UL1 RR024131). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
These data were presented in abstract form at the 18th Conference on Retroviruses and Opportunistic Infections (Paper #391), Boston, MA.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Flegal KM. Carroll MD. Ogden CL. Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Tien PC. Schneider MF. Cole SR, et al. Antiretroviral therapy exposure and insulin resistance in the Women's Interagency HIV study. J Acquir Immune Defic Syndr. 2008;49:369–376. doi: 10.1097/qai.0b013e318189a780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shikuma CM. Yang Y. Glesby MJ, et al. Metabolic effects of protease inhibitor-sparing antiretroviral regimens given as initial treatment of HIV-1 Infection (AIDS Clinical Trials Group Study A5095) J Acquir Immune Defic Syndr. 2007;44:540–550. doi: 10.1097/QAI.0b013e318031d5a0. [DOI] [PubMed] [Google Scholar]
- 4.Schambelan M. Benson CA. Carr A, et al. Management of metabolic complications associated with antiretroviral therapy for HIV-1 infection: Recommendations of an International AIDS Society–USA panel. J Acquir Immune Defic Syndr. 2002;31:257–275. doi: 10.1097/00126334-200211010-00001. [DOI] [PubMed] [Google Scholar]
- 5.Tien PC. Schneider MF. Cole SR, et al. Antiretroviral therapy exposure and incidence of diabetes mellitus in the Women's Interagency HIV Study. AIDS. 2007;21:1739–1745. doi: 10.1097/QAD.0b013e32827038d0. [DOI] [PubMed] [Google Scholar]
- 6.Profenno LA. Porsteinsson AP. Faraone SV. Meta-analysis of Alzheimer's disease risk with obesity, diabetes, and related disorders. Biol Psychiatry. 2010;67:505–512. doi: 10.1016/j.biopsych.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Schuur M. Henneman P. van Swieten JC, et al. Insulin-resistance, metabolic syndrome are related to executive function in women in a large family-based study. Eur J Epidemiol. 25:561–568. doi: 10.1007/s10654-010-9476-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stolk RP. Breteler MM. Ott A, et al. Insulin and cognitive function in an elderly population. The Rotterdam Study. Diabetes Care. 1997;20:792–795. doi: 10.2337/diacare.20.5.792. [DOI] [PubMed] [Google Scholar]
- 9.Bruehl H. Sweat V. Hassenstab J. Polyakov V. Convit A. Cognitive impairment in nondiabetic middle-aged and older adults is associated with insulin resistance. J Clin Exp Neuropsychol. 32:487–493. doi: 10.1080/13803390903224928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rasgon NL. Kenna HA. Wroolie TE, et al. Insulin resistance and hippocampal volume in women at risk for Alzheimer's disease. Neurobiol Aging. 2011;32(11):1942–1948. doi: 10.1016/j.neurobiolaging.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valcour V. Shikuma C. Watters M. Sacktor N. Cognitive impairment in older HIV-1-seropositive individuals: Prevalence and potential mechanisms. AIDS. 2004;18(Suppl 1):S79–86. doi: 10.1097/00002030-200401001-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valcour V. Shikuma CM. Shiramizu BT, et al. Diabetes, insulin resistance, and dementia among HIV-1-infected patients. J Acquir Immune Defic Syndr. 2005;38:31–36. doi: 10.1097/00126334-200501010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matthews DR. Hosker JP. Rudenski AS. Naylor BA. Treacher DF. Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 14.Valcour V. Sacktor NC. Paul RH, et al. Insulin resistance is associated with cognition among HIV-1-infected patients: The Hawaii Aging With HIV Cohort. J Acquir Immune Defic Syndr. 2006;43:405–410. doi: 10.1097/01.qai.0000243119.67529.f5. [DOI] [PubMed] [Google Scholar]
- 15.Ances BM. Vaida F. Rosario D, et al. Role of metabolic syndrome components in HIV-associated sensory neuropathy. AIDS. 2009;23:2317–2322. doi: 10.1097/QAD.0b013e328332204e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Becker JT. Kingsley L. Mullen J, et al. Vascular risk factors, HIV serostatus, and cognitive dysfunction in gay and bisexual men. Neurology. 2009;73:1292–1299. doi: 10.1212/WNL.0b013e3181bd10e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Craft S. Watson GS. Insulin and neurodegenerative disease: Shared and specific mechanisms. Lancet Neurol. 2004;3:169–178. doi: 10.1016/S1474-4422(04)00681-7. [DOI] [PubMed] [Google Scholar]
- 18.Barkan SE. Melnick SL. Preston-Martin S, et al. The Women's Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9:117–125. [PubMed] [Google Scholar]
- 19.Reitan R, editor. Manual for Administration of Neuropsychological Test Batteries for Adults and Children. Neuropsychological Laboratories, Inc.; Tucson, AZ: 1978. [Google Scholar]
- 20.Heaton R, editor; Grant I, editor; Matthews C, editor. Comprehensive Norms for an Expanded Halstead-Reitan Battery. Psychological Assessment Resources, Inc.; Odessa, FL: 1991. [Google Scholar]
- 21.Smith WL. Philippus MJ. Guard HL. Psychometric study of children with learning problems and 14-6 positive spike EEG patterns, treated with ethosuximide (Zarontin) and placebo. Arch Dis Child. 1968;43:616–619. doi: 10.1136/adc.43.231.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Comalli PE., Jr Wapner S. Werner H. Interference effects of Stroop color-word test in childhood, adulthood, and aging. J Genet Psychol. 1962;100:47–53. doi: 10.1080/00221325.1962.10533572. [DOI] [PubMed] [Google Scholar]
- 23.Wilkinson: Wide Range Achievement Test 3—Administrative Manual. Jastak Associates, Inc.; Wilmington, DE: 1993. [Google Scholar]
- 24.Manly JJ. Touradji P. Tang MX. Stern Y. Literacy and memory decline among ethnically diverse elders. J Clin Exp Neuropsychol. 2003;25:680–690. doi: 10.1076/jcen.25.5.680.14579. [DOI] [PubMed] [Google Scholar]
- 25.Anthony K. Reed LJ. Dunn JT, et al. Attenuation of insulin-evoked responses in brain networks controlling appetite and reward in insulin resistance: The cerebral basis for impaired control of food intake in metabolic syndrome? Diabetes. 2006;55:2986–2992. doi: 10.2337/db06-0376. [DOI] [PubMed] [Google Scholar]
- 26.Craft S. Asthana S. Cook DG, et al. Insulin dose-response effects on memory and plasma amyloid precursor protein in Alzheimer's disease: Interactions with apolipoprotein E genotype. Psychoneuroendocrinology. 2003;28:809–822. doi: 10.1016/s0306-4530(02)00087-2. [DOI] [PubMed] [Google Scholar]
- 27.den Heijer T. Vermeer SE. van Dijk EJ, et al. Type 2 diabetes and atrophy of medial temporal lobe structures on brain MRI. Diabetologia. 2003;46:1604–1610. doi: 10.1007/s00125-003-1235-0. [DOI] [PubMed] [Google Scholar]
- 28.Unger JW. Livingston JN. Moss AM. Insulin receptors in the central nervous system: localization, signalling mechanisms and functional aspects. Prog Neurobiol. 1991;36:343–362. doi: 10.1016/0301-0082(91)90015-s. [DOI] [PubMed] [Google Scholar]
- 29.Gatto NM. Henderson VW. St John JA. McCleary C. Hodis HN. Mack WJ. Metabolic syndrome and cognitive function in healthy middle-aged and older adults without diabetes. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2008;15:627–641. doi: 10.1080/13825580802036936. [DOI] [PMC free article] [PubMed] [Google Scholar]