Abstract
Pediatric HIV-1 infection is characterized by rapid disease progression and without antiretroviral therapy (ART), more than 50% of infected children die by the age of 2 years. However, a small subset of infected children progresses slowly to disease in the absence of ART. This study aimed to identify functional characteristics of HIV-1-specific T cell responses that distinguish children with rapid and slow disease progression. Fifteen perinatally HIV-infected children (eight rapid and seven slow progressors) were longitudinally studied to monitor T cell polyfunctionality. HIV-1-specific interferon (IFN)-γ+ CD8+ T cell responses gradually increased over time but did not differ between slow and rapid progressors. However, polyfunctional HIV-1-specific CD8+ T cell responses, as assessed by the expression of four functions (IFN-γ, CD107a, TNF-α, MIP-1β), were higher in slow compared to rapid progressors (p=0.05) early in infection, and was associated with slower subsequent disease progression. These data suggest that the quality of the HIV-specific CD8+ T cell response is associated with the control of disease in children as has been shown in adult infection.
According to UNAIDS, an estimated 370,000 children became newly infected with HIV in 20071 and HIV-1 infection is the underlying reason for more than one-third of deaths in children younger than 5 years. The majority of these infections are through mother-to-child-transmission. In contrast to adults, the natural history of HIV-1 disease is very rapid in children, and without antiretroviral treatment (ART), more than 50% of HIV-1-infected children will die within the first 2 years of life.2–4 Several factors might account for this rapid disease progression in children, including their immature neonatal immune system and the transmission of viruses that have adapted to the mother's HLA class I genotype and include viral escape variants within cytotoxic T lymphocyte (CTL) epitopes restricted by those HLA class I alleles shared between mother and child.4–6 However, a small proportion of children can control the virus for years without the need of ART. The correlates of this delayed disease progression in children are not understood.
Superior control of HIV-1 replication in long-term nonprogressors has been associated with CD8+ T cells that express multiple effector functions upon recognition of cognate antigen.7–9 In addition, the functional capabilities of the T cells in response to antigen can be influenced by several factors, including the intensity of T cell receptor (TCR)-mediated signaling and antigen sensitivity.10–12 Whereas individuals who progress slowly to disease (long-term nonprogressors) display polyfunctional CD8+ T cells, polyfunctionality has also been shown to depend inversely on viral load; thus it is still unclear whether polyfunctionality is a cause or effect of viral control.13,14 Very little is known about the characteristics of T cell polyfunctionality in children during the transition from early to chronic pediatric HIV infection. Here, we analyzed T cell functionality in HIV-1-infected children with rapid and slow disease progression over the first 3–4 years of life, using samples from a previously described pediatric cohort.6,15 We observed that children with delayed disease progression had more polyfunctional HIV-1-specific CD8+ T cells than children with rapid disease progression. These studies suggest that the quality of the antiviral CD8+ T cell response can make an important contribution to outcome in pediatric HIV-1 infection.
The study was approved by the Ethics Committee of the University of KwaZulu-Natal and the Institutional Review Board of the Massachusetts General Hospital. The mothers gave written informed consent for participation of their children in the study.
Fifteen perinatally HIV-1-infected infants were longitudinally studied from birth. This pediatric study cohort including exclusion and inclusion criteria has been previously described.6,15–17 Briefly, we enrolled HIV-1-infected infants born to HIV-1-infected mothers at St. Mary's and Prince Mshiyeni hospitals in Durban, South Africa between 2003 and 2005. The HIV-1-seropositive mothers were recruited in the last trimester of pregnancy. The mothers and children received single-dose nevirapine at the onset of labor and within 48 h of birth, respectively, according to the HIVNET 012 protocol.18,19
Study participants were subsequently enrolled into a clinical study, as described previously,16 and were either followed longitudinally prior to initiation of deferred ART (arm A) or received 1 year of ART following diagnosis at birth (arm B). All infants enrolled onto the study received long-term ART once World Health Organization (WHO) clinical and/or immunological criteria for antiretroviral treatment were met.20 Taking into account that 85% of HIV-1-infected children progressed to CD4 ≤20% by 1 year in the absence of ART,16 we defined rapid progressors as children who met WHO criteria to receive ART within 12 months of birth or within 12 months of ART cessation, and slow progressors as children not meeting WHO criteria to receive ART for ≥36 months. Because progression was rapid in the absence of ART in the majority of these children, we analyzed the remaining seven children who did not require ART after 36 months (slow progressors) and comparison was made with eight children who progressed rapidly to disease by 12 months in the absence of ART (rapid progressors). The peripheral blood mononuclear cell (PBMC) samples from the 15 individuals were assayed at three time points for CD8+ T cell polyfunctionality: early (median of 4 months, range 2–6 months), intermediate (median of 16 months, range 13–23 months), and late (median of 38 months, range 25–55 months). Clinical characteristics of the study participants are shown in Table 1.
Table 1.
Characteristics of the Study Participants
| |
|
Early |
Intermediate |
Late |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Participants | HLA type | Age (months) | VLa | CD4%/Abs | Age (months) | VL | CD4%/Abs | Age (months) | VL | CD4%/Abs |
| Arm A (n=6) | ||||||||||
| Slow-progressors | ||||||||||
| A-517C | A3002/6802 B1510/4201 Cw03/1701 |
4 | 5,620,000 | 25/2671 | 18 | >750000 | 27/1745 | 48 | 40,535 | 36/1716 |
| A-133C | A6801/7408 B5802/8101 Cw04/0602 |
6 | 180,000 | 38/2884 | 16 | 40,600 | 32/2130 | 55 | 18,300 | 32/1612 |
| Rapid-progressors | ||||||||||
| A-298C | A29/6802 B13/1401 Cw06/08 |
6 | 318,000 | 11/831 | 14b | 1,050 | 17/1716 | 33 | <50 | 23/2220 |
| A-562C | A0205/3002 B1402/5801 Cw07/0802 |
6 | 11,860 | 10/581 | 13 | 519 | 29/2316 | 27 | <50 | 27/2460 |
| A-458C | A4301/6802 B1503/1510 Cw03/18 |
3 | 583,000 | 17/1198 | 20 | <50 | 43/2333 | 29 | <50 | 48/2653 |
| A-447C | A03/6802 B1401/4701 Cw0602/0802 |
2 | 7,820,000 | 25/1206 | 20 | 717 | 33/1709 | 25 | <50 | 34/1617 |
| Arm B (n=9) | ||||||||||
| Slow-progressors | ||||||||||
| B-114C | A0301/24 B07/08 Cw07/07 |
N/Ac | N/A | N/A | 23 | 249,000 | 24/1761 | 46 | 11,800 | 19/702 |
| B-380C | A2301/6802 B1510/1510 Cw03/08 |
5 | 101 | 40/2214 | 20 | 65,200 | 31/1352 | 38 | 373,000 | 30/790 |
| B-559C | A2301/6802 B0801/5801 Cw07/07 |
4 | <50 | 53/2433 | 21 | 973,000 | 25/1310 | 38 | 356,000 | 21/1275 |
| B-586C | A0101/6802 B1510/8101 Cw08/1801 |
2 | 387 | 38/2620 | 15 | 137,000 | 30/2896 | 42 | >750000 | 18/922 |
| B-021C | A0205/29 B4201/44 Cw0202/1701 |
5 | <50 | 33/1587 | 16 | >750000 | 33/2179 | 38 | 99,895 | 24/1435 |
| Rapid-progressors | ||||||||||
| B-001C | A0101/3001 B4201/8101 Cwl7/1801 |
4 | <50 | 54/3137 | 21 | 236,000 | 25/1898 | 47 | <50 | 29/1409 |
| B-222C | A29/6802 B0702/1302 Cw0602/07 |
N/A | N/A | N/A | 13 | 56,900 | 11/233 | N/A | N/A | NA |
| B-675C | A6601/6802 B1510/39 Cw03/12 |
4 | 1,290 | 47/2194 | 16 | 714,000 | 14/1540 | 37 | <50 | 37/2666 |
| B-732C | A3002/6602 B4201/45 Cwl601/1701 |
3 | 2,000 | 22/1873 | 15 | 654,000 | 17/1032 | N/A | N/A | NA |
Plasma HIV RNA measurement; dilutions for quantification of high viral load (VL) >750,000 was done for some and not all patients due to inadequate sample availability.
Terms in bold indicate time on ART, applicable to Arm A rapid-progressor children at the intermediate and late time points; all Arm B children at the early time point and Arm B rapid-progressors at the late time point.
N/A, sample not available for measurement.
Plasma viral loads were measured using the Roche Amplicor Monitor assay (version 1.5) and CD4 counts were determined from fresh whole blood using Tru-Count technology as previously described.15
DNA for HLA typing was done by DNA polymerase chain reaction (PCR) using sequence-specific primers as previously described.21
Ex vivo measurement of T cells for interferon (IFN)-γ production was undertaken using an IFN-γ ELISPOT assay as previously reported.6,15,21 A panel of 410 overlapping peptides (18-mers with a 10 amino acid overlap) spanning the entire HIV-1 clade C consensus sequence and defined optimal peptides matching CD8+ T cell epitopes described for the HLA of the patients were used in the ELISPOT assays.
Ex vivo measurement of T cells for expression of multiple effector markers was undertaken. In brief, freshly thawed cryopreserved PBMCs were resuspended to 1–2×106 cells/ml in R10 media [RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum (FCS), 100 U/ml penicillin, 1.7 mM sodium glutamate, and 5.5 ml HEPES buffer] and rested for 2 h at 37○C; 5% CO2. One million cells were stimulated with 2 μg/ml of each peptide corresponding to described optimal CD8+ T cell epitopes that represented immunodominant responses in the study subjects (see Table 2; Fig. 1B) in the presence of costimulatory antibodies (anti-CD49 and anti-CD28, 1 μg/ml; BD Biosciences). Negative control tube with PBMCs alone and positive control containing PBMCs stimulated with 2 μg/ml of phorbol myristate acetate (PMA) and 1 μl of ionomycin (1 mg/ml; AG scientific) were included in the assays. Anti-CD107a-PE-Cy7 (BD-Biosciences) antibody was added and incubated for 30 min at 37○C, 5% CO2, followed by the addition of Brefaldin A (10 μg/ml, Sigma-Aldrich, St Louis, MO) and Golgi stop (2.5 μg/ml, Sigma-Aldrich) and incubated at 37○C, 5% CO2, for a total of 6 h. Cells were then washed with phosphate-buffered saline (PBS; 2% PBS/FCS), stained for intracellular amine groups to differentiate between live and dead (violet viability dye (Invitrogen), and incubated for 30 min at 4○C. Following incubation, cells were washed and T cell functionality was assessed by staining for four functions using the following antibody panel: anti-CD19 Pacific Blue (Caltag), anti-CD3-PE Cy5.5 (Caltag), anti-CD4-APC, anti-CD8-APC Cy7, anti-IFN-γ-PE CY7, anti-TNF α-Alexa 700, and anti-MIP-1β-PE (all from BD Biosciences) as described previously.14 Cells were washed and fixed in 1% paraformaldehyde (Fix Perm A, Caltag) for 20 min. Following washing in PBS, cells were permeabilized (Fix Perm B, Caltag) and stained intracellularly with the following antibodies: anti-IFN-γ PE Cy7, anti-TNF-α-Alexa 700, and anti-MIP-1β PE; they were then incubated for 20 min in the dark at room temperature, washed twice with PBS, and resuspended in 200 μl of PBS before acquisition on LSRII.
Table 2.
Intracellular IFN-γ Measurement of CD8+ T Cell in Response to Dominant Epitopes Targeted by Slow- and Rapid-Progressor Children over Time of Infection
| Participants | Optimal epitope | Epitope sequence | Early | Intermediate | Late |
|---|---|---|---|---|---|
| % IFN-γ+ CD8+ T cells | |||||
| Arm A (n=6) | |||||
| A-517C | B42-TL9 (Gag) | TPQDLNTML | 0.00 | 0.33 | 0.11 |
| CW3-YL9 (Gag) | YVDRFFKTL | 0.16 | 0.00 | 0.00 | |
| A-133C | B81-TL9 (Gag) | TPQDLNTML | 0.08 | 0.37 | 1.05 |
| A-298-C | A29-SY9 (Env) | SFDPIPIHY | 0.45 | 0.00 | 0.04 |
| A29-RW10 (Gag) | RQANFLGKIW | 0.02 | 0.00 | 0.12 | |
| A-562C | B58-TW10 (Gag) | TSTLQEQIAW | 0.16 | 0.06 | 0.07 |
| A-458C | CW3-YL9 (Gag) | YVDRFFKTL | 0.36 | 0.11 | 0.00 |
| A-447C | CW8-TL9 (Gag) | TPQDLNTML | 0.86 | 0.11 | 0.00 |
| Arm B (n=9) | |||||
| B-114C | CW7-KY11 (Nef) | KRQEILDLWVY | NDa | 0.16 | 2.35 |
| A23-RW8 (Nef) | RYPLTFGW | ND | 0.32 | 0.26 | |
| B-380C | B1510-GL9 (Gag) | GHQAAMQML | 0.00 | 0.46 | 1.70 |
| B1510-VL10 (Gag) | VHQAISPRTL | 0.00 | 0.13 | 0.20 | |
| A23-RW8 (Nef) | RYPLTFGW | 0.00 | 0.22 | 0.96 | |
| B-559C | B58-TW10 (Gag) | TSTLQEQIAW | 0.10 | 0.12 | 0.40 |
| CW7-KY11 (Nef) | KRQEILDLWVY | 0.00 | 3.10 | 0.96 | |
| B58-KW11 (Env) | KAYETEVHNVW | 0.14 | 1.56 | 0.25 | |
| B58-KAF9 (Nef) | KAAFDLSFF | 0.42 | 0.81 | ND | |
| B-586C | B81-TL9 (Gag) | TPQDLNTML | 0.05 | 0.64 | 0.52 |
| B-021C | A2-YI9 (Pol) | YTAFTIPSI | 0.06 | 2.18 | 0.08 |
| A29-SY9 (Env) | SFDPIPIHY | 0.28 | 1.93 | 0.16 | |
| B42-TL9 (Gag) | TPQDLNTML | 0.00 | 0.39 | 0.74 | |
| B-001C | B81-TL9 (Gag) | TPQDLNTML | 0.00 | 3.17 | 0.47 |
| B-222C | B7-TL10 (Nef) | TPGPGVRYPL | ND | 0.12 | ND |
| B-675C | CW3-YL9 (Gag) | YVDRFFKTL | 0.09 | 3.42 | 0.19 |
| B-732C | B45-GV11 (Nef) | GEVGFPVRPQV | 0.00 | 0.73 | ND |
| B7-RI10 (Env) | RPNNNTRKSI | 0.00 | 1.19 | ND | |
Dotted line separates slow-progressor from rapid-progressor children in both Arm A and Arm B groups. Terms in bold indicate time on ART as detailed in Table 1.
ND, not determined.
IFN-γ, interferon γ.
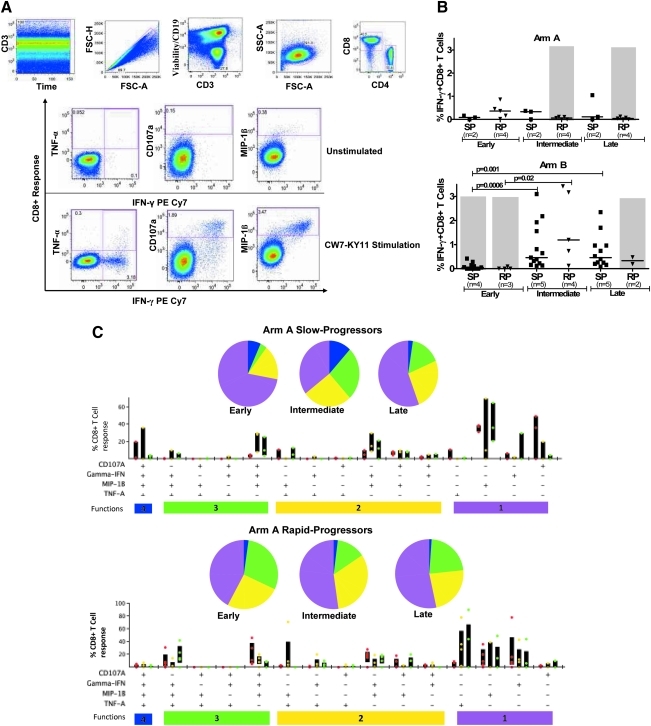
FIG. 1.
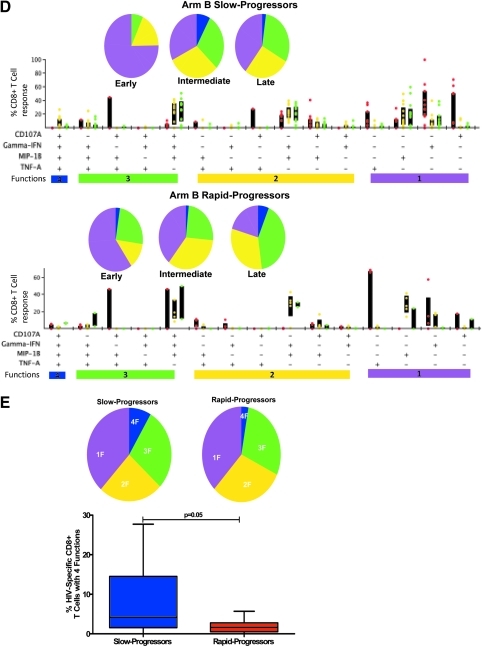
Measurement of HIV-1-specific CD8+ T cell functionality by multicolor staining in slow-progressor and rapid-progressor children. (A) Representative gating scheme for identification of polyfunctional HIV-1-specific CD8+ T cells following stimulation with HIV-1 peptide is shown for one individual. Initial gating was on the CD3 versus time to eliminate random laser events, followed by removal of doublets by forward scatter height (FSC-H) versus area (FSC-A). Live/dead and B cells were discriminated by the use of viability dye and anti-CD19, respectively, versus CD3; subsequently the lymphocyte population was gated, followed by identification of CD8+ and CD4+ T cells. Gates for each of the measured four CD8+ T cell functions were set based on the negative control (unstimulated, media only). Boolean gating was used to create a full array of the 16 possible response patterns when testing four functions. (B) Longitudinal intracellular measurement of interferon (IFN)-γ expression by CD8+ T cells. Measurement of CD8+ IFN-γ+ CD8+ T cells by intracellular cytokine staining (ICS) in arm A (top panel) and arm B (bottom panel) slow (SP) and rapid-progressor (RP) children throughout the first 3–4 years of life. Dot plots show median values of the different epitopes (as shown in Table 2) targeted by different individuals in each group. The shaded area in gray shows time on antiretroviral therapy (ART). (C, D) Representative functional composition of HIV-1-specific CD8+ T cell responses in slow-progressor and rapid-progressor children. The functional flavor of the individual response patterns to IFN-γ, MIP-1β, CD107a, and TNF-α is denoted by colored dots; red (early), yellow (intermediate), and green (late time points) for arm A (C) and arm B (D) children. The proportion contributed by the response pattern from slow-progressor (top panel) and rapid-progressor (bottom panel) individuals and the interquartile ranges are shown. The response patterns for each individual and the epitopes studied (as depicted in Table 2) were grouped and color coded by the number of functions and summarized in pie chart form where each slice of the pie represents the fraction of the total epitope-specific response that consist of CD8+ T cells with the respective number of functions. (E) Summarized comparison of the overall proportions of polyfunctional CD8+ T cells in slow-progressor and rapid-progressor children. Top panel: Polyfunctionality as depicted on the pie charts was obtained by grouping slow-progressors (left pie chart) and rapid-progressors (right pie chart) at comparable time points while not on ART. For arm A this was the early time point (slow progressors, n of individuals=2, n of epitopes=3; rapid progressors, n of individuals=4, n of epitopes=5) and for arm B this was the intermediate time-point (slow progressors, n of individuals=4, n of epitopes=13; rapid progressors, n of individuals=4, n of epitopes=5). The pie charts represent the medians of the different functions for the two groups at the stipulated time points. Bottom panel: The contribution of HIV-1-specific CD8+ T cells with four functions to the overall virus-specific CD8+ T cell response was compared between the combined slow (n of individuals=7, n of epitopes=16) and rapid (n of individuals=8, n of epitopes=10) progressor children.
Between 500,000 and 1,000,000 events were acquired per sample and data analysis was performed using FlowJo version 8.8.2 (TreeStar, Inc.). Initial gating was on CD3 versus time to eliminate random laser events, followed by removal of doublets by forward scatter height (FSC-H) versus area (FSC-A). Live/dead and B cells were discriminated by the use of viability dye and CD19 in the Pacific Blue channel, respectively; subsequently the lymphocyte population was gated, followed by identification of CD8+ and CD4+ T cells. For functionality analysis, gates for each of the measured four CD8+ T cell functions (IFN-γ, TNF-α, MIP-1β, and CD107a) were set based on the negative control (unstimulated, media only).
Boolean gating was used to create a full array of the 16 possible response patterns when testing four functions. Data reported here are after background subtraction and were further analyzed using SPICE 5 and PESTLE software programs (provided by Mario Roederer and Joshua Nozzi, NIAID, NIH).
Statistical analyses and graphs were plotted on Graphpad prism (version 5). The Mann–Whitney test was used to compare differences between the medians in the expression of IFN-γ by T cells between slow- and rapid-progressor children. The same test was also used to compare differences between the medians in proportions of CD8+ T cells expressing multiple functions.
Nine of the 15 enrolled infants described here received short-term ART in the first 12 months of life as part of the primary clinical study; the remaining infants did not receive immediate ART upon diagnosis.16 Children were assigned into one of four groups (Table 1) on the basis of whether they were randomized into early ART or not and whether or not they remained off ART for >36 months. We previously reported in this same cohort of children that the majority of in utero-infected infants mount CD8+ T cell responses in the first days of life15; however, disease progression is still rapid in most untreated HIV-1-infected children.16 We first monitored the persistence of HIV-1-specific T cell responses over the first 3–4 years postinfection using the IFN-γ ELISPOT assay in the 15 slow-progressor and rapid-progressor children, and confirmed immunodominant responses by flow cytometry using intracellular cytokine staining (ICS) (Table 2). IFN-γ+ CD8+ T cell responses (gated as shown in Fig. 1A) increased over the course of infection in untreated children, and decreased following initiation of ART. However, no significant differences in the studied IFNγ+ CD8+ T cell responses were noted between slow-progressor and rapid-progressor children (Fig. 1B).
Untreated children (all arm A) had significantly higher IFN-γ-producing CD8+ T cells than the children who received immediate ART upon diagnosis (all arm B) at the early time point (median 0.16% vs. 0.00%; p=0.036; Mann–Whitney, data not shown); in addition, there was a significant increase in the responses in arm B children when treatment was stopped at the intermediate time point (median 0.0% vs. 0.7%; p<0.0001, Mann–Whitney, data not shown), resulting in higher HIV-1-specific IFN-γ+ CD8+ T cell responses in children in arm B who discontinued ART compared to those in the untreated group (median 0.16% vs. 0.7%; p=0.024, Mann–Whitney, data not shown). Due to the small volumes of blood that was received from HIV-1-infected children, we were not able to perform ICS and ELISPOT analysis at the same time point for most samples. At those occasions in which we were able to perform both assays, the results of the assays were closely correlated (p<0.0001, R=0.7). Taken together, these data demonstrate dynamic changes in the frequency of IFN-γ+ HIV-1-specific CD8+ T cell frequencies in response to HIV-1 replication, but the magnitude of these IFN-γ+ responses did not allow differentiation between rapid and slow progressors.
Because IFN-γ expression alone could not discriminate disease outcome in this cohort of HIV-1-infected children, we next evaluated T cell polyfunctionality (as represented in Fig. 1A), defined as the ability of HIV-1-specific CD8+ T cells to produce multiple cytokines and chemokines in response to viral antigen. HIV-1-specific CD8+ T cell polyfunctionality has previously been associated with viral control in adults.8 Slow progressors in the delayed treatment group displayed more polyfunctional (four functions) CD8+ T cells in the first 6 months of life than rapid progressors, which increased at the intermediate time point and decreased at the last time point. Rapid progressors in the same group had less polyfunctional HIV-1-specific CD8+ T cells than slow progressors, which decreased with disease progression and did not improve with ART at the intermediate and late time points (Fig. 1C). Slow progressors in the immediate treated group had more monofunctional to three function-expressing T cells at the early time point while on ART, and gained more functions (up to four functions) only when viral load increased at the intermediate time point, subsequently decreasing with disease progression at the late time point. The proportion of ≤3 functions did not differ between slow and rapid progressors in the immediate treated group; however, CD8+ T cells expressing four functions were lower at the early and intermediate time point and increased at the late time point when ART was resumed (Fig. 1D).
Next, we evaluated if the proportion of CD8+ T cells expressing all four functions could predict disease outcome in these children (Fig. 1E). Slow progressors were analyzed by combining arm A (early time point) and arm B (immediate time point) groups when off ART and determining the proportions of HIV-1-specific CD8+ T cells expressing a different number of functions; a similar analysis was undertaken on rapid progressors. No significant differences were noted for one, two, and three functions (≤3 functions) between slow- and rapid-progressor children; however, there were more polyfunctional (four functions) HIV-1-specific CD8+ T cells in slow progressors than in rapid progressors (p=0.05, Mann–Whitney; Fig. 1E). Taken together these data suggest that with continuous viral stimulation in the absence of ART, polyfunctionality of HIV-1-specific CD8+ T cells may predict slow disease progression in children.
A small group of perinatally HIV-1-infected children maintain immunological control and display slow disease progression. Multiple factors may contribute to delayed HIV-1 disease progression in adults, however, very little is known about these factors in pediatric infection. Here we assessed T cell functionality in the slow-progressor children compared to rapid progressors, and we observed low levels of polyfunctional CD8+ T cells in rapid compared to slow progressors.
As demonstrated in adults studies, differences were noted between the quality of the HIV-1-specific CD8+ T cell response in slow and rapid disease progression in children. Expression of IFN-γ alone by virus-specific CD8+ T cells, as has been traditionally measured by ELISPOT and ICS assays, was not sufficient to discriminate between slow and rapid progressors. We noted that individuals who received short-term ART (arm B) had higher frequencies of IFN-γ+ CD8+ T cell responses when treatment was interrupted, in comparison to untreated children (arm A). Augmentation of T cell responses following interruption of ART has been well documented.22,23 However, it cannot be ruled out that qualitative differences exist in the T cell responses generated by these children. This may, in part, account for the rapid progression to disease observed in the majority of infected children despite early detection of IFN-γ+ CD8+ T cell responses in the first months of life.15,24
Indeed, differences were observed when more CD8+ T cell functions in response to antigen were measured by multiparameter flow assays; HIV-1-specific CD8+ T cell responses in slow progressors displayed a higher proportion of polyfunctional T cell responses than rapid progressors. Notably, these responses were different at the earliest time point off ART when viral loads were not yet different between the two groups. This finding is consistent with previous observations in studies of adult long-term nonprogressors who displayed polyfunctional HIV-1-specific CD8+ T cells.8 An alternative explanation for this result may be that slow progressors had more antigen-specific CD8+ T cells than rapid progressors as the number of epitopes targeted by individuals between the two groups was variable. Here, we detected polyfunctional HIV-1-specific CD8+ T cell responses in children younger than 6 months of age. In contrast, in a recent study, polyfunctional responses were less common in children younger than 24 months and were hardly detected in children less than 12 months.25 A possible explanation for these differences could be viral subtype or host genetic differences, timing of infection, as most infants in our cohort were infected in utero, and also that peptides corresponding to optimal CD8+ T cell epitopes and not pools of overlapping HIV-1 peptides were used in our study.
The heterogeneity of the cohort studied here in terms of ART, and the limited number of patients, did not allow us to establish a clear-cut relationship between polyfunctional HIV-1-specific CD8+ T cell responses and viral load, their maintenance over the time of infection, and changes in responses on ART. An additional limitation in our study is that the heterogeneity of slow and rapid progression noted here may have been confounded by ART with some receiving deferred treatment (n=6) while the rest (n=9) were initiated on treatment at the time of diagnosis and then discontinued after 1 year. Therefore our observations need to be interpreted cautiously as they may not be generalized to the natural course of disease in the absence of ART. In previous studies, the presence of three or more functions depicted polyfunctionality and was associated with slow disease progression. In our analysis, the effect of polyfunctionality as assessed by three functions did not differ between slow and rapid progressors but was seen only with regards to four functions. Follow-up studies to address these questions in a homogeneous cohort with large numbers of therapy-naive children are warranted. However, these may be difficult to undertake in future because the high risk of rapid progression in children requires immediate ART intervention following diagnosis,16,26 which is now provided to all HIV-1-infected children in South Africa.
This study assessed T cell polyfunctionality in a pediatric cohort, HIV infected at birth, and followed from early through to chronic stages for slow versus rapid progression. We conclude that the quality of the HIV-1-specific CD8+ T cell response is associated with immunological outcome in pediatric HIV-1 infection as has previously been shown for adult infection. The introduction of early ART in infants following diagnosis as currently recommended will overlook the few children likely to be able to spontaneously control replication of the virus. However, early initiation of ART will preserve T cell functionality in the majority of rapid-progressor children and is clearly justified given the difficulty in discriminating between these two groups during routine clinical care. The potential to discontinue early ART following the initial period of rapid disease progression in children is currently under investigation.26
Acknowledgments
We would like to thank all the mothers and children for participation in this study, the clinic team at St Mary's Hospital antenatal clinic and Prince Mshiyeni Hospital, and the HIV Pathogenesis Programme (HPP) team: K. Mgwenya, T. Sikhakhane, N. Linda, N. Maphalala, N. Nkupi, J. Gaza, P. Mkhwanyana, L. Nxele, M. Nyawo, M. Van der Stok, D. Ramduth, E. Govender, T. Moodley, N. Ismail, K. Nair, K. Bishop, N. Mlotshwa, S. Reddy, and N. Phungula. The authors are also grateful to S. Bryan and L. Werner for their statistical analysis input and to M. Mureithi for the helpful editing of this manuscript. This study was funded by the Doris Duke Charitable Foundation (P.J.R.G. and M.A.), the Wellcome Trust (to P.J.R.G.), Bristol Myers Squibb Secure the Future (RES116/01 to P.J.R.G.), and the South African DST/NRF Chair in Systems Biology of HIV/AIDS (to T.N.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.UNAIDS: Report on the global AIDS epidemic. 2008.
- 2.Brahmbhatt H. Kigozi G. Wabwire-Mangen F, et al. Mortality in HIV-infected and uninfected children of HIV-infected and uninfected mothers in rural Uganda. J Acquir Immune Defic Syndr. 2006;41:504–508. doi: 10.1097/01.qai.0000188122.15493.0a. [DOI] [PubMed] [Google Scholar]
- 3.Newell ML. Coovadia H. Cortina-Borja M. Rollins N. Gaillard P. Dabis F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: A pooled analysis. Lancet. 2004;364:1236–1243. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 4.Prendergast A. Tudor-Williams G. Jeena P. Burchett S. Goulder P. International perspectives, progress, and future challenges of paediatric HIV infection. Lancet. 2007;370:68–80. doi: 10.1016/S0140-6736(07)61051-4. [DOI] [PubMed] [Google Scholar]
- 5.Goulder PJ. Brander C. Tang Y, et al. Evolution and transmission of stable CTL escape mutations in HIV infection. Nature. 2001;412:334–338. doi: 10.1038/35085576. [DOI] [PubMed] [Google Scholar]
- 6.Thobakgale CF. Prendergast A. Crawford H, et al. Impact of HLA in mother and child on disease progression of pediatric human immunodeficiency virus type 1 infection. J Virol. 2009;83:10234–10244. doi: 10.1128/JVI.00921-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almeida JR. Price DA. Papagno L, et al. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J Exp Med. 2007;204:2473–2485. doi: 10.1084/jem.20070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Betts MR. Nason MC. West SM, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seder RA. Darrah PA. Roederer M. T-cell quality in memory and protection: Implications for vaccine design. Nat Rev Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 10.Almeida JR. Sauce D. Price DA, et al. Antigen sensitivity is a major determinant of CD8+ T-cell polyfunctionality and HIV-suppressive activity. Blood. 2009;113:6351–6360. doi: 10.1182/blood-2009-02-206557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Betts MR. Price DA. Brenchley JM, et al. The functional profile of primary human antiviral CD8+ T cell effector activity is dictated by cognate peptide concentration. J Immunol. 2004;172:6407–6417. doi: 10.4049/jimmunol.172.10.6407. [DOI] [PubMed] [Google Scholar]
- 12.La Gruta NL. Turner SJ. Doherty PC. Hierarchies in cytokine expression profiles for acute and resolving influenza virus-specific CD8+ T cell responses: Correlation of cytokine profile and TCR avidity. J Immunol. 2004;172:5553–5560. doi: 10.4049/jimmunol.172.9.5553. [DOI] [PubMed] [Google Scholar]
- 13.Rehr M. Cahenzli J. Haas A, et al. Emergence of polyfunctional CD8+ T cells after prolonged suppression of human immunodeficiency virus replication by antiretroviral therapy. J Virol. 2008;82:3391–3404. doi: 10.1128/JVI.02383-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Streeck H. Brumme ZL. Anastario M, et al. Antigen load and viral sequence diversification determine the functional profile of HIV-1-specific CD8+ T cells. PLoS Med. 2008;5:e100. doi: 10.1371/journal.pmed.0050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thobakgale CF. Ramduth D. Reddy S, et al. Human immunodeficiency virus-specific CD8+ T-cell activity is detectable from birth in the majority of in utero-infected infants. J Virol. 2007;81:12775–12784. doi: 10.1128/JVI.00624-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mphatswe W. Blanckenberg N. Tudor-Williams G, et al. High frequency of rapid immunological progression in African infants infected in the era of perinatal HIV prophylaxis. AIDS. 2007;21:1253–1261. doi: 10.1097/QAD.0b013e3281a3bec2. [DOI] [PubMed] [Google Scholar]
- 17.Prendergast A. Mphatswe W. Tudor-Williams G, et al. Early virological suppression with three-class antiretroviral therapy in HIV-infected African infants. AIDS. 2008;22:1333–1343. doi: 10.1097/QAD.0b013e32830437df. [DOI] [PubMed] [Google Scholar]
- 18.Guay LA. Musoke P. Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354:795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 19.Jackson JB. Musoke P. Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: 18-month follow-up of the HIVNET 012 randomised trial. Lancet. 2003;362:859–868. doi: 10.1016/S0140-6736(03)14341-3. [DOI] [PubMed] [Google Scholar]
- 20.http://www.who.int/hiv/pub/guidelines/WHOpaediatric.pdf http://www.who.int/hiv/pub/guidelines/WHOpaediatric.pdf
- 21.Kiepiela P. Leslie AJ. Honeyborne I, et al. Dominant influence of HLA-B in mediating the potential co-evolution of HIV and HLA. Nature. 2004;432:769–775. doi: 10.1038/nature03113. [DOI] [PubMed] [Google Scholar]
- 22.Ortiz GM. Wellons M. Brancato J, et al. Structured antiretroviral treatment interruptions in chronically HIV-1-infected subjects. Proc Natl Acad Sci USA. 2001;98:13288–13293. doi: 10.1073/pnas.221452198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papasavvas E. Ortiz GM. Gross R, et al. Enhancement of human immunodeficiency virus type 1-specific CD4 and CD8 T cell responses in chronically infected persons after temporary treatment interruption. J Infect Dis. 2000;182:766–775. doi: 10.1086/315748. [DOI] [PubMed] [Google Scholar]
- 24.Lohman BL. Slyker JA. Richardson BA, et al. Longitudinal assessment of human immunodeficiency virus type 1 (HIV-1)-specific gamma interferon responses during the first year of life in HIV-1-infected infants. J Virol. 2005;79:8121–8130. doi: 10.1128/JVI.79.13.8121-8130.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang S. Dunkley-Thompson J. Tang Y, et al. Deficiency of HIV-Gag-specific T cells in early childhood correlates with poor viral containment. J Immunol. 2008;181:8103–8111. doi: 10.4049/jimmunol.181.11.8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Violari A. Cotton MF. Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]