Abstract
Tissue regeneration into a three-dimensional scaffold requires the stimulation of blood vessel ingrowth. We have employed a freely interconnecting porous scaffold developed by us to determine the utility of a covalently bound heparin surface coating for the delivery of vascular endothelial growth factor (VEGF) and platelet-derived growth factor BB (PDGF-BB) in vivo. The heparin surface was shown to release VEGF far more rapidly than PDGF-BB in vitro (VEGF: 75 ng/h for 24 h; PDGF-BB: 86 pg/h for >7 days). In rat subcutaneous implants, at 10 days the heparin surface alone increased vessel ingrowth substantially (p<0.05 vs. unmodified scaffold), release of VEGF resulted in a further increase (p<0.05 vs. heparinized scaffold), whereas PDGF-BB had no additional effect. The increase induced by the combination of growth factors was similar to VEGF alone. After 2 months, PDGF-BB, but not VEGF delivery, resulted in a substantial increase in vascularization above that induced by heparin (p<0.05). At the longer time point the combination of growth factors was similar to PDGF-BB. However, only the combination of growth factors significantly elevated the number of ingrowing arterioles (p<0.05 vs. heparinized scaffold). Thus, the covalent modification of a porous scaffold with heparin allows for the differential release of VEGF and PDGF-BB that results in both a rapid and sustained increase in scaffold vascularization.
Introduction
The successful replacement of nonfunctional tissue via guided in situ regeneration into a three-dimensional (3D) scaffold requires the concomitant formation of a neovasculature to supply the cells within the newly formed tissue with oxygen and nutrients. The new network will need to be established rapidly and to persist; in most instances, it is envisaged that neovascularization will be achieved through angiogenesis. The exogenous delivery of growth factors1,2 is the most widely investigated means of stimulating angiogenesis. In 3D scaffold-based tissue regeneration it is most sensible that the growth factors be delivered from the implanted scaffold, as this should generate a gradient of the factors, thus stimulating vessel growth towards the desired site.2 However, it has become increasingly clear that there are a number of additional critical design requirements for effective delivery of pro-angiogenic growth factors from scaffolds. These include the need to sustain delivery over an extended period (weeks to months) to allow for the possibility of stable neovessel network generation3,4 and to avoid the potential safety concerns of bolus delivery of large doses of growth factors (e.g., vascular endothelial growth factor [VEGF] has been reported to produce hemangiomas).5 As a corollary to the above, the scaffold should also sequester the factors away from the cellular milieu, as the half-lives of factors can be very short when they are in solution in vivo, with that of basic fibroblast growth factor (bFGF) and VEGF being less than 1 h after intravenous injection.6,7
Furthermore, an ability to deliver combinations of growth factors is desirable, as though single factor delivery has been shown to be angiogenic, the results of clinical trials that resulted from this research have been disappointing.8 It is now generally supposed that this lack of efficacy resulted in part from an initial under appreciation of the complexity of the angiogenic process9,10 whereby there is interplay between a number of angiogenic factors in creating a stable, functional vascular network. Recently, the delivery of bFGF and platelet-derived growth factorplatelet-derived growth factor (PDGF) was shown to generate long-lasting vascular systems (>1 year), whereas VEGF, bFGF, and PDGF alone did not.9 Coincident to the ability to deliver growth factor combinations is the capacity to stage their delivery to more closely simulate that that occurs physiologically. Staged delivery of VEGF followed by PDGF-BB from blown PLGA/PLG foam/bead composites resulted in stable vasculature being formed for up to 4 weeks after implantation.11
Physiologically, growth factors are found to bind to glycosaminoglycans (e.g., heparan sulfate and heparin) and extracellular matrix proteins (e.g., collagen and fibronectin).12. This presumably serves in part to prolong their duration; thus, the utilization of these types of molecules as delivery vehicles for growth factors has begun to be explored in the field of therapeutic angiogenesis.2 Heparin has been shown to bind a large array of growth factors and accordingly heparin has been conjugated to biopolymers in the form of hydrogels, porous scaffolds, and fibers to immobilize factors such as VEGF, basic fibroblast growth, PDGF-BB, and keratinocyte growth factor.13–15 These derivatized scaffolds were shown to influence cellular and vessel growth after implantation.
We have developed a scaffold with well-defined interconnected porosity that allows for quantitative analysis of vessel ingrowth into the scaffold.4,16–18 Covalent coupling of heparin to the porous surface was found to be inherently pro-angiogenic. In the current study we utilized this system to characterise the binding of VEGF and PDGF-BB to the heparinized surface and the ability of this combination to stimulate angiogenesis into the porous scaffold for up to 2 months.
Materials and Methods
Growth factors
VEGF165 was produced as described in detail previously.4 In brief, human VEGF165 was expressed in Escherichia coli expression host BL21 (DE3) pLysS. After lysis, solubilization in urea, and dimerization, the dimeric protein was purified on a heparin agarose affinity column.
Human PDGF-BB was purchased from PeproTech, Inc. (Rocky Hill, NJ).
Production and heparin surface modification of porous polyurethane
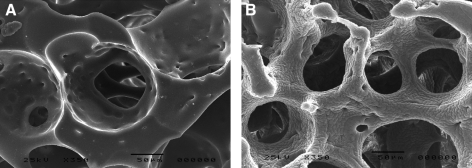
Polyurethane (PU) discs with a diameter of 5.4 mm and a thickness of 2 mm with well-defined open porosity (82% porosity, 157±1 μm pores) were produced by a variation on the phase inversion/porogen extraction technique, as described previously17 (Fig. 1). In brief, tubular moulds were pre-packed with gelatin beads and the interstices infiltrated with PU solution. After demolding, polymer precipitation, and porogen removal by extensive washing in water, the resultant porous cylinders were dried and sliced into discs.
FIG. 1.
Scanning electron micrographs of cross sections of uncoated (A) and heparin-coated (B) porous PU discs. The rough appearance of the heparin coating results from sample preparation for scanning electron microscopy. PU, polyurethane.
Details of the covalent heparinization of the porous discs have also been previously described.16 Briefly, PU discs were surface-grafted by cerium-ion initiated copolymerization with acrylic acid and acrylamide, aminated by reaction with ethylene diamine after carbodiimide activation of the carboxylic acid groups, and heparinized by reductive amination using nitrous acid de-aminated heparin.
Treated PU discs were rinsed in distilled water, washed overnight in phosphate-buffered saline (PBS, pH 7.4), sterilized by immersion in 70% ethanol for 12 h, and air-dried under sterile conditions until loading with growth factors.
Growth factor elution
Either VEGF (12 μg)19 or PDGF-BB (1.8 μg), or combination thereof, was allowed to passively adsorb overnight onto the heparinized surface at 4°C. Non-heparin-coated or heparin coated PU control discs were loaded with 40 μL of PBS in the same manner and left overnight at 4°C.
The discs (four replicates per group) were then individually washed three times at 37°C (15 min each) and the growth factor present in the washes quantified. Each disc was then placed in 10 mL PBS (equivalent to 200× volumes of a single disc) at 37°C and constantly agitated, and aliquots were taken across 8 days. Discs were then placed in PBS containing 1 M NaCl (high salt elution). VEGF and PDGF-BB content was assayed with growth factor-specific ELISA (R&D, Minneapolis, MN).
In vivo angiogenesis assay
All animal experiments were approved by the Animal Research and Ethics Committee of the University of Cape Town and were in compliance with the Principles of Laboratory Care and the guidelines for the care and use of laboratory animals (NIH publication no. 86–23). After anesthesia (ketamine/xylazine) and shaving of the animal, 1 cm longitudinal incisions were made through the skin at either side of the dorsal midline and a subcutaneous pocket for each disc was prepared by gentle blunt dissection (using aseptic technique). The discs were implanted with each rat (Wistar) receiving only one disc of each group (eight rats were utilized per time point resulting in eight disc replicates per group), and the skin incisions were closed with single 3/0 Prolene sutures. After 10 days or 2 months days of implantation, the animals were sacrificed by inhalation of carbon dioxide. Discs were explanted with their surrounding capsules, halved into equal semi-cylindrical sections, and fixed (one half of each disc fixed in zinc fixative20 and the other in 10% buffered formalin) for 24 h before both were transferred to a 70% EtOH solution before processing.
Immunohistochemistry
After fixation in zinc or 10% formalin fixative, all samples were processed through graded alcohol and then embedded in paraffin wax. Cross sections (3 μm thick) from the mid-region of the device were dewaxed with 2,2,4-trimethylpentane. For detection of blood vessels, zinc-fixed sections were incubated with a 1:50 dilution of mouse anti-rat CD31 primary antibody (RDI Research Diagnostics, Inc., Flanders, NJ) in 1% bovine serum albumin (Jacksons Immunoresearch, West Grove, PA) in PBS. The primary antibody was then detected with a mouse on rat alkaline phosphatase-polymer kit (Biocare Medical, Concord, CA) as per manufacturer's instructions. For detection of mural cells, formalin-fixed sections were incubated with a 1:25 dilution of mouse anti-rat α-smooth muscle cell actin (α-SMC actin; Fitzgerald Industries International, Acton, MA) in PBS. The primary antibody was then detected with a 1:250 dilution of an Alexa488 conjugated goat anti-mouse secondary antibody (Jackson Immunoresearch).
Histological analysis
CD31-stained samples were loaded into a Nikon Coolscope (Nikon Corp., Tokyo, Japan), and an image of the complete mid region cross-section was acquired at a 100× magnification (thus, a micrograph was generated that encompasses a 5.4×2 mm cross section for each replicate). All of the ensuing analysis was performed with Visiopharm Integrated Systems (Visiopharm A/S, Hørsholm, Denmark). Briefly, the software was trained to automatically detect CD31-positive structures; all analyses were assessed by an observer for accuracy (presence of a lumen was prerequisite in assigning staining to vasculature) and, if necessary, manually corrected. Vascular area of each vessel was defined as the area occupied by both the endothelium and lumen. Vascular area and vessel number were normalized to total ingrowth area. Total ingrowth area was extrapolated from gravimetric determination of the volume of disc occupied by PU scaffold, namely, 18%.16 The heparin coating does not significantly decrease available ingrowth area.16 Vascular area is given as the percentage of ingrowth area occupied by vessels, whereas vessel density is given as number of vessels per mm2 of ingrowth area.
SMC actin-stained sections were imaged on a Nikon 90i fluorescence microscope (Nikon Corp.) at 40× magnification and an entire cross-sectional area was captured using the stitching facility in the Nikon software. SMC actin-positive cells were automatically detected using Visiopharm software.
Statistical analysis
For quantitative analysis, one-way analysis of variance was performed when more than two groups were compared and two-tailed student t-tests were used to assess differences between two groups. p<0.05 was considered significant. Data are expressed as mean values±standard error.
Results
Release kinetics of VEGF and PDGF-BB from heparinized porous PU
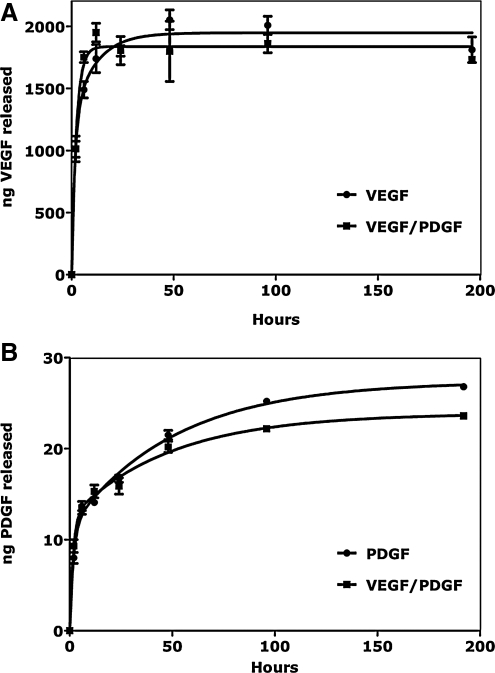
There was a substantial difference in both the binding and elution of VEGF and PDGF-BB to the heparinized PU (Fig. 2). Initial washing eluted 72% (±13%) of loaded VEGF, a further 16% (±1%) eluted over approximately 24 h with a nominal rate of 75 ng/h (±0.026 ng/h). Only 1% of VEGF loaded was detected in the high salt displacement eluant. For PDGF-BB, only 1% of loaded growth factor could be detected in the pre-wash eluant and a further 0.9% (±0.1%) was eluted over 7 days at a nominal rate of 86 pg/h (±0.11 pg/h). Half (50%±1%) of the remaining PDGF-BB was eluted with high salt. There were no significant differences in the amount of relative growth factor eluted at the various time points for single or dual growth factor-loaded heparinized PU.
FIG. 2.
Elution profiles of VEGF and PDGF-BB from heparin-modified PU discs at 37°C over 8 days. (A) Elution of VEGF from discs loaded with VEGF alone (circles) and with VEGF and PDGF-BB (squares). (B) Elution of PDGF-BB from discs loaded with PDGF-BB alone (circles) and with VEGF and PDGF-BB (squares) (n=4). VEGF, vascular endothelial growth factor; PDGF-BB, platelet-derived growth factor BB.
Neovascularization after subcutaneous implantation of heparinized porous PU for 10 days
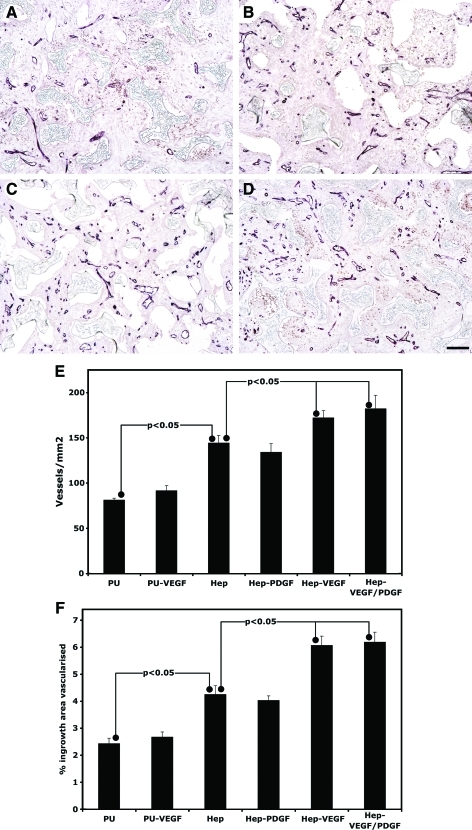
The ingrowth of vessels into unmodified PU discs (Fig. 3) was similar in nature to that previously observed for tubular PU implanted in the same manner.4 Addition of heparin or heparin plus growth factors resulted in a substantial increase in vascularization at 10 days. Quantification for both vessel number and area of ingrowth tissue vascularized showed similar patterns. Delivery of VEGF in nonheparinized PU discs did not increase vascularization of the scaffold. Addition of heparin alone resulted in a significant increase in vessel number (78%±10%) and area vascularized (75%±13%) over that seen in unmodified PU. Loading of heparinized PU with VEGF or VEGF plus PDGF-BB resulted in significant increases in vessel number (VEGF: 19%±5%; VEGF/PDGF-BB 26%±10%) and area vascularized (VEGF: 43%±8%; VEGF/PDGF-BB 46%±9%) over that observed for heparinized PU. This represents increases in vessel ingrowth for these treatments relative to unmodified PU of 112%±10% and 124%±18% for VEGF and VEGF plus PDGF-BB, respectively. Similarly area vascularized increased by 150%±14% and 155%±15%, respectively. There was no influence of PDGF-BB bound to heparin on vascularization above that caused by the addition of heparin. Additionally, the vascularization induced by addition of both VEGF plus PDGF-BB to heparinized discs was not significantly increased over that caused by addition of VEGF alone.
FIG. 3.
Vascularization within porous PU discs after subcutaneous implantation for 10 days. Representative micrographs of CD31 immunostained explants of uncoated PU discs (A) and heparin-coated PU discs (B) and heparin-coated discs loaded with VEGF (C) or VEGF and PDGF-BB (D) (bar represents 50 μm). Vessel ingrowth was quantified for both vessel density (E) and percentage of ingrowth area vascularized (F) (n=8). Color images available online at www.liebertonline.com/tea
Neovascularization after subcutaneous implantation of heparinized porous PU for 2 months
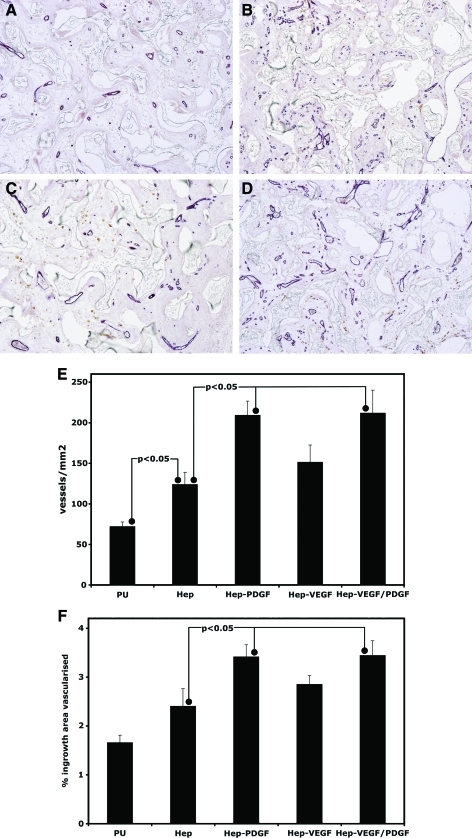
The level of vascularization present within the treated and untreated porous PU after 2 months was assessed in a similar manner (Fig. 4). The vessels appeared similar in morphology to that observed at 10 days, although there appeared to be a greater proportion of smaller vessels. At 2 months, addition of heparin alone now only resulted in a significant increase in vessel number (72%±26%) but not in area vascularized over that in unmodified PU. Vascularization after loading of heparinized PU with VEGF significantly increased above that of PU alone for vessel number (110%±24%) and area vascularized (72%±10%) but did not increase significantly relative to heparinized PU. At 2 months, addition of PDGF-BB or VEGF plus PDGF-BB did increase vascularization relative to heparinized PU for vessel numbers (PDGF-BB: 69%±17%; VEGF/PDGF-BB: 71%±23%) and area vascularized (PDGF-BB: 42%±10%; VEGF/PDGF-BB: 43%±13%). These represent increases in vascularization for these treatments relative to unmodified PU with numbers of vessels being increased by 191%±29% and 199%±39% for PDGF-BB and VEGF plus PDGF-BB, respectively. Similarly area vascularized increased by 105%±15% and 108%±18%, respectively. The vascularization induced by addition of VEGF plus PDGF-BB was not significantly increased over that caused by addition of PDGF-BB alone.
FIG. 4.
Vascularization within porous PU discs after subcutaneous implantation for 2 months. Representative micrographs of CD31 immunostained explants of uncoated PU discs (A) and heparin-coated PU discs loaded with PDGF-BB (B) or VEGF (C) or VEGF and PDGF-BB (D) (bar represents 50 μm). Vessel ingrowth was quantified for both vessel density (E) and percentage of ingrowth area vascularized (F) (n=8). Color images available online at www.liebertonline.com/tea
Arteriolization after subcutaneous implantation of heparinized porous PU for 2 months
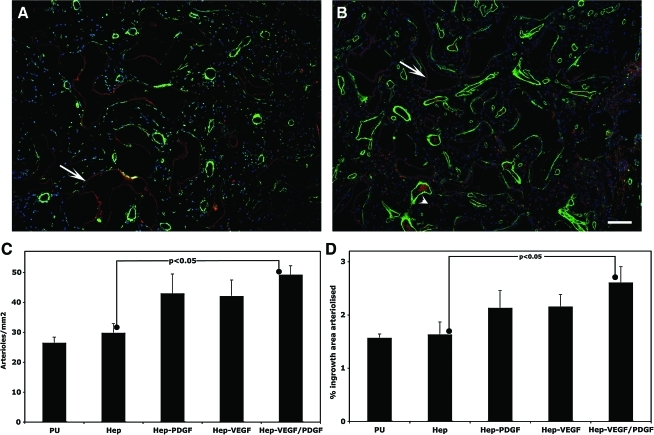
The 2-month implants were also assessed for arteriole content by quantifying SMC actin-containing vessels (Fig. 5). The 10 day explants had large populations of myofibroblasts (data not shown) that precluded accurate analysis of arteriole levels. Quantitation indicated that at 2 months all growth factor containing treatments had a trend towards an increase in arteriole numbers and area of ingrowth tissue occupied by arterioles but that only the combination of VEGF plus PDGF-BB was significantly elevated. The number of arterioles for this group rose relative to heparinized PU by 66%±22% and the area occupied by arterioles by 68%±16%.
FIG. 5.
Arteriolization of tissue within porous PU discs after implantation for 2 months. Representative fluorescent micrographs of α-smooth muscle cell actin immunostained explants of heparin-coated PU discs (A) and heparin-coated PU discs loaded with VEGF and PDGF-BB (B) (bar represents 50 μm). Arrows and arrow heads indicate autofluorescing heparin coating and putative erythrocytes/macrophages respectively. Arteriole ingrowth was quantified for both vessel density (C) and percentage of ingrowth area vascularized (D) (n=8). Color images available online at www.liebertonline.com/tea
Discussion
The heparin-based delivery system described here was shown to be able to deliver two distinct growth factors at markedly different rates. Utilizing this system in a quantitative subcutaneous angiogenesis assay, it was further demonstrated that there was a marked increase in vascularization at 10 days for discs releasing VEGF from heparin, with a further and substantial increase in response to PDGF-BB observed only at 2 months. Importantly, the combination of the two growth factors resulted in a sustained increase in vascularization over the entire period.
The very similar amounts of each growth factor eluted whether in the context of a single or dual loading of the two factors on to the heparinised surface is possibly suggestive of different binding sites being available for PDGF-BB and VEGF. The substantially slower release kinetics of PDGF-BB from heparin and its more efficient binding to the derivatized surface in vitro presumably translated into PDGF-BB being delivered for an extended period during implantation as compared to VEGF.
The presence of a heparin surface alone on the PU discs caused a significant increase in vessels at 10 days. This outcome has been previously observed by us to persist out to 4 weeks in our model. In this present study, however, the vascularization levels at 2 months indicate a potential diminishment in the angiogenic response to the heparin surface alone with only the number of vessels but not the area vascularized being significantly increased. Heparin has been incorporated into a variety of matrices and increased the angiogenic response.16,21,22. Heparin modified fibrin,23 alginate,24 gelatin,25 and collagen matrices14 have all been reported to show similar effects. The mechanism by which heparin stimulates vascularization is not clear but it seems reasonable to suggest that it may result form the trapping and stabilization of endogenously released growth factors. It might be that at the later time point there has been a general reduction in growth factor production due to, for example, the resolution of the wound healing associated inflammatory response26 and the observed marked reduction in myofibroblasts.
The delivery of potent pro-angiogenic VEGF27 in conjunction with heparin resulted in dramatic increases in vessel number and area vascularized at 10 days relative to the unmodified PU discs with the former doubling and the latter resulting in an even more marked response. Although a significant proportion of the VEGF was released during the wash steps in vitro and thus a pronounced bolus effect would be expected in vivo, no increase in vascularization was seen in unmodified discs loaded with VEGF. This strongly suggests that sustained release is needed for stimulated vessel ingrowth.4,11 However, the heparinized surface only allowed for a relatively short release period for VEGF in vitro and it appears that release for an extended period is required for persistent vessel presence.3,4 Thus, this is a likely reason for the lack of a significant increase in vascularization over heparinized discs at 2 months.
Delivery of PDGF-BB alone from heparinized surfaces did not generate additional vessels at the earlier time point, but by 2 months there was a significant increase above that observed for heparinized discs alone. This increase was considerable with the number of vessels triple that seen in unmodified discs and the area vascularized having doubled. There was not a significant increase in arterioles as might be expected from this growth factor with its well-established ability to recruit mural cells.28 A pronounced angiogenic rather than an arteriogenic effect has not been generally observed when PDGF-BB has been delivered either as a gene29 or as a protein by controlled release from poly(lactide-co-glycolide) discs.11 This indicates that the precise context of delivery is of importance where in this study PDGF-BB was most likely released at a very low dosage over an extended period in the presence of heparin. A previous study that delivered PDGF-BB from heparinized demineralised bone scaffolds observed increased vessel levels above controls at 14 days postimplantation.15 However, the effect of the heparin was not assessed, making it difficult to directly compare these findings with ours. PDGF-BB has been shown to directly promote angiogenesis in vitro possibly via interaction with its receptor PDGF-R beta that are present on endothelial cells.30–32 Perhaps of greater relevance to this study is the ability of PDGF-BB to induce increased expression of VEGF from a variety of cells, including mural cells, endothelial precursor cells, and tumor cells.33–36 It has been suggested that PDGF-BB enhances glioma angiogenesis in part by increasing VEGF expression33 and it has also been recently shown that teratomas and embryoid bodies that have constitutively activated PDGF-R beta have increased vascularization that could be blocked by neutralizing antibodies against VEGF and its receptor VEGF-R2.37 Thus, it is possible that prolonged release of low levels of PDGF-BB gradually increased the levels of VEGF present in the disc and that this VEGF may have been stabilized by the heparin surface.
The dual release of both growth factors resulted in elevated levels at both time points but at levels similar to those observed for VEGF at 10 days and PDGF-BB at 2 months. There was therefore no indication of synergy for the two factors and it is possible that the vessels observed at each time point for the dual factor group resulted in each instance from the angiogenic effect of the relevant individual factor. However, there was a significantly increased level of arteriolization in this group at 2 months, suggestive of a persistence of vessels that were induced earlier allowing for greater mural cell investment. Similar results for arteriolization were observed when these two growth factors were released together from poly-(lactide-co-glycide) sponges11; however, as noted above there was no indication in this study of an angiogenic response to PDGF-BB.
Although vessel number remained relatively constant between the two time points, the area vascularized had decreased from 6% at 10 days to 3.5% at 2 months. This indicates a reduction in size as a result of vessel maturation and might reflect increased intussusception of large vessels and/or sprouting of smaller vessels.
In conclusion, surface modification of a well-defined openly porous scaffold with heparin allows for a robust means of exploring the angiogenic potential of a wide range of heparin binding growth factors. In this particular study, the staged release of VEGF and PDGF-BB resulted in a sustained elevation of vascularization within a scaffold from as early as 10 days to at least 2 months after implantation. The growth factor-induced vascularization in this system appears to be enhanced by the presence of the inherently pro-angiogenic carrier, namely, heparin.
Acknowledgments
This work was supported by a grant from the South African Medical Research Council.
Disclosure Statement
No competing financial interests exist.
References
- 1.Mikos A.G. Herring S.W. Ochareon P. Elisseeff J. Lu H.H. Kandel R., et al. Engineering complex tissues. Tissue Eng. 2006;12:3307. doi: 10.1089/ten.2006.12.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tayalia P. Mooney D.J. Controlled growth factor delivery for tissue engineering. Adv Mater. 2009;21:3269. doi: 10.1002/adma.200900241. [DOI] [PubMed] [Google Scholar]
- 3.Baluk P. Lee C.G. Link H. Ator E. Haskell A. Elias J.A., et al. Regulated angiogenesis and vascular regression in mice overexpressing vascular endothelial growth factor in airways. Am J Pathol. 2004;165:1071. doi: 10.1016/S0002-9440(10)63369-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies N. Dobner S. Bezuidenhout D. Schmidt C. Beck M. Zisch A.H., et al. The dosage dependence of VEGF stimulation on scaffold neovascularisation. Biomaterials. 2008;29:3531. doi: 10.1016/j.biomaterials.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Zisch A.H. Lutolf M.P. Hubbell J.A. Biopolymeric delivery matrices for angiogenic growth factors. Cardiovasc Pathol. 2003;12:295. doi: 10.1016/s1054-8807(03)00089-9. [DOI] [PubMed] [Google Scholar]
- 6.Lazarous D.F. Shou M. Scheinowitz M. Hodge E. Thirumurti V. Kitsiou A.N., et al. Comparative effects of basic fibroblast growth factor and vascular endothelial growth factor on coronary collateral development and the arterial response to injury. Circulation. 1996;94:1074. doi: 10.1161/01.cir.94.5.1074. [DOI] [PubMed] [Google Scholar]
- 7.Whalen G.F. Shing Y. Folkman J. The fate of intravenously administered bFGF and the effect of heparin. Growth Factors. 1989;1:157. doi: 10.3109/08977198909029125. [DOI] [PubMed] [Google Scholar]
- 8.Simons M. Bonow R. Chronos N. Cohen D. Giordano F. Hammond H., et al. Clinical trials in coronary angiogenesis: issues, problems, consensus: an expert panel summary. Circulation. 2000;102:E73. doi: 10.1161/01.cir.102.11.e73. [DOI] [PubMed] [Google Scholar]
- 9.Cao R. Brakenhielm E. Pawliuk R. Wariaro D. Post M.J. Wahlberg E., et al. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat Med. 2003;9:604. doi: 10.1038/nm848. [DOI] [PubMed] [Google Scholar]
- 10.Yancopoulos G. Davis S. Gale N. Rudge J. Wiegand S. Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 11.Richardson T. Peters M. Ennett A. Mooney D. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 12.Taipale J. Keski-Oja J. Growth factors in the extracellular matrix. FASEB J. 1997;11:51. doi: 10.1096/fasebj.11.1.9034166. [DOI] [PubMed] [Google Scholar]
- 13.Nillesen S.T. Geutjes P.J. Wismans R. Schalkwijk J. Daamen W.F. van Kuppevelt T.H. Increased angiogenesis in acellular scaffolds by combined release of FGF2 and VEGF. J Control Release. 2006;116:e88. doi: 10.1016/j.jconrel.2006.09.065. [DOI] [PubMed] [Google Scholar]
- 14.Steffens G.C. Yao C. Prevel P. Markowicz M. Schenck P. Noah E.M., et al. Modulation of angiogenic potential of collagen matrices by covalent incorporation of heparin and loading with vascular endothelial growth factor. Tissue Eng. 2004;10:1502. doi: 10.1089/ten.2004.10.1502. [DOI] [PubMed] [Google Scholar]
- 15.Sun B. Chen B. Zhao Y. Sun W. Chen K. Zhang J., et al. Crosslinking heparin to collagen scaffolds for the delivery of human platelet-derived growth factor. J Biomed Mater Res B Appl Biomater. 2009;91:366. doi: 10.1002/jbm.b.31411. [DOI] [PubMed] [Google Scholar]
- 16.Bezuidenhout D. Davies N. Black M. Schmidt C. Oosthuysen A. Zilla P. Covalent surface heparinization potentiates porous polyurethane scaffold vascularization. J Biomater Appl. 2010;24:401. doi: 10.1177/0885328208097565. [DOI] [PubMed] [Google Scholar]
- 17.Bezuidenhout D. Davies N. Zilla P. Effect of well defined dodecahedral porosity on inflammation and angiogenesis. ASAIO J. 2002;48:465. doi: 10.1097/00002480-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt C. Bezuidenhout D. Beck M. Van der Merwe E. Zilla P. Davies N. Rapid three-dimensional quantification of VEGF-induced scaffold neovascularisation by microcomputed tomography. Biomaterials. 2009;30:5959. doi: 10.1016/j.biomaterials.2009.07.044. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt C. Bezuidenhout D. Higham L. Zilla P. Davies N. Induced Chronic Hypoxia negates the pro-angiogenic effect of surface immobilized heparin in a polyurethane porous scaffold. J Biomed Mater Res A. 2011;98:621. doi: 10.1002/jbm.a.33150. [DOI] [PubMed] [Google Scholar]
- 20.Beckstead J. A simple technique for preservation of fixation-sensitive antigens in paraffin-embedded tissues. J Histochem Cytochem. 1994;42:1127. doi: 10.1177/42.8.8027531. [DOI] [PubMed] [Google Scholar]
- 21.Fujita M. Ishihara M. Simizu M. Obara K. Ishizuka T. Saito Y., et al. Vascularization in vivo caused by the controlled release of fibroblast growth factor-2 from an injectable chitosan/non-anticoagulant heparin hydrogel. Biomaterials. 2004;25:699. doi: 10.1016/s0142-9612(03)00557-x. [DOI] [PubMed] [Google Scholar]
- 22.Ishihara M. Obara K. Ishizuka T. Fujita M. Sato M. Masuoka K., et al. Controlled release of fibroblast growth factors and heparin from photocrosslinked chitosan hydrogels and subsequent effect on in vivo vascularization. J Biomed Mater Res. 2003;64A:551. doi: 10.1002/jbm.a.10427. [DOI] [PubMed] [Google Scholar]
- 23.Sakiyama ES. Hubbell J. Development of fibrin derivatives for controlled release of heparin-binding growth factors. J Control Release. 2000;65:389. doi: 10.1016/s0168-3659(99)00221-7. [DOI] [PubMed] [Google Scholar]
- 24.Tanihara M. Suzuki Y. Yamamoto E. Noguchi A. Mizushima Y. Sustained release of basic fibroblast growth factor and angiogenesis in a novel covalently crosslinked gel of heparin and alginate. J Biomed Mater Res. 2001;56:216. doi: 10.1002/1097-4636(200108)56:2<216::aid-jbm1086>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 25.Doi K. Matsuda T. Enhanced vascularization in a microporous polyurethane graft impregnated with basic fibroblast growth factor and heparin. J Biomed Mater Res. 1997;34:361. doi: 10.1002/(sici)1097-4636(19970305)34:3<361::aid-jbm11>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 26.Singer A. Clark R. Cutaneous wound healing. N Engl J Med. 1999;341:738. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 27.Elcin A.E. Elcin Y.M. Localized angiogenesis induced by human vascular endothelial growth factor-activated PLGA sponge. Tissue Eng. 2006;12:959. doi: 10.1089/ten.2006.12.959. [DOI] [PubMed] [Google Scholar]
- 28.Benjamin L. Hemo I. Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998;125:1591. doi: 10.1242/dev.125.9.1591. [DOI] [PubMed] [Google Scholar]
- 29.Hao X. Mansson-Broberg A. Blomberg P. Dellgren G. Siddiqui A.J. Grinnemo K.H., et al. Angiogenic and cardiac functional effects of dual gene transfer of VEGF-A165 and PDGF-BB after myocardial infarction. Biochem Biophys Res Commun. 2004;322:292. doi: 10.1016/j.bbrc.2004.07.101. [DOI] [PubMed] [Google Scholar]
- 30.Battegay E.J. Rupp J. Iruela-Arispe L. Sage E.H. Pech M. PDGF-BB modulates endothelial proliferation and angiogenesis in vitro via PDGF beta-receptors. J Cell Biol. 1994;125:917. doi: 10.1083/jcb.125.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heldin C.H. Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79:1283. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- 32.Sato N. Beitz J.G. Kato J. Yamamoto M. Clark J.W. Calabresi P., et al. Platelet-derived growth factor indirectly stimulates angiogenesis in vitro. Am J Pathol. 1993;142:1119. [PMC free article] [PubMed] [Google Scholar]
- 33.Guo P. Hu B. Gu W. Xu L. Wang D. Huang H.J., et al. Platelet-derived growth factor-B enhances glioma angiogenesis by stimulating vascular endothelial growth factor expression in tumor endothelia and by promoting pericyte recruitment. Am J Pathol. 2003;162:1083. doi: 10.1016/S0002-9440(10)63905-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sufen G. Xianghong Y. Yongxia C. Qian P. bFGF and PDGF-BB have a synergistic effect on the proliferation, migration and VEGF release of endothelial progenitor cells. Cell Biol Int. 2011;35:545. doi: 10.1042/CBI20100401. [DOI] [PubMed] [Google Scholar]
- 35.Matei D. Kelich S. Cao L. Menning N. Emerson R.E. Rao J., et al. PDGF BB induces VEGF secretion in ovarian cancer. Cancer Biol Ther. 2007;6:1951. doi: 10.4161/cbt.6.12.4976. [DOI] [PubMed] [Google Scholar]
- 36.Stavri G.T. Hong Y. Zachary I.C. Breier G. Baskerville P.A. Yla-Herttuala S., et al. Hypoxia and platelet-derived growth factor-BB synergistically upregulate the expression of vascular endothelial growth factor in vascular smooth muscle cells. FEBS Lett. 1995;358:311. doi: 10.1016/0014-5793(94)01458-d. [DOI] [PubMed] [Google Scholar]
- 37.Magnusson P.U. Looman C. Ahgren A. Wu Y. Claesson-Welsh L. Heuchel R.L. Platelet-derived growth factor receptor-beta constitutive activity promotes angiogenesis in vivo and in vitro. Arterioscler Thromb Vasc Biol. 2007;27:2142. doi: 10.1161/01.ATV.0000282198.60701.94. [DOI] [PubMed] [Google Scholar]