Abstract
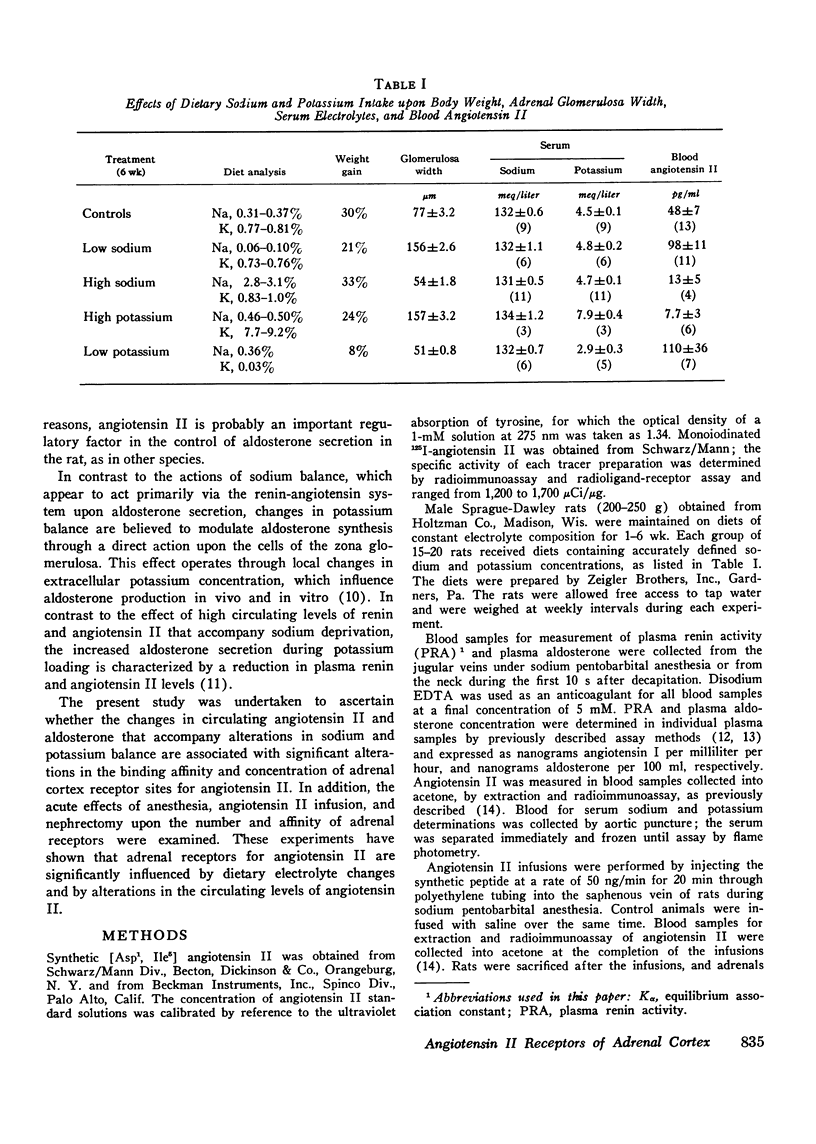
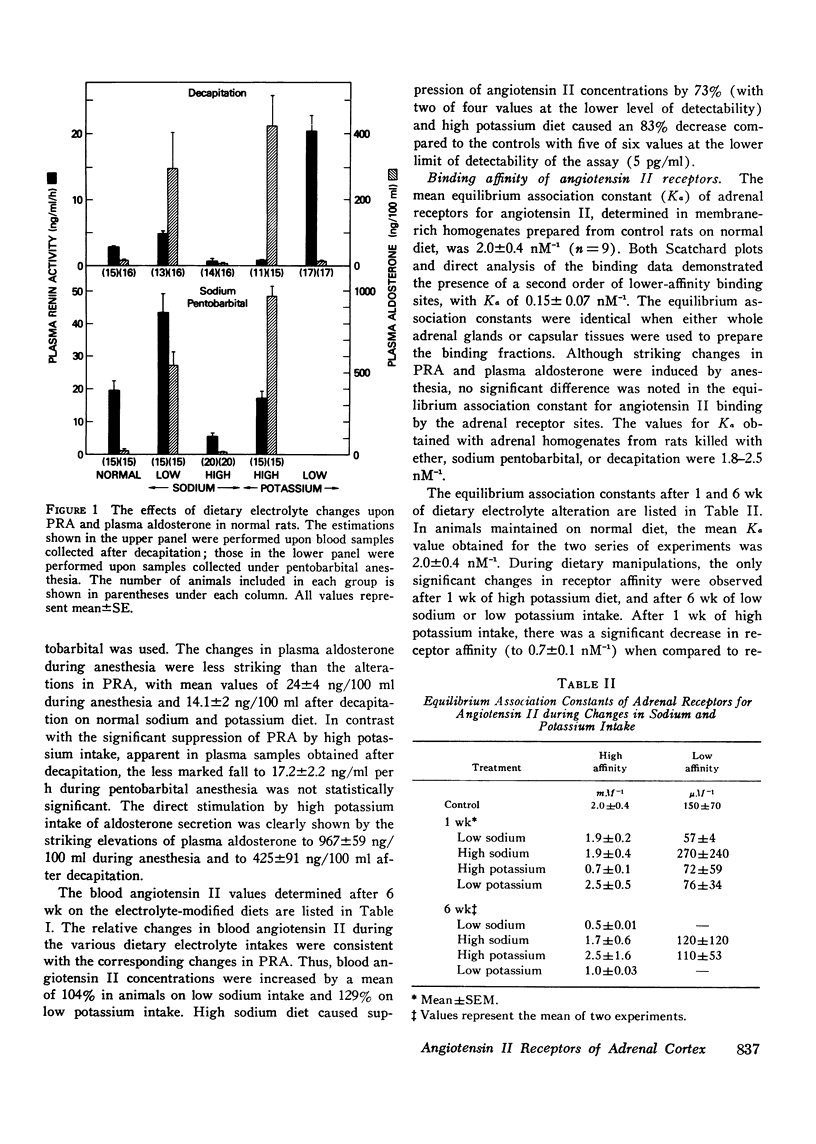
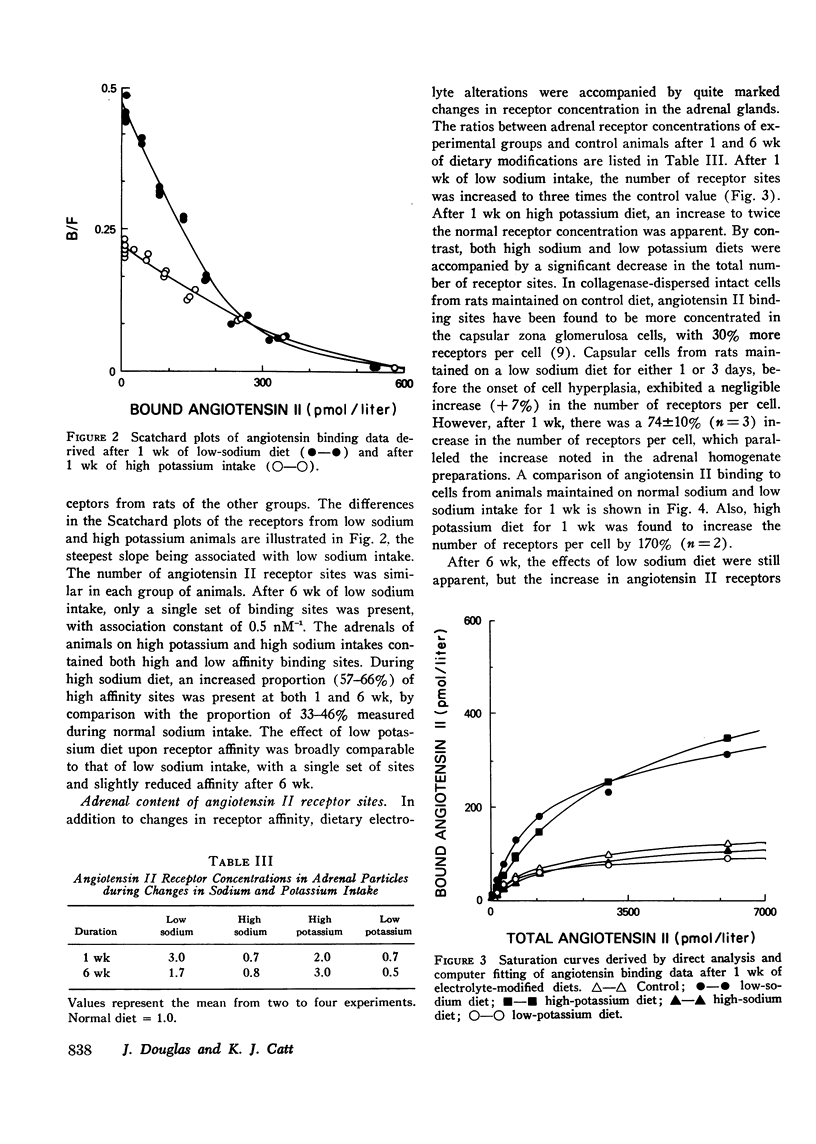
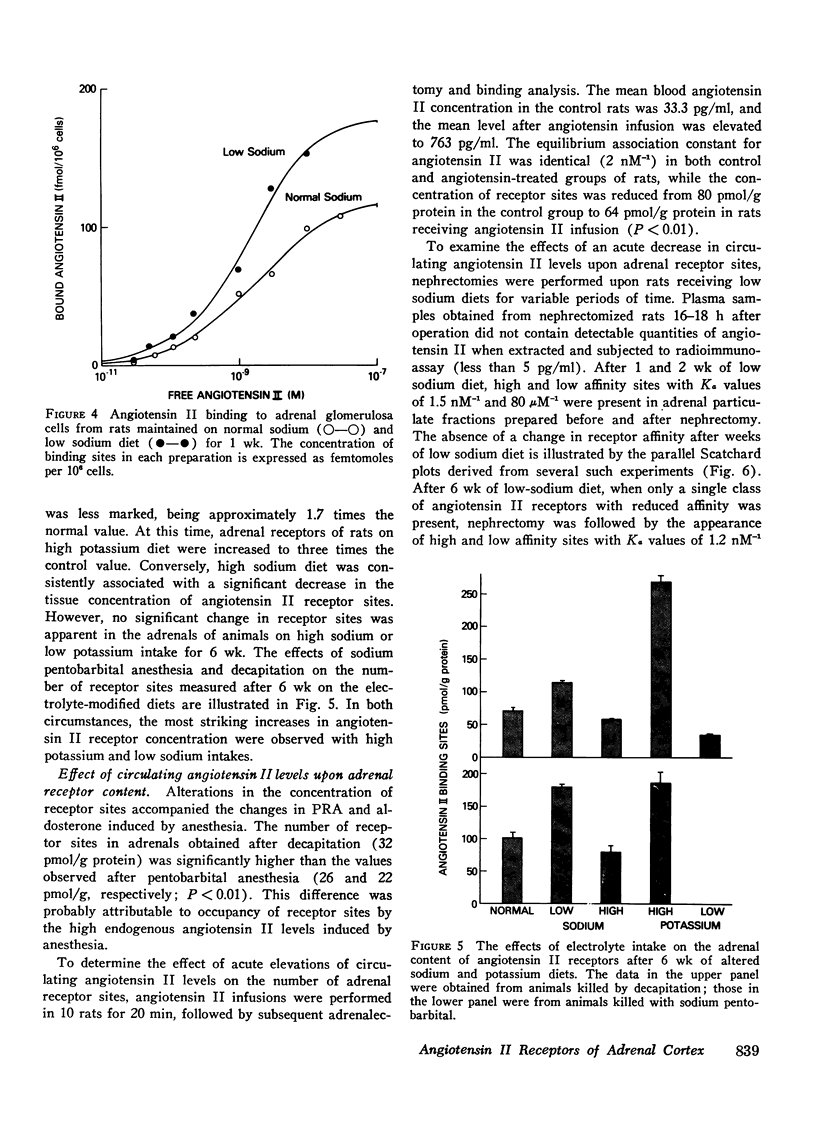
The binding affinity and concentration of specific angiotensin II receptor sites of rat adrenal cortical cells and homogenates were determined after 1 and 6 wk of altered sodium and potassium intake. Sodium deprivation caused marked increases in plasma renin, blood angiotensin II, and plasma aldosterone, and was accompanied by a significant increase (+74%) in the number of specific angiotensin II receptor sites per adrenal cortical cell. High potassium intake was followed by increased serum potassium and markedly elevated plasma aldosterone, with subnormal levels of renin and angiotensin II and a 170% increase in the number of angiotensin II receptors per cell after 1 wk. Sodium loading and potassium deprivation were followed by the opposite effect upon adrenal receptors, with reduction of the angiotensin II-binding capacity. None of the dietary electrolyte changes were accompanied by an ancrease in receptor affinity above the control value of 2 nM-1. A decrease in receptor affinity was noted after 6 wk of either low sodium or low potassium intake, when the renin and angiotensin II levels were increased by 104-129%. The adrenals of normal rats infused acutely with synthetic angiotensin II, or anesthetized with ether or sodium pentobarbital, which markedly increased plasma renin activity, contained fewer angiotensin receptors. These reductions in binding site concentration were not accompanied by changes in affinity and were attributed to occupancy by angiotensin II. These studies have demonstrated that chronic changes in sodium or potassium balance and acute changes in blood angiotensin II levels can exert modulating effects upon the adrenal content and/or affinity of specific receptor sites for angiotensin II.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell W. B., Brooks S. N., Pettinger W. A. Angiotensin II- and angiotensin 3-induced aldosterone release vivo in the rat. Science. 1974 May 31;184(4140):994–996. doi: 10.1126/science.184.4140.994. [DOI] [PubMed] [Google Scholar]
- Coleman T. G., McCaa R. E., McCaa C. S. Effect of angiotensin II on aldosterone secretion in the conscious rat. J Endocrinol. 1974 Mar;60(3):421–427. doi: 10.1677/joe.0.0600421. [DOI] [PubMed] [Google Scholar]
- Davis W. W., Burwell L. R., Bartter F. C. Inhibition of the effects of angiotensin II on adrenal steroid production by dietary sodium. Proc Natl Acad Sci U S A. 1969 Jul;63(3):718–723. doi: 10.1073/pnas.63.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meyts P. Cooperative properties of hormone receptors in cell membranes. J Supramol Struct. 1976;4(2):241–258. doi: 10.1002/jss.400040211. [DOI] [PubMed] [Google Scholar]
- Douglas J., Saltman S., Fredlund P., Kondo T., Catt K. J. Receptor binding of angiotensin II and antagonists. Correlation with aldosterone production by isolated canine adrenal glomerulosa cells. Circ Res. 1976 Jun;38(6 Suppl 2):108–112. doi: 10.1161/01.res.38.6.108. [DOI] [PubMed] [Google Scholar]
- Dufau M. L., Crawford J. D., Kliman B. Effect of high sodium intake on the response of the rat adrenal to angiotensin II. Endocrinology. 1969 Feb;84(2):462–463. doi: 10.1210/endo-84-2-462. [DOI] [PubMed] [Google Scholar]
- Dufau M. L., Kliman B. Acute effects of angiotensin-II-amide on aldosterone and corticosterone secretion by morphine-pentobarbital treated rats. Endocrinology. 1968 Jul;83(1):180–183. doi: 10.1210/endo-83-1-180. [DOI] [PubMed] [Google Scholar]
- GROSS F., BRUNNER H., ZIEGLER M. RENIN-ANGIOTENSIN SYSTEM, ALDOSTERONE, AND SODIUM BALANCE. Recent Prog Horm Res. 1965;21:119–177. [PubMed] [Google Scholar]
- Ganong W. F., Boryczka A. T., Shackelford R. Effect of renin on adrenocortical sensitivity to ACTH and angiotensin II in dogs. Endocrinology. 1967 Apr;80(4):703–706. doi: 10.1210/endo-80-4-703. [DOI] [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Roth J., Neville D. M., Jr, de Meyts P., Buell D. N. Insulin-dependent regulation of insulin receptor concentrations: a direct demonstration in cell culture. Proc Natl Acad Sci U S A. 1974 Jan;71(1):84–88. doi: 10.1073/pnas.71.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glossmann H., Baukal A. J., Catt K. J. Properties of angiotensin II receptors in the bovine and rat adrenal cortex. J Biol Chem. 1974 Feb 10;249(3):825–834. [PubMed] [Google Scholar]
- Hollenberg N. K., Chenitz W. R., Adams D. F., Williams G. H. Reciprocal influence of salt intake on adrenal glomerulosa and renal vascular responses to angiotensin II in normal man. J Clin Invest. 1974 Jul;54(1):34–42. doi: 10.1172/JCI107748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T., Woo J., Haning R., Horton R. A radioimmunoassay for aldosterone in human peripheral plasma including a comparison of alternate techniques. J Clin Endocrinol Metab. 1972 Jan;34(1):106–112. doi: 10.1210/jcem-34-1-106. [DOI] [PubMed] [Google Scholar]
- Ketelslegers J. M., Knott G. D., Catt K. J. Kinetics of gonadotropin binding by receptors of the rat testis. Analysis by a nonlinear curve-fitting method. Biochemistry. 1975 Jul 15;14(14):3075–3083. doi: 10.1021/bi00685a006. [DOI] [PubMed] [Google Scholar]
- Kinson G. A., Singer B. Sensitivity to angiotensin and adrenocorticotrophic hormone in the sodium deficient rat. Endocrinology. 1968 Nov;83(5):1108–1116. doi: 10.1210/endo-83-5-1108. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lesniak M. A., Roth J., Gorden P., Gavin J. R., 3rd Human growth hormone radioreceptor assay using cultured human lymphocytes. Nat New Biol. 1973 Jan 3;241(105):20–22. doi: 10.1038/newbio241020a0. [DOI] [PubMed] [Google Scholar]
- Menard J., Catt K. J. Measurement of renin activity, concentration and substrate in rat plasma by radioimmunoassay of angiotensin I. Endocrinology. 1972 Feb;90(2):422–430. doi: 10.1210/endo-90-2-422. [DOI] [PubMed] [Google Scholar]
- Oelkers W., Brown J. J., Fraser R., Lever A. F., Morton J. J., Robertson J. I. Sensitization of the adrenal cortex to angiotensin II in sodium-deplete man. Circ Res. 1974 Jan;34(1):69–77. doi: 10.1161/01.res.40.4.69. [DOI] [PubMed] [Google Scholar]
- Sealey J. E., Clark I., Bull M. B., Laragh J. H. Potassium balance and the control of renin secretion. J Clin Invest. 1970 Nov;49(11):2119–2127. doi: 10.1172/JCI106429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielman W. S., Davis J. O. The renin-angiotensin system and aldosterone secretion during sodium depletion in the rat. Circ Res. 1974 Oct;35(4):615–624. doi: 10.1161/01.res.35.4.615. [DOI] [PubMed] [Google Scholar]
- Thurston H., Laragh J. H. Prior receptor occupancy as a determinant of the pressor activity of infused angiotensin II in the rat. Circ Res. 1975 Jan;36(1):113–117. doi: 10.1161/01.res.36.1.113. [DOI] [PubMed] [Google Scholar]


