Abstract
Circulating levels of dehydroepiandrosterone, a major adrenal steroid, show a marked age-related decrease in both humans and nonhuman primates. Because this decrease has been implicated in age-related cognitive decline, we administered supplementary dehydroepiandrosterone to perimenopausal rhesus macaques (Macaca mulatta) to test for cognitive benefits. Although recognition memory improved, there was no benefit to spatial working memory. To address the limitations of this study we developed a hormone supplementation regimen in aged male macaques that more accurately replicates the 24-hr androgen profiles of young animals. We hypothesize that this more comprehensive physiological hormone replacement paradigm will enhance cognitive function in the elderly.
Introduction
The adrenal androgen dehydroepiandrosterone (DHEA) and its ester, dehydroepiandrosterone sulfate (DHEAS), decline markedly during aging, and this decrease has been linked to many age-related changes, including cognitive decline. Despite positive correlations between cognition and endogenous DHEA in aged humans,1,2 and beneficial effects of supplemental DHEA on cognitive performance in aged rodents,3,4 DHEA supplementation therapy appears to have little effect on memory in the elderly.5 One possible explanation for this lack of efficacy is an age-related decline in the conversion of DHEA to estradiol (E2) in memory-associated regions of the brain.6 Consistent with this view, the expression level of mRNA encoding the enzyme 3β-hydroxysteroid dehydrogenase (3BHSD2) declines significantly with age in the rhesus macaque (Macaca mulatta) hippocampus.7 Therefore, after menopause DHEA is less likely to be converted to E2.
In the present study, we used aged premenopausal female rhesus macaques (18–24 years old, n=8) to investigate if an earlier intervention of DHEA supplementation may preserve or improve cognition. The animals were trained to perform a spatial working memory task (Delayed Response [DR]) and a recognition memory task (Delayed Match-to-Sample [DMS]) using a touchscreen computer. In DR, the animal is shown a square on the left or right side of a screen. After a delay, squares are shown on the left and right side of the screen and the animal must select the side that was shown originally. In DMS, the animal is shown a picture in the center of the screen. After a delay, two pictures are shown and the animal must choose the picture shown originally. In both tasks, correct responses are rewarded with a treat and incorrect responses result in a timeout period. Animals were trained to a criterion of 85% correct at a 5-sec delay before baseline performance was assessed. The animals were tested both prior to treatment and after 2 months of daily morning oral DHEA supplementation (5 mg, Sigma-Aldrich Corp, St. Louis, MO). This treatment improved performance in the DMS task; in the DR task, improvement was seen at short delays of 5 sec but not on the more cognitively challenging longer delays, indicating a general lack of effect on working memory.
We suggest three possible explanations for the limited effect of DHEA supplementation on cognition. First, the usage of pre- or perimenopausal females meant that the animals may have had sufficient circulating E2 to maintain normal cognitive function, and so any potential benefits of DHEA-derived E2 may have been overshadowed by the endogenous ovary-derived E2. Second, because rhesus macaques undergo menopause relatively later in life than women,8 3BHSD2 expression and activity may already have been highly attenuated in these animals despite their premenopausal status. Consequently, cognition-associated brain areas of premenopausal macaques may have limited capacity to perform the first step of the conversion of DHEA to E2. Third, the dose and time of DHEA administration may have been suboptimal for accurately recreating the youthful pattern of DHEA and DHEAS in the circulation.9,10
To address all three of these concerns, we developed a novel oral hormone supplementation paradigm to further investigate the efficacy of steroid treatment on cognition in the aged. We used aged male rhesus macaques to avoid the confounding effect of variable E2 concentrations across the menstrual cycle. Old male macaques have reduced circulating testosterone (T) levels.11 Furthermore, they show reduced expression of 3BHSD2 in the hippocampus,7 and consequently may be less able to centrally convert DHEA to T. Therefore, we supplemented the animals with both DHEA and T. Note that aromatase, the enzyme responsible for conversion of T to E2, does not seem to decline with age, and therefore it is expected that this portion of the intracrine pathway may remain intact in old age.7 Furthermore, to address the third limitation of the prior study, we administered the two hormones at physiologically appropriate times of day; T was administered orally at the start of the night and DHEA was administered orally in the morning.11 To confirm that physiologically normal hormone profiles were established, a 24-hr remote blood sampling paradigm12 was used to monitor the effects of hormone supplementation at varying times and various doses to accurately match those of young control males.
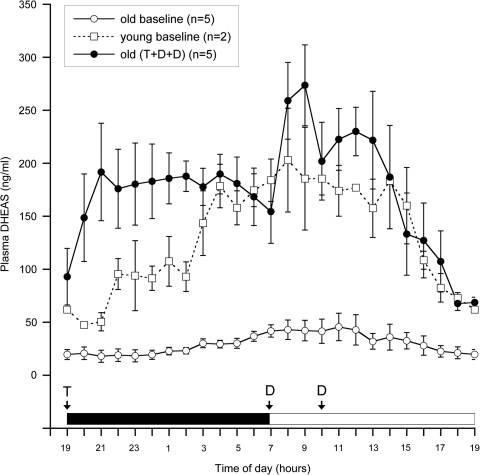
Briefly, young (6–7 years, n=2) and old animals (20–25 years, n=5) were implanted with an intravenous catheter in the right subclavian vein. Catheter tubing was routed to a swivel assembly located at the top of the animal's cage, allowing for unrestricted movement and undisturbed blood collection from an adjacent room. The animals were housed on a 12:12 light:dark cycle, with lights on from 0700–1900 hr. Blood was collected hourly over a 24-hr period from 1900 hr to 1900 hr into EDTA-treated borosilicate tubes. Plasma was isolated and levels of T, cortisol, and DHEAS were assessed by electrochemiluminescence, using the Elecsys 2010 System (Roche Diagnostics, Indianapolis, IN). The old animals were then supplemented with various doses of T (Sigma-Aldrich Corp, St. Louis, MO) and DHEA, given at various times of the day. Once an optimal dose and time of administration were established, the animals were treated for 5 consecutive days with a combination of T and DHEA, and their circulating DHEAS profiles were determined (Fig. 1). The hormone supplementation paradigm consisted of oral T administration (12 mg/kg body weight) just before lights off at 1900 hr, followed by two oral DHEA administrations (0.04 mg/kg body weight) just after 700 hr and 1000 hr in the morning.
FIG. 1.
Effect of testosterone (T) and dehydroepiandrosterone (DHEA) administration on plasma dehydroepiandrosterone sulfate (DHEAS) levels in aged male rhesus macaques. Each data point represents the mean, and the vertical lines represent standard errors of the mean (SEMs). Blood samples were collected remotely every hour for 24 hr, starting at 1900 hr, from young (6–7 years old) and old (20–25 years old) males. The light cycle is represented on the abscissa, with a black bar indicating lights off and an open bar indicating lights on. Baseline plasma DHEAS levels of young and old animals are shown as open squares and open circles, respectively. Plasma DHEAS levels of the same old animals after oral T administration (12 mg/kg body weight) and two oral DHEA administrations (0.04 mg/kg body weight) are shown as filled circles. Note that the times of T and DHEA administration are indicated in the figure by T and D, respectively; the blood sampling was initiated on the fifth consecutive day of hormone treatment. The data demonstrate a pragmatic oral hormone replacement paradigm that elevates plasma DHEAS levels to those observed in young animals, while maintaining a clear circadian pattern.
Previously, it has been shown that when testosterone is administered orally in oil, significant quantities bypass the liver, presumably because of its uptake by the lymphatic system, and elevate circulating T concentrations.13 Consequently, we suspended the T in sesame oil at a concentration of 120 mg/mL, and then mixed it with ∼12 grams of chocolate or placed inside a 5-gram cookie, based on the animal's preference. Similarly, we suspended the DHEA in sesame oil (2 mg/mL) and mixed it with chocolate or placed it inside a cookie. Our preliminary studies indicated that a single administration of DHEA failed to produce a sustained elevation of circulating DHEAS, confirming that the single-dose DHEA paradigm used in the premenopausal females was not ideal at reproducing the sustained elevation of DHEAS that is typically observed in young animals9,10; in contrast, the plasma DHEAS levels resulting from the new double-dose DHEA supplementation paradigm closely followed those of the young control males.
T secretion in male macaques, as well as men, shows a 24-hr pattern, with peak levels occurring during the night; they also show a significant age-related decrease.10,11,14 As expected, when we administered T to the old animals, their plasma T levels increased to young levels within an hour of oral administration. Due to the relatively high clearance rate of testosterone, this elevation was not sustained throughout the night; however, plasma levels of dihydrotestosterone (DHT), the more active metabolite of T, closely resembled that of young males after T administration (data not shown).
Interestingly, T alone resulted in a nocturnal increase in circulating DHEAS levels. This increase was replicated when DHT was administered in place of T (data not shown), suggesting it is an effect of androgen receptor activation and not due to back-conversion to DHEAS. This novel finding demonstrates that the combined treatment of night-time T and morning DHEA not only raises morning DHEAS levels and nocturnal T/DHT levels to those observed in young adults, but also that the circadian rhythms of DHEAS, T, and DHT are all preserved, thereby maintaining normal physiologically youthful patterns. The findings also demonstrate that feedback mechanisms exist between the adrenal and gonadal steroid systems, as previously observed in human females,15,16 but not yet closely studied or observed in males. Note that plasma cortisol levels also showed a clear circadian pattern, with a diurnal peak, but as expected the 24-hr cortisol profiles were similar in all animals regardless of age or hormonal treatment (data not shown).
We propose that this steroid regimen of combined T and DHEA supplementation at physiologically appropriate times of the day closely mimics the circadian hormone profiles of young animals and so represents an improved paradigm for assessing the physiological effects of hormone supplementation during aging. Furthermore, because T has the potential to be converted both to E2, as well as to androgenic metabolites (DHT, 3α-diol) that have been implicated in improved cognitive performance,17–19 we predict that this protocol will result in significant memory improvement and will provide a basis for further investigation of the interaction of hormone systems during aging.
Acknowledgments
We wish to thank the ONPRC Endocrine Technology and Support Core for help with the hormone measurements. This work was supported by National Institutes of Health (NIH) grants AG-023477, AG-029612, AG-036670, and RR-000163.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Davis SR. Shah SM. McKenzie DP. Kilkarni J. Davison SL. Bell RJ. Dehydroepiandrosterone sulfate levels are associated with more favorable cognitive function in women. J Clin Endocrinol Metab. 2008;93:801–808. doi: 10.1210/jc.2007-2128. [DOI] [PubMed] [Google Scholar]
- 2.Sanders JL. Cappola AR. Arnold AM. Boudreau RM. Chaves PH. Robbins J. Cushman M. Newman AB. Concurrent change in dehydroepiandrosterone sulfate and functional performance in the oldest-old: Results from the Cardiovascular Health Study All Stars study. J Gerontol A Biol Sci Med Sci. 2010;65:976–981. doi: 10.1093/gerona/glq072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flood JF. Roberts E. Dehydroepiandrosterone sulfate improves memory in aging mice. Brain Res. 1988;448:178–181. doi: 10.1016/0006-8993(88)91116-x. [DOI] [PubMed] [Google Scholar]
- 4.Markowski M. Ungeheuer M. Bitran D. Locurto C. Memory-enhancing effects of DHEAS in aged mice on a win-shift water escape task. Physiol Behav. 2001;72:521–525. doi: 10.1016/s0031-9384(00)00446-7. [DOI] [PubMed] [Google Scholar]
- 5.Grimley-Evans J. Malouf R. Huppert F. van Niekerk JK. Dehydroepiandrosterone (DHEA) supplementation for cognitive function in healthy elderly people. Cochrane Database Syst Rev. 2006;2006;18:CD006221. doi: 10.1002/14651858.CD006221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sorwell KG. Urbanski HF. Dehydroepiandrosterone and age-related cognitive decline. Age (Dordr) 2010;32:61–67. doi: 10.1007/s11357-009-9113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorwell KG. Kohama SG. Urbanski HF. Perimenopausal regulation of steroidogenesis in the nonhuman primate. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.05.004. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Downs JL. Urbanski HF. Neuroendocrine changes in the aging reproductive axis of female rhesus macaques (Macacaca mulatta) Biol Reprod. 2006;75:539–546. doi: 10.1095/biolreprod.106.051839. [DOI] [PubMed] [Google Scholar]
- 9.Downs JL. Mattison JA. Ingram DK. Urbanski HF. Effect of age and caloric restriction on circadian adrenal steroid rhythms in rhesus macaques. Neurobiol Aging. 2008;29:1412–1422. doi: 10.1016/j.neurobiolaging.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Urbanski HF. Role of circadian neuroendocrine rhythms in the control of behavior and physiology. Neuroendocrinology. 2011;93:211–222. doi: 10.1159/000327399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Urbanski HF. Sorwell K. Age-related changes in neuroendocrine rhythmic function in the rhesus macaque. Age (Dodr) 2012 doi: 10.1007/s11357-011-9352-z. (Special Issue: Primate Models of Aging. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Urbanski HF. Circadian variation in the physiology and behavior of humans and nonhuman primates. In: Raber J, editor. Animal Models of Behavioral Analysis. Springer; New York: 2011. pp. 217–235. Neuromethods 50. [Google Scholar]
- 13.Amory JK. Bremner WJ. Oral testosterone in oil plus dutasteride in men: A pharmacokinetic study. J Clin Endocrinol Metab. 2005;90:2610–2317. doi: 10.1210/jc.2004-1221. [DOI] [PubMed] [Google Scholar]
- 14.Schlatt S. Pohl CR. Ehmcke J. Ramaswamy S. Age-related changes in diurnal rhythms and levels of gonadotropins, testosterone, and inhibin B in male rhesus monkeys (Macaca mulatta) Biol Reprod. 2008;79:93–99. doi: 10.1095/biolreprod.107.066126. [DOI] [PubMed] [Google Scholar]
- 15.Pluchino N. Genazzani AD. Barnardi F. Casarosa E. Pieri M. Palumbo M. Picciarelli G. Gabbinini M. Luisi M. Genazzani AR. Tibolone, transdermal estradiol or oral estrogen-progestin therapies: Effects on circulating allopregnanonlone, cortisol and dehydroepiandrosterone levels. Gynecol Endocrinol. 2005;20:144–149. doi: 10.1080/09513590400021169. [DOI] [PubMed] [Google Scholar]
- 16.Crawford S. Santoro N. Laughlin GA. Sowers MF. McConnell D. Sutton Turrell K. Weiss G. Vuga M. Randolph J. Lasley B. Circulating dehydroepiandrosterone sulfate concentrations during the menopausal transition. J Clin Endocrinol Metab. 2009;94:2945–2951. doi: 10.1210/jc.2009-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rapp PR. Morrison JH. Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003;23:5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spritzer MD. Galea LA. Testosterone and dihydrotestosterone, but not estradiol, enhance survival of new hippocampal neurons in adult male rats. Dev Neurobiol. 2007;67:1321–1333. doi: 10.1002/dneu.20457. [DOI] [PubMed] [Google Scholar]
- 19.Frye CA. Edinger KL. Lephart ED. Walf AA. 3alpha-androstanediol, but not testosterone, attenuates age-related decrements in cognitive, anxiety, and depressive behavior of male rats. Front Age Neurosci. 2010;2:15. doi: 10.3389/fnagi.2010.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]