Abstract
Background
Type-1 long-QT syndrome (LQTS) is caused by loss-of-function mutations in the KCNQ1-encoded IKs cardiac potassium channel. We evaluated the effect of location, coding type, and biophysical function of KCNQ1 mutations on the clinical phenotype of this disorder.
Methods and Results
We investigated the clinical course in 600 patients with 77 different KCNQ1 mutations in 101 proband-identified families derived from the US portion of the International LQTS Registry (n=425), the Netherlands’ LQTS Registry (n=93), and the Japanese LQTS Registry (n=82). The Cox proportional hazards survivorship model was used to evaluate the independent contribution of clinical and genetic factors to the first occurrence of time-dependent cardiac events from birth through age 40 years. The clinical characteristics, distribution of mutations, and overall outcome event rates were similar in patients enrolled from the 3 geographic regions. Biophysical function of the mutations was categorized according to dominant-negative (>50%) or haploinsufficiency (≤50%) reduction in cardiac repolarizing IKs potassium channel current. Patients with transmembrane versus C-terminus mutations (hazard ratio, 2.06; P<0.001) and those with mutations having dominant-negative versus haploinsufficiency ion channel effects (hazard ratio, 2.26; P<0.001) were at increased risk for cardiac events, and these genetic risks were independent of traditional clinical risk factors.
Conclusions
This genotype–phenotype study indicates that in type-1 LQTS, mutations located in the transmembrane portion of the ion channel protein and the degree of ion channel dysfunction caused by the mutations are important independent risk factors influencing the clinical course of this disorder.
Keywords: electrocardiography, genetics, long-QT syndrome
The hereditary long-QT syndrome (LQTS) is characterized by prolonged ventricular repolarization on the ECG and arrhythmia-related syncope and sudden death.1 Mutations in 1 or more of several ion channel genes are known to cause this disorder,2 with mutations in the KCNQ1 gene causing the type-1 long-QT syndrome.3,4 The KCNQ1 gene codes for the potassium channel protein responsible for the slow component of the delayed rectifier repolarizing current (IKs). Mutations involving this gene result in reduction of the repolarizing IKs current and lengthening of the QT interval.3
Functional IKs channels result from the coassembly of 4 subunits into a tetrameric protein channel that is transported to the myocyte membrane. Each subunit contains 6 membrane-spanning domains (S1 to S6) flanked by amino (N)- and carboxyl (C)-terminus regions. Two distinct biophysical mechanisms mediate the reduced IKs current in patients with KCNQ1 mutations: (1) coassembly or trafficking defects in which mutant subunits are not transported properly to the cell membrane and fail to incorporate into the tetrameric channel, with the net effect being a ≤50% reduction in channel function (haploinsufficiency)5; and (2) formation of defective channels involving mutant subunits with the altered channel protein transported to the cell membrane, resulting in a dysfunctional channel having >50% reduction in channel current (dominant-negative effect).6
Limited prior studies involving relatively small numbers of patients with type-1 LQTS have been reported with conflicting results on the relationship between various KCNQ1 mutations and the clinical outcome.7,8 We hypothesized that the location, coding type, and functional effect of the channel mutation would have important influence on the phenotypic manifestations and clinical course of patients with this disorder. To test this hypothesis, we investigated the clinical aspects of a large cohort of subjects having a spectrum of KCNQ1 mutations categorized by their location, coding type, and type of biophysical ion channel dysfunction.
Methods
Study Population
The study population of 600 subjects with genetically confirmed KCNQ1 mutations was derived from 101 proband-identified families with the type-1 LQTS disorder. The proband in each family had QTc prolongation not due to a known cause. The subjects were drawn from the US portion of the International LQTS Registry (n=425), the Netherlands’ LQTS Registry (n=93), and the Japanese LQTS Registry (n=82). All subjects or their guardians provided informed consent for the genetic and clinical studies.
Phenotype Characterization
Routine clinical and ECG parameters were acquired at the time of enrollment in each of the registries. Follow-up was censored at age 41 years to avoid the influence of coronary disease on cardiac events. Measured parameters on the first recorded ECG included QT and R-R intervals in milliseconds, with QT corrected for heart rate by Bazett’s formula. The QTc interval was expressed in its continuous form and categorized into 3 levels: <500, 500 to 530, and >530 ms. Clinical data were collected on prospectively designed forms with information on demographic characteristics, personal and family medical history, ECG findings, therapy, and end points during long-term follow-up. LQTS-related cardiac events included syncope, aborted cardiac arrest, or unexpected sudden death without a known cause. Data common to all 3 LQTS registries involving genetically identified patients with type-1 genotype were electronically merged into a common database for the present study.
Genotype Characterization
The KCNQ1 mutations were identified with the use of standard genetic tests performed in academic molecular-genetic laboratories including the Functional Genomics Center, University of Rochester Medical Center, Rochester, NY; Baylor College of Medicine, Houston, Tex; Mayo Clinic College of Medicine, Rochester, Minn; Boston Children’s Hospital, Boston, Mass; Laboratory of Molecular Genetics, National Cardiovascular Center, Suita, Japan; and Department of Clinical Genetics, Academic Medical Center, Amsterdam, Netherlands.
Genetic alterations of the amino acid sequence were characterized by location and by the specific mutation (missense, splice site, in-frame insertions/deletions, nonsense, stop codon, and frameshift). The transmembrane region of the KCNQ1-encoded channel was defined as the coding sequence involving amino acid residues from 120 through 355 (S5-pore-S6 region 285 to 355), with the N-terminus region defined before residue 120 and the C-terminus region after residue 355. Nineteen study patients had intron mutations predicted to disrupt the canonical splice-site domains. Fifty-one subjects died of sudden cardiac death at a young age but did not have genotype studies. These 51 subjects were assumed to have the same KCNQ1 mutation as other affected members of their respective family. Twelve subjects had 2 mutations, one in the KCNQ1 gene and a second mutation in another LQTS ion channel gene; these 12 subjects are described separately and are not included in any of the tables or outcome analyses. Subjects with Jervell and Lange-Nielsen syndrome with deafness and 2 KCNQ1 mutations as well as those with 1 known KCNQ1 mutation and congenital deafness are not included in the present study.
The biophysical function of the mutant channels was classified as having dominant-negative effect (>50% reduction in function) or haploinsufficiency (≤50% reduction in function) on the basis of the following: (1) cellular expression studies for those with missense (n=21) and nonsense (n=2) mutations reported in the literature, with the functional information derived exclusively from heterologous expression studies; and (2) assumed loss of function for identified nonsense, splice site, in-frame deletion, and frameshift mutations (n=10) that have not yet been functionally characterized. Forty-one missense mutations and the 3 intron mutations that have not been functionally reported in cellular expression studies were categorized as unknown in terms of type of functional perturbation.
Statistical Analysis
Differences in the univariate characteristics by specific groupings were evaluated by standard statistical methods. The primary end point was time to syncope, aborted cardiac arrest, or sudden death, whichever occurred first. The cumulative probability of a first cardiac event was assessed by the Kaplan-Meier method with significance testing by the log-rank statistic. The Cox proportional hazards survivorship model was used to evaluate the independent contribution of clinical and genetic factors to the first occurrence of time-dependent cardiac events from birth through age 40 years.9 Stratified and unstratified Cox regression models, allowing for time-dependent covariates, were fit to estimate the adjusted hazard ratio of each factor as a predictor of first cardiac events. We observed that sex was not proportional as a function of age with crossover in risk at age 13 years on univariate Kaplan-Meier analysis. To relax the assumption of proportional hazards for sex over the entire age range, separate nonparametric baseline hazard functions were allowed for male and female subjects via the stratified Cox model; then, to summarize the sex effect, sex was modeled in an unstratified Cox model as a time-dependent covariate (via an interaction with time), allowing for different hazard ratios by sex before and after age 13 years.
Because almost all the subjects were first- and second-degree relatives of probands, the effect of lack of independence between subjects was evaluated in the Cox model with grouped jackknife estimates for family membership.10 All grouped jackknife standard errors for the covariate risk factors fell within 3% of those obtained from the unadjusted Cox model, and therefore only the Cox model findings are reported.
Patients who died suddenly at a young age from suspected LQTS and who did not have an ECG for QTc measurement were identified in the Cox models as “QTc missing.” Prespecified covariate interactions were evaluated. The influence of time-dependent β-blocker therapy (the age at which β-blocker therapy was initiated) on outcome was determined by adding this variable to the final Cox model containing the various covariates.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Total Study Population
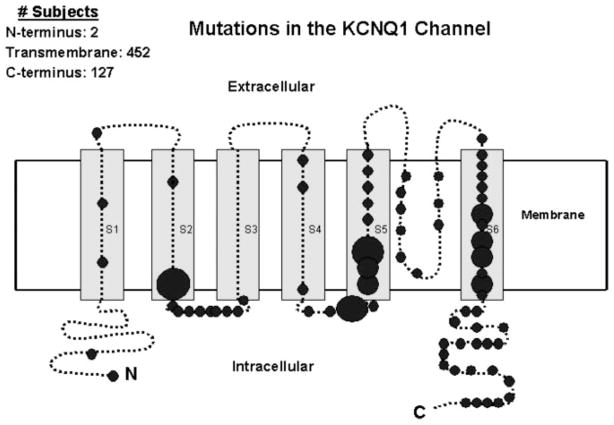
The spectrum and number of KCNQ1 mutations by location, type of mutation, and functional effect are presented in Table 1, with the location frequency of the mutations presented diagrammatically in Figure 1. A total of 77 different KCNQ1 mutations were identified. A majority of the mutations were localized to the S1 to S6 transmembrane domains (66%), and missense (single amino acid substitutions) accounted for 81% of all the mutations.
TABLE 1.
KCNQ1 Mutations by Location and Coding, Type of Mutation, and Functional Effect
| Location and Coding* | No. of Subjects† | Type of Mutation | Functional Effect‡ |
|---|---|---|---|
| N-terminus | |||
| M1V | 1 | Missense | Unknown |
| G57V | 1 | Missense | Unknown |
| Transmembrane | |||
| W120C | 2 | Missense | Unknown |
| T144A | 7 | Missense | Unknown |
| A150fs/133 [del CT 451-452] | 2 | Frameshift | Haploinsufficiency |
| E160K | 3 | Missense | Unknown |
| G168R | 44 | Missense | Unknown |
| Y171X [513 C>G] | 6 | Nonsense | Haploinsufficiency |
| R174H | 2 | Missense | Unknown |
| A178P | 5 | Missense | Dominant-negative effect (a) |
| Y184S | 18 | Missense | Unknown |
| G185S | 10 | Missense | Unknown |
| G189E | 2 | Missense | Unknown |
| G189R | 4 | Missense | Dominant-negative effect (b) |
| R190Q | 4 | Missense | Haploinsufficiency (b, c) |
| L191fs/90 [del TGCGC 572-576] | 8 | Frameshift | Haploinsufficiency |
| R195fs/40 [del G 585] | 2 | Frameshift | Haploinsufficiency |
| S225L | 13 | Missense | Dominant-negative effect (d) |
| A226V | 3 | Missense | Unknown |
| R237P | 1 | Missense | Unknown |
| D242N | 3 | Missense | Unknown |
| R243C | 13 | Missense | Haploinsufficiency (e) |
| V254 mol/L | 59 | Missense | Dominant-negative effect (b, f) |
| R258C | 1 | Missense | Haploinsufficiency |
| R259C | 1 | Missense | Haploinsufficiency (g) |
| L266P | 15 | Missense | Unknown |
| G269D | 35 | Missense | Dominant-negative effect (h) |
| G269S | 25 | Missense | Haploinsufficiency (i) |
| L273F | 6 | Missense | Dominant-negative effect (a) |
| I274V | 1 | Missense | Unknown |
| S277L | 3 | Missense | Unknown |
| Y278H | 2 | Missense | Unknown |
| E284K | 2 | Missense | Unknown |
| G292D | 3 | Missense | Unknown |
| F296S | 2 | Missense | Unknown |
| G306R | 2 | Missense | Dominant-negative effect (b, j) |
| V310I | 1 | Missense | Unknown |
| T312I | 14 | Missense | Dominant-negative effect (a) |
| G314S | 8 | Missense | Dominant-negative effect (h, k, l, m) |
| Y315C | 10 | Missense | Dominant-negative effect (d, n) |
| Y315S | 1 | Missense | Dominant-negative effect (h, m) |
| D317G | 3 | Missense | Unknown |
| P320H | 1 | Missense | Unknown |
| T322 mol/L | 2 | Missense | Unknown |
| G325R | 3 | Missense | Unknown |
| delF340 [del CTT 1017-1019] | 7 | In-frame deletion | Haploinsufficiency |
| A341E | 9 | Missense | Dominant-negative effect (b) |
| A341V | 20 | Missense | Dominant-negative effect (o) |
| P343S | 1 | Missense | Dominant-negative effect (p) |
| A344A/sp [1032 G>A] | 27 | Splice site | Haploinsufficiency |
| A344V | 17 | Missense | Unknown |
| S349W | 15 | Missense | Unknown |
| L353P | 4 | Missense | Unknown |
| C-terminus | |||
| Q357H | 3 | Missense | Unknown |
| R360G | 3 | Missense | Unknown |
| S373P | 7 | Missense | Unknown |
| K393N | 10 | Missense | Unknown |
| R397W | 5 | Missense | Unknown |
| P400fs/62 [ins C 1201-1022] | 6 | Frameshift | Haploinsufficiency |
| P448fs/13 [ins G 1344-1345] | 11 | Frameshift | Haploinsufficiency |
| I517T | 3 | Missense | Unknown |
| R518X [1552 C>T] | 11 | Nonsense | Haploinsufficiency (q) |
| M520R | 3 | Missense | Unknown |
| V524G | 4 | Missense | Unknown |
| Q530X [1588 C>T] | 13 | Nonsense | Haploinsufficiency (q) |
| R562 mol/L | 2 | Missense | Unknown |
| S566F | 3 | Missense | Unknown |
| I567S | 6 | Missense | Unknown |
| S571fs/20 [del C 1714] | 3 | Frameshift | Haploinsufficiency |
| R591C | 5 | Missense | Unknown |
| R591H | 6 | Missense | Haploinsufficiency (r) |
| R594Q | 11 | Missense | Haploinsufficiency (q) |
| D611Y | 10 | Missense | Haploinsufficiency (s) |
| A636fs/28 [del C 1909] | 2 | Frameshift | Haploinsufficiency |
| Intron | |||
| IVS2+1 G>A | 2 | Splice site | Unknown |
| IVS4+5 G>A | 2 | Splice site | Unknown |
| IVS7+5 G>A | 15 | Splice site | Unknown |
The numbers and letters refer to the amino acid coding of the mutant channel protein. The brackets contain the nucleotide code for deletions, frameshift, splice site, and nonsense mutations.
Included in this table are 52 subjects who died suddenly at a young age. These subjects were from families with a known KCNQ1 mutation and were assumed to have their respective family mutation.
Dominant-negative effect is associated with >50% reduction whereas haploinsufficiency is associated with <50% reduction in ion channel repolarizing current. See text for details. Letters in parentheses refer to references that are available in the online-only Data Supplement.
Figure 1.
Frequency and location of 74 different mutations in the KCNQ1 potassium channel involving 581 subjects. The 19 subjects with 3 intron mutations are not included in this diagram. The α subunit involves the N-terminus (N), 6 membrane-spanning segments, and the C-terminus portion (C). The size of the circles reflect the number of subjects with mutations at the respective locations, with the small circles indicating <15, medium-sized circles 15 to 30, and large circles >30 subjects.
The phenotypic characteristics of patients enrolled in each of the 3 registries and by location and type of mutation are presented in Table 2. The clinical characteristics of the patients were similar among the 3 registries except for QTc duration and frequency of β-blocker use. The QTc interval was longer and cardiac events and β-blocker use were more frequent in patients with mutations in the transmembrane than in the C-terminus location. β-Blockers were used less frequently in patients from the Japanese registry than in patients from the other 2 registries. The frequency of first cardiac events was higher in those with than without missense mutations. The clinical characteristics of the 19 subjects possessing intron mutations resembled those with transmembrane and missense mutations.
TABLE 2.
Phenotypic Characteristics by Source of Subjects, Location of Mutation, and Type of Mutation
| Characteristics | Source of Subjects
|
Location of Mutation
|
Missense Mutation
|
Intron Mutation (n=19) | ||||
|---|---|---|---|---|---|---|---|---|
| United States (n=425) | Netherlands (n=93) | Japan (n=82) | Trans Membrane (n=452) | C-Terminus (n=127) | Yes (n=483) | No (n=98) | ||
| Female, % | 57 | 53 | 54 | 57 | 51 | 54 | 62 | 63 |
| ECG at enrollment | ||||||||
| QTc†‡, ms | 488±58 | 450±45 | 472±46 | 485±53 | 460±61 | 481±59 | 471±38 | 478±60 |
| Therapy, % | ||||||||
| β-Blockers†‡ | 45 | 34 | 26 | 45 | 28 | 42 | 38 | 37 |
| Pacemaker | 2.4 | 0 | 0 | 1.5 | 2.4 | 1.4 | 3.1 | 0 |
| Sympathectomy | 0.5 | 0 | 0 | 0.4 | 0 | 0.4 | 0 | 0 |
| Defibrillator | 6.4 | 3.2 | 0 | 5.8 | 3.1 | 5.2 | 5.1 | 0 |
| First cardiac event*‡§, % | 41 | 37 | 38 | 45 | 21 | 43 | 26 | 42 |
| Syncope‡ (n=200) | 35 | 31 | 29 | 38 | 17 | 36 | 21 | 32 |
| Aborted cardiac arrest (n=15) | 1.9 | 1.1 | 7.3 | 2.9 | 0.8 | 2.5 | 2.0 | 5.3 |
| Death (n=23) | 4.0 | 5.5 | 1.2 | 4.0 | 3.1 | 4.2 | 2.0 | 5.3 |
| Ever cardiac event, % | ||||||||
| Syncopeठ| 35 | 31 | 31 | 39 | 17 | 37 | 21 | 33 |
| Aborted cardiac arrest† | 2.4 | 15 | 8.8 | 5.3 | 3.2 | 5.4 | 2.0 | 11 |
| Death | 11 | 14 | 2.4 | 10 | 6.3 | 11 | 4.1 | 26 |
Plus-minus values are mean±SD. Percentages >10 are rounded to a whole number. The 600 subjects in this table include 51 subjects who died suddenly at a young age, were from families with known KCNQ1 mutation, and were assumed to have the family mutation. Patients with intron mutations are categorized separately and are not included in the location or missense categories. Seven subjects with transmembrane mutations and 1 with C-terminus mutations had missing data about the date of the first cardiac event. Eight subjects with missense mutations had missing data about the date of the first cardiac event. Numbers in parentheses indicate the total number of specific first cardiac events from the 3 sources of patients.
First cardiac event was syncope, aborted cardiac arrest, or sudden death, whichever occurred first.
P<0.01 for the comparison of characteristics among the 3 sources of subjects.
P<0.01 for the comparison of characteristics between the 2 locations of the mutations.
P<0.01 for the comparison of characteristics between missense yes and no.
The QTc interval was significantly longer in the 12 patients with 2 mutations than in those with only single KCNQ1 mutations (570±70 versus 480±60 ms; P<0.01). All 12 patients with 2 mutations experienced at least 1 cardiac event.
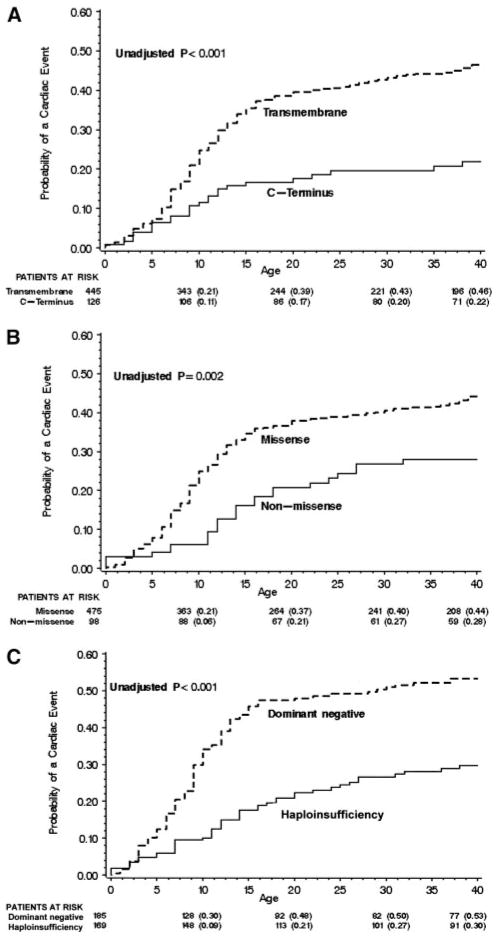
The cumulative probabilities of first cardiac event by location and type of mutation are presented in Figure 2A and 2B, respectively. Significantly higher event rates were found in subjects with transmembrane than C-terminus mutations and in those with than without missense mutations, with the most rapid increase in event rates occurring during ages 7 to 20 years. In patients with transmembrane-localized mutations, the event rates for patients with mutations localized to the pore region (S5-pore-S6) were nearly identical to those with nonpore mutations (data not shown).
Figure 2.
Kaplan-Meier estimate of the cumulative probability of a first cardiac event by location (A), type (B), and biophysical function of the mutation (C).
The findings from the Cox regression analysis for location and type of mutation are presented in Table 3. The clinical risk factors associated with first cardiac events involved males before age 13 years, females after age 13 years, and longer QTc intervals. Mutations located in the transmembrane region of the channel made significant and independent contributions to the risk model, but missense mutations were not an independent risk factor. Three different intron mutations were present in 19 subjects from 4 families, and these intron mutations made a meaningful but nonsignificant contribution to the risk model. Prespecified interactions were investigated for their effect on cardiac events, and no significant interactions were found for transmembrane location by type of mutation, transmembrane location by QTc, or mutation type by QTc. Time-dependent β-blocker use was associated with a significant 74% reduction in the risk of first cardiac events (P<0.001).
TABLE 3.
Cox Regression With Multiple Predictor Variables Including Location and Type of Mutations for First Cardiac Event
| Variable | Hazard Ratio | 95% CI | P |
|---|---|---|---|
| Netherlands:United States | 1.15 | 0.74–1.78 | 0.55 |
| Japan:United States | 1.45 | 0.98–2.16 | 0.07 |
| Male <13 y:female <13 y | 1.72 | 1.25–2.38 | <0.001 |
| Female 13–40 y:male 13–40 y | 2.27 | 1.30–3.96 | <0.01 |
| QTc 500–530 ms:QTc <500 ms | 2.04 | 1.41–2.96 | <0.001 |
| QTc >530 ms:QTc <500 ms | 3.25 | 2.25–4.69 | <0.001 |
| QTc missing*:QTC <500 ms | 2.26 | 1.57–3.25 | <0.001 |
| Transmembrane:C-terminus | 2.06 | 1.36–3.12 | <0.001 |
| Missense yes:no | 1.33 | 0.86–2.05 | 0.20 |
| Intron:C-terminus | 2.45 | 0.98–6.11 | 0.06 |
| Time-dependent β-blocker use | 0.26 | 0.14–0.49 | <0.001 |
The Cox analysis involved 592 subjects with 445 transmembrane, 126 C-terminus, 2 N-terminus, and 19 intron mutations; 8 subjects were not included in this Cox analysis because of missing data about the date of their first cardiac event.
QTc missing category involves 47 subjects who died suddenly at a young age without a prior ECG.
Biophysical Function and Outcome
The clinical implications of disordered biophysical function of the mutant KCNQ1 channels were investigated in a subset of 356 subjects with known or suspected alteration in ion channel function (see Methods for functional categorization). The clinical characteristics of patients with dominant-negative and haploinsufficiency ion channel dysfunction are presented in Table 4. Patients with mutations having dominant-negative ion current effects had a longer QTc interval and a higher frequency of cardiac events than subjects with mutations resulting in haploinsufficiency. The cumulative probabilities of a first cardiac event by the biophysical function of the mutations are presented in Figure 2C. As shown in Table 5, patients with mutations having dominant-negative functional effects experienced a significantly greater risk for cardiac events than those with haploinsufficiency (hazard ratio, 2.26; 95% CI, 1.56 to 3.25; P<0.001) after adjustment for relevant covariates including QTc and gender effects by age group. β-Blocker use was associated with a significant 79% reduction in first cardiac events in this subset of patients. Because substantial colinearity exists for transmembrane mutations, missense mutations, and mutations with dominant-negative biophysical function, the individual effects of these 3 mutation parameters could not be ascertained reliably in the same Cox model.
TABLE 4.
Phenotypic Characteristics by Biophysical Function of the KCNQ1 Mutations in 356 Subjects
| Characteristics | Dominant-Negative Effect (n=187) | Haploinsufficiency (n=169) |
|---|---|---|
| Female, % | 51 | 61 |
| ECG at enrollment | ||
| QTc,* ms | 500±60 | 470±50 |
| Therapy, % | ||
| β-Blockers | 47 | 37 |
| Pacemaker | 1.1 | 4.1 |
| Sympathectomy | 0.5 | 0 |
| Defibrillator | 4.8 | 7.7 |
| First cardiac event*, % | 53 | 27 |
| Syncope | 45 | 22 |
| Aborted cardiac arrest | 2.1 | 3.0 |
| Death | 5.3 | 2.4 |
Percentages >10 are rounded to a whole number. Two subjects had missing data about the date of their first cardiac event.
P<0.01.
TABLE 5.
Cox Regression With Multiple Predictor Variables Including Ion Channel Dysfunction for First Cardiac Events
| Variable | Hazard Ratio | 95% CI | P |
|---|---|---|---|
| Netherlands:United States | 2.78 | 1.48–5.23 | <0.01 |
| Japan:United States | 1.63 | 1.02–2.63 | 0.04 |
| Male <13 y:female <13 y | 1.94 | 1.29–2.91 | <0.01 |
| Female 13–40 y:male 13–40 y | 1.95 | 0.99–3.87 | 0.06 |
| QTc 500–530 ms:QTc <500 ms | 1.88 | 1.18–2.99 | <0.01 |
| QTc >530 ms:QTc <500 ms | 3.22 | 2.06–5.05 | <0.001 |
| QTc missing*:QTc <500 ms | 2.07 | 1.29–3.33 | <0.01 |
| Dominant-negative:haploinsufficiency | 2.26 | 1.56–3.25 | <0.001 |
| Time-dependent β-blocker use | 0.21 | 0.09–0.48 | <0.001 |
The analysis involved 354 subjects with known or suspected ion channel dysfunction; 2 subjects were not included because of missing data about the date of their first cardiac event.
The QTc missing category involves 26 patients who died suddenly at a young age without a prior ECG.
Discussion
The main results of the present study from 600 patients having a spectrum of KCNQ1 mutations derived from 3 LQTS registries are significantly higher cardiac event rates in patients with transmembrane mutations and in patients with mutations having a putative dominant-negative effect on the repolarizing IKs current. The effect of these genetically determined factors is independent of traditional clinical risk factors and of β-blocker therapy.
Since 1995, when the first 2 genes responsible for LQTS were identified,11,12 molecular genetic studies have revealed a total of 9 forms of congenital LQTS caused by mutations in genes involving potassium channel (LQT-1, -2, -5, -6, and -7), sodium channel (LQT-3, -9), and calcium channel proteins (LQT-8) as well as a membrane-adapter protein (LQT-4).2,13 Genotype–phenotype studies have enabled us to stratify risk and to treat more specifically patients with LQT-1, LQT-2, and LQT-3 subtypes of this genetic disorder. LQT-1, the most common form of LQTS, accounts for ≈50% of genotyped patients4,14 and has more variable expressivity and incomplete penetrance than the other forms.15 Mutation location and knowledge of the functional effects of the mutation provide additional risk information beyond the clinical risk factors and the genotype, at least for LQT-1, and this information should contribute to improved risk stratification and more focused management of these higher-risk patients.
Mutations in KCNQ1 are responsible for defects in the slowly activating component of the delayed rectifier current IKs.16 This current is the main repolarizing current at increased heart rate and is highly sensitive to catecholamines.3 We speculate that IKs channels with transmembrane mutations might have reduced responsiveness to the regulatory β-adrenergic signaling of the ion-conduction pathway with more impairment of shortening of the QTc with exercise-related tachycardia than mutations in the C-terminus region.
Functional IKs channels result from the coassembly of 4 KCNQ1-encoded subunits. A mutated gene encodes a protein with aberrant function, and the presence of both normal and abnormal proteins in the ion channel contributes to a >50% reduction in ion channel function (dominant-negative effect). An alternative mechanism of reduced repolarizing KCNQ1 K+ current is the inability of mutated subunits to coassemble with normal gene products, such as occurs with a trafficking defect, resulting in a ≤50% reduction in channel function (haploinsufficiency). With only 1 exception,17 this is the case for all studied truncating mutations leading to incomplete proteins. Our assumption that truncated proteins (based on frameshift nonsense mutations) lead to haploinsufficiency seems justified. The biophysical effect of missense mutations is unpredictable, and both haploinsufficiency and dominant-negative effects have been described. In the absence of reported biophysical studies, missense mutations were classified as unknown.
Previous attempts to identify a genotype–phenotype relationship for KCNQ1 mutations failed to reach consensus on the clinical outcome of the type and site of mutations.7,8 Relatively small numbers and different ethnic background of the previously reported patients with the LQT-1 genotype might be responsible for the discrepant results. The present larger study allows us to demonstrate for the first time that the biophysical effect clearly affects the clinical outcome (ie, dominant-negative mutations are associated with a more severe phenotype than are mutations conferring haploinsufficiency [Figure 2C], even after adjustment for relevant covariates [Table 5]). The risk observed in 19 subjects with 3 different intron mutations was not quite significant (P=0.06), possibly because of small numbers, but the magnitude of the risk effect was similar to the risk accompanying transmem-brane mutations. Although these intron mutations produced splice-site alterations predicted to affect the transmembrane portion of the ion channel, we used a separate categorization of intron mutations in view of the limited understanding of the structural alterations and functional effects resulting from these exon-skipping intron mutations.
A few additional findings from this large genotype–phenotype study of type-1 LQTS patients emphasize high risk for first cardiac events during adolescence, a crossover in risk by sex at approximately age 13 years, and a lower rate of first cardiac events in the adult years than in the younger years. These findings are not especially new,18,19 but the present study highlights their presence in type-1 LQTS.
Study Limitations
The present study used the biophysical function of mutations reported in the literature in only a portion of the mutations that were included (see references associated with Table 1 in the online-only Data Supplement). The published studies were from many different laboratories with the use of different cellular heterologous expression systems involving Xenopus oocytes and other cells at both room and physiological temperatures. Although such nonuniform testing may have contributed to some inconsistency in the categorized biophysical function, the finding of a significantly higher event rate in mutations with dominant-negative than with haploinsufficient effects (hazard ratio, 2.26; P<0.001) is unlikely to have resulted from the nonuniform testing. Unfortunately, we did not have the resources to perform such uniform testing in all 77 mutations presented in the present study.
Once a mutation was identified in KCNQ1, thorough genetic sequencing was not performed routinely in all the ion channel genes to look for second mutations. Thus, some of the patients included in the analysis may have had a second mutation in addition to the identified KCNQ1 mutation. It is estimated that ≈10% of genotype LQTS patients may carry a second mutation, and those with >1 mutation could contribute to some of the findings in our study. In addition, it is possible that some of the reported mutations (Table 1) are simply uncommon sequence mutations, but this is relatively unlikely because all the subjects in the present study were derived from families in which the proband had QTc prolongation not due to a known cause.
The outcome analyses included subjects from families with a known KCNQ1 mutation who died suddenly and unexpectedly at a young age and were classified as LQTS-related death with the same mutation that was present in the family. It is possible that a few of these subjects could have died from a non-LQTS cause or had an LQTS mutation different from the family mutation, but we think the error rate is likely to be small. The number of deaths and aborted cardiac arrest events is small, and there is insufficient power to evaluate the risk association of the genotype characteristics with these endpoint events in a multivariate time-dependent model.
Conclusions
The present study confirms that in patients with type-1 LQTS, longer QTc intervals are associated with higher cardiac event rates and that male patients are generally younger than female patients at first cardiac events.20,21 The new findings from the present study are that transmembrane mutations and mutations with dominant-negative functional effect adversely influence the outcome of this disorder independent of traditional clinical risk factors and β-blocker therapy. The present study was not designed to assess the effectiveness of different therapies in patients with KCNQ1 mutations. The findings presented do not provide justification for using specific genotype characteristics to identify patients for implanted defibrillator therapy.
Supplementary Material
CLINICAL PERSPECTIVE.
Type-1 long-QT syndrome is caused by loss-of-function mutations in the KCNQ1-encoded IKs cardiac potassium channel. In the present study involving 600 patients having a spectrum of KCNQ1 mutations derived from 3 long-QT syndrome registries, we found that cardiac event rates are increased significantly in patients with mutations located in the transmembrane region of the potassium channel and in patients with mutations having a putative dominant-negative effect on the repolarizing IKs current. The effects of these genetically determined factors are independent of traditional clinical risk factors and of β-blocker therapy. Mutation location and knowledge of functional effects of the mutation provide additional risk information beyond the clinical risk factors and the genotype, at least for type-1 long-QT syndrome, and this information should contribute to improved risk stratification and more focused management of these higher-risk patients.
Acknowledgments
We thank David J. Tester, Senior Research Technologist, Sudden Death Genomics Laboratory, Mayo Clinic College of Medicine, Rochester, Minn, for the detailed review and assistance he provided on the terminology and nomenclature for the annotated mutations presented in Table 1.
Sources of Funding
This study was supported in part by (1) research grants HL-33843 and HL-51618 from the National Institutes of Health, Bethesda, Md (Dr Moss); (2) Ministry of Education, Culture, Sports, Science, and Technology Leading Project for Biosimulation and health sciences research grant (H18-Research on Human Genome-002) from the Ministry of Health, Labor, and Welfare, Japan (Dr Shimizu); (3) grant 2000.059 from the Nederlandse Hartstichting, Amsterdam, the Netherlands (Dr Wilde); and (4) research grants from the National Institutes of Health (HD42569), American Heart Association (Established Investigator Award), CJ Foundation for SIDS, and Dr Scholl Foundation (Dr Ackerman).
Footnotes
The online-only Data Supplement, consisting of references, is available with this article at http://circ.ahajournals.org/cgi/content/full/CIRCULATIONAHA.106.665406/DC1.
Disclosures
Dr Ackerman is a consultant for Clinical Data (formerly Genaissance Pharmaceuticals) with respect to the FAMILION genetic test for cardiac ion channel mutations. The other authors report no conflicts.
Note Added in Proof
After this article was accepted for publication, we noted the recent article by Tsuji et al, in which the A344A/sp [1032G>A] mutation that we categorized as haploinsufficient (Table 1) was reported to have a weak dominant-negative effect.22 We reran the KCNQ1 data recategorizing the 27 A344A/sp [1032 G>A] mutations as dominant-negative. Negligible changes occurred in the results as presented in Table 5 and Figure 2C; the hazard ratio for dominant-negative:haploinsufficiency (Table 5) was unchanged at 2.26 (P<0.001).
References
- 1.Moss AJ. Long QT syndrome. JAMA. 2003;289:2041–2044. doi: 10.1001/jama.289.16.2041. [DOI] [PubMed] [Google Scholar]
- 2.Wilde AA, Bezzina CR. Genetics of cardiac arrhythmias. Heart. 2005;91:1352–1358. doi: 10.1136/hrt.2004.046334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanguinetti MC. Long QT syndrome: ionic basis and arrhythmia mechanism in long QT syndrome type 1. J Cardiovasc Electrophysiol. 2000;11:710–712. doi: 10.1111/j.1540-8167.2000.tb00035.x. [DOI] [PubMed] [Google Scholar]
- 4.Tester DJ, Will ML, Haglund CM, Ackerman MJ. Compendium of cardiac channel mutations in 541 consecutive unrelated patients referred for long QT syndrome genetic testing. Heart Rhythm. 2005;2:507–517. doi: 10.1016/j.hrthm.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 5.Bianchi L, Priori SG, Napolitano C, Surewicz KA, Dennis AT, Memmi M, Schwartz PJ, Brown AM. Mechanisms of I(Ks) suppression in LQT1 mutants. Am J Physiol. 2000;279:H3003–H3011. doi: 10.1152/ajpheart.2000.279.6.H3003. [DOI] [PubMed] [Google Scholar]
- 6.Shalaby FY, Levesque PC, Yang WP, Little WA, Conder ML, Jenkins-West T, Blanar MA. Dominant-negative KvLQT1 mutations underlie the LQT1 form of long QT syndrome. Circulation. 1997;96:1733–1736. doi: 10.1161/01.cir.96.6.1733. [DOI] [PubMed] [Google Scholar]
- 7.Zareba W, Moss AJ, Sheu G, Kaufman ES, Priori S, Vincent GM, Towbin JA, Benhorin J, Schwartz PJ, Napolitano C, Hall WJ, Keating MT, Qi M, Robinson JL, Andrews ML. Location of mutation in the KCNQ1 and phenotypic presentation of long QT syndrome. J Cardiovasc Electrophysiol. 2003;14:1149–1153. doi: 10.1046/j.1540-8167.2003.03177.x. [DOI] [PubMed] [Google Scholar]
- 8.Shimizu W, Horie M, Ohno S, Takenaka K, Yamaguchi M, Shimizu M, Washizuka T, Aizawa Y, Nakamura K, Ohe T, Aiba T, Miyamoto Y, Yoshimasa Y, Towbin JA, Priori SG, Kamakura S. Mutation site-specific differences in arrhythmic risk and sensitivity to sympathetic stimulation in the LQT1 form of congenital long QT syndrome: multicenter study in Japan. J Am Coll Cardiol. 2004;44:117–125. doi: 10.1016/j.jacc.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 9.Cox DR. Regression models and life-tables. J Stat Soc [B] 1972;34:187–220. [Google Scholar]
- 10.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York, NY: Springer-Verlag; 2000. [Google Scholar]
- 11.Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81:299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 12.Wang Q, Shen J, Li Z, Timothy K, Vincent GM, Priori SG, Schwartz PJ, Keating MT. Cardiac sodium channel mutations in patients with long QT syndrome, an inherited cardiac arrhythmia. Hum Mol Genet. 1995;4:1603–1607. doi: 10.1093/hmg/4.9.1603. [DOI] [PubMed] [Google Scholar]
- 13.Vatta M, Ackerman MJ, Ye B, Makielski JC, Ughanze EE, Taylor EW, Tester DJ, Balijepalli RC, Foell JD, Li Z, Kamp TJ, Towbin JA. Mutant caveolin-3 induces persistent late sodium current and is associated with long-QT syndrome. Circulation. 2006;114:2104–2112. doi: 10.1161/CIRCULATIONAHA.106.635268. [DOI] [PubMed] [Google Scholar]
- 14.Splawski I, Shen J, Timothy KW, Lehmann MH, Priori S, Robinson JL, Moss AJ, Schwartz PJ, Towbin JA, Vincent GM, Keating MT. Spectrum of mutations in long-QT syndrome genes: KVLQT1, HERG, SCN5A, KCNE1, and KCNE2. Circulation. 2000;102:1178–1185. doi: 10.1161/01.cir.102.10.1178. [DOI] [PubMed] [Google Scholar]
- 15.Shimizu W, Noda T, Takaki H, Nagaya N, Satomi K, Kurita T, Suyama K, Aihara N, Sunagawa K, Echigo S, Miyamoto Y, Yoshimasa Y, Nakamura K, Ohe T, Towbin JA, Priori SG, Kamakura S. Diagnostic value of epinephrine test for genotyping LQT1, LQT2, and LQT3 forms of congenital long QT syndrome. Heart Rhythm. 2004;1:276–283. doi: 10.1016/j.hrthm.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 16.Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, Keating MT. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature. 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- 17.Aizawa Y, Ueda K, Wu LM, Inagaki N, Hayashi T, Takahashi M, Ohta M, Kawano S, Hirano Y, Yasunami M, Kimura A, Hiraoka M. Truncated KCNQ1 mutant, A178fs/105, forms hetero-multimer channel with wild-type causing a dominant-negative suppression due to trafficking defect. FEBS Lett. 2004;574:145–150. doi: 10.1016/j.febslet.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 18.Hobbs JB, Peterson DR, Moss AJ, McNitt S, Zareba W, Goldenberg I, Qi M, Robinson JL, Sauer AJ, Ackerman MJ, Benhorin J, Kaufman ES, Locati EH, Napolitano C, Priori SG, Towbin JA, Vincent GM, Zhang L. Risk of aborted cardiac arrest or sudden cardiac death during adolescence in the long-QT syndrome. JAMA. 2006;296:1249–1254. doi: 10.1001/jama.296.10.1249. [DOI] [PubMed] [Google Scholar]
- 19.Moss AJ, Schwartz PJ, Crampton RS, Tzivoni D, Locati EH, MacCluer J, Hall WJ, Weitkamp L, Vincent GM, Garson A., Jr The long QT syndrome: prospective longitudinal study of 328 families. Circulation. 1991;84:1136–1144. doi: 10.1161/01.cir.84.3.1136. [DOI] [PubMed] [Google Scholar]
- 20.Locati EH, Zareba W, Moss AJ, Schwartz PJ, Vincent GM, Lehmann MH, Towbin JA, Priori SG, Napolitano C, Robinson JL, Andrews M, Timothy K, Hall WJ. Age- and sex-related differences in clinical manifestations in patients with congenital long-QT syndrome: findings from the International LQTS Registry. Circulation. 1998;97:2237–2244. doi: 10.1161/01.cir.97.22.2237. [DOI] [PubMed] [Google Scholar]
- 21.Zareba W, Moss AJ, Locati EH, Lehmann MH, Peterson DR, Hall WJ, Schwartz PJ, Vincent GM, Priori SG, Benhorin J, Towbin JA, Robinson JL, Andrews ML, Napolitano C, Timothy K, Zhang L, Medina A. Modulating effects of age and gender on the clinical course of long QT syndrome by genotype. J Am Coll Cardiol. 2003;42:103–109. doi: 10.1016/s0735-1097(03)00554-0. [DOI] [PubMed] [Google Scholar]
- 22.Tsuji K, Akao M, Ishii TM, Ohno S, Makiyama T, Takenaka K, Doi T, Haruna Y, Yoshida H, Nakashima T, Kita T, Horie M. Mechanistic basis for the pathogenesis of long QT syndrome associated with a common splicing mutation in KCNQ1 gene. J Mol Cell Cardiol. 2007;42:662–669. doi: 10.1016/j.yjmcc.2006.12.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.